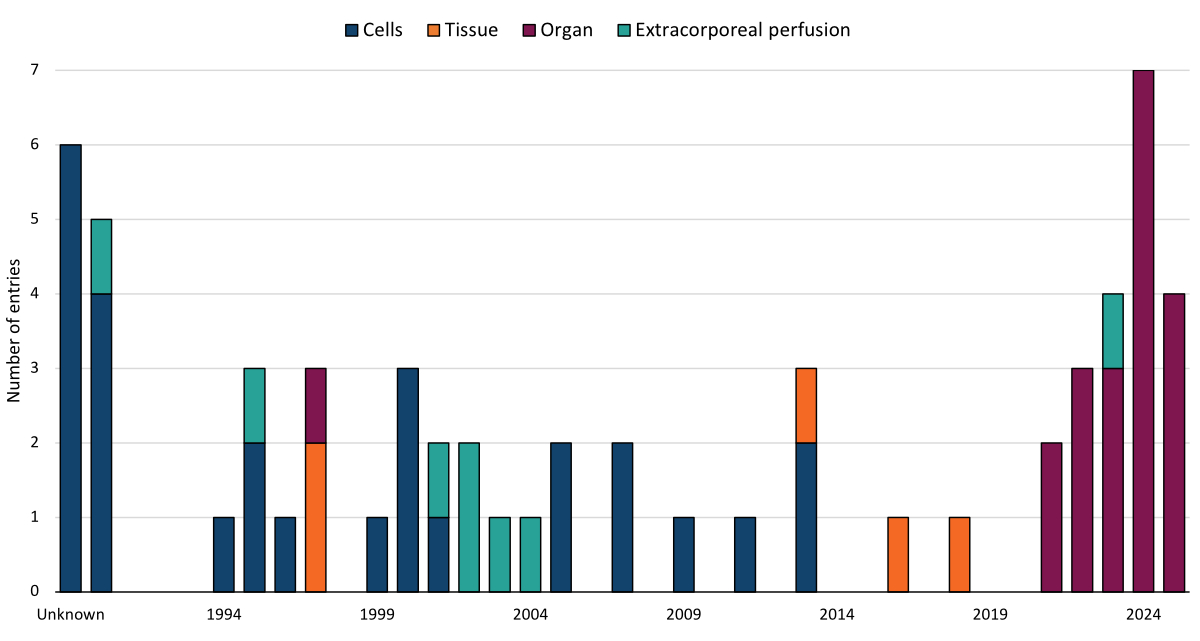
Figure 1Type of transplantation, by year, from 1990 to July 2025. Data from the International Human Xenotransplantation Inventory.
DOI: https://doi.org/https://doi.org/10.57187/s.4945
antibody-mediated rejection
end-stage kidney disease
Food and Drug Administration
gene-edited
International Xenotransplantation Association
nonhuman primate
porcine cytomegalovirus
the Transplantation Society
World Health Organization
Organ allotransplantation remains the definitive treatment for end-stage organ failure, yet the persistent shortage of human donor organs leads to prolonged waiting times and significant mortality among patients on transplant waiting lists. In 2024, 75 patients died while awaiting transplantation in Switzerland, and 1,331 patients were still on the waiting list at the end of the year [1]. Although the principle of presumed consent is scheduled to be implemented in Switzerland in 2027 [2], the shortage of donor organs is expected to remain a major barrier to meeting the needs of an ageing population. This imbalance between organ supply and demand is exacerbated by the increasing prevalence of chronic diseases, which increases the need for transplantation and reduces the number of medically suitable donors [3].
In this context, xenotransplantation, particularly the use of gene-edited (GE) pigs as organ sources, has emerged as a promising alternative to human donation [4-7]. A more readily available organ supply could allow transplantation at earlier stages of organ failure, improving patients’ quality of life and long-term outcomes [8]. Nevertheless, the clinical application of xenotransplantation faces considerable challenges, primarily due to pronounced immunological and haematological incompatibilities between species. In addition, concerns persist regarding the perceived risk of zoonosis (the transmission of infectious diseases from animals to humans), especially given the immunosuppressive therapies required to prevent graft rejection.
After decades of preclinical research in nonhuman primates (NHPs), significant progress in gene editing and novel immunosuppressive strategies has led to a series of landmark studies. These achievements have brought xenotransplantation into the spotlight, particularly following the first transplants of gene-edited porcine hearts and kidneys into living human recipients in recent years [9].
According to data from the International Human Xenotransplantation Inventory, a registry that collects information on all xenotransplantation procedures in humans worldwide, cellular and tissue-based xenotransplantation has represented the majority of clinical activity over the past two decades. These procedures primarily involve islet of Langerhans transplantation trials and other cell types [10]. Building on encouraging preclinical results from pig-to-NHP models, the first porcine kidney xenotransplantation study in a human was conducted in 2021 in the USA. It involved the transplantation of genetically modified kidneys with a single gene knockout into two recipients who were brain-dead and maintained on circulatory and respiratory support (table 1) [11, 12].
Table 1Current gene edits in pigs for xenotransplantation.
| GE type | Description of genetic modifications |
| 1-GE | Knockout of the main carbohydrate xenoantigen present in pigs, similar to the human ABO blood group antigen |
| 6-GE | Knockout of three major carbohydrate xenoantigens; insertion of one human coagulation inhibitory gene and two complement regulatory genes |
| 10-GE | Same three carbohydrate knockouts as in 6-GE, plus knockout of the porcine growth hormone receptor. Transgenic expression of two human coagulation inhibitory genes, two human complement regulatory genes, one anti-inflammatory agent, and one innate immunity regulator |
| 69-GE | As in 10-GE, with additional inactivation of 59 porcine endogenous retrovirus sequences |
This study confirmed the absence of hyperacute rejection over a 54-hour observation period and marked the beginning of clinical trials in solid organ xenotransplantation. Over the past three years, a total of two hearts, five kidneys, and one liver from genetically modified pigs have been transplanted into living human recipients, making solid organ xenotransplantation the main current clinical xenotransplantation activity (figure 1).

Figure 1Type of transplantation, by year, from 1990 to July 2025. Data from the International Human Xenotransplantation Inventory.
Many experts anticipated that a kidney xenograft would be the first to receive clinical approval from the U.S. Food and Drug Administration (FDA), as kidney failure is not immediately life-threatening and patients can return to dialysis in case of xenograft failure. However, the FDA approved a porcine heart xenotransplantation in 2022 under a compassionate use authorisation. This first case, performed at the University of Maryland in Baltimore, involved a 57-year-old patient with end-stage heart failure who was ineligible for allotransplantation. The patient received a 10-GE porcine heart along with a novel immunosuppressive regimen based on an anti-CD40 agent. The graft initially functioned well and showed no signs of hyperacute rejection. However, acute diastolic dysfunction occurred on day 49 following administration of intravenous immunoglobulin (IVIg) for suspected porcine cytomegalovirus (pCMV) reactivation. Life support was withdrawn on day 60. Autopsy findings did not suggest classical acute rejection but revealed antibody-mediated endothelial injury [13].
The following year, the same institution performed a second porcine heart xenotransplantation in a 58-year-old patient with end-stage heart failure who was also deemed ineligible for conventional treatment. This patient also received a 10-GE porcine heart under compassionate use approval. The immunosuppressive protocol included an anti-CD154 agent, which has shown superior efficacy in NHP models [14], and enhanced screening of source animals was conducted to minimise the risk of zoonotic infection. Although the graft initially functioned well, signs of antibody-mediated rejection (AMR) appeared on biopsy by day 13. The clinical course was complicated by an episode of cardiorespiratory arrest and noninfectious diarrhoea requiring total parenteral nutrition. Rapid deterioration of cardiac function occurred on day 29, and the patient died on day 40. Biopsies did not show evidence of pCMV involvement, but AMR was again implicated in the graft failure [15].
Following additional studies in human recipients who were brain-dead, five porcine kidney xenotransplants have been performed in living patients since March 2024. The first was carried out at Massachusetts General Hospital in Boston in a 62-year-old patient with end-stage kidney disease (ESKD) and a history of failed allotransplantation. The patient received a 69-GE porcine kidney with an intensified immunosuppressive protocol including T- and B-cell depletion, terminal complement blockade, and an anti-CD154 agent. The graft functioned well and eliminated the need for haemodialysis until the patient, who had a history of coronary artery disease, experienced an unexpected fatal cardiac event on day 52 [16].
One month later, a team at New York University performed a kidney xenotransplant in a 54-year-old patient with combined end-stage heart and kidney failure who had received a left ventricular assist device (LVAD) one week earlier. The patient received a 1-GE porcine kidney with conventional immunosuppression, and the graft was removed on day 47 due to complications related to complex haemodynamics associated with the LVAD [17]. Several months later, the same team transplanted a 10-GE porcine kidney into a 53-year-old patient ineligible for allotransplantation due to HLA hypersensitisation. Despite conventional immunosuppression, the graft showed progressive dysfunction and was explanted after 130 days, representing the longest duration of a porcine kidney in a living human at the time [18]. The cause of graft failure appeared to be rejection precipitated by lowered immunosuppression in the setting of a bacterial infection [19].
At the time of writing, three patients worldwide are living with porcine kidney xenografts, all derived from genetically modified pigs: (1) a patient at Massachusetts General Hospital in Boston, USA, who received a 69-GE kidney on January 25, 2025 [20]; (2) a patient at Xijing Hospital in Xi’an, China, who received a 6-GE kidney on March 6, 2025 [21]; and (3) a patient at Massachusetts General Hospital who received a 69-GE kidney on June 14, 2025 [22]. In addition to classical tacrolimus–mycophenolate–prednisone-based triple immunosuppression, both American patients are maintained on an anti-CD154 regimen, which appears to be a key component of immunosuppressive strategies in xenotransplantation [14, 23]. Details regarding the immunosuppressive treatment of the Chinese patient are not yet available.
A single case of porcine liver xenotransplantation has been reported, involving the transplantation of a 10-GE liver into a 71-year-old patient following a right lobectomy for hepatocellular carcinoma. Early reports mention normalisation of hepatic function and no signs of rejection after 13 days, but no further updates on the patient’s course are available [24].
The first cases of clinical xenotransplantation have been limited by the significant comorbidities of the recipients, which considerably complicated their overall medical management due to issues unrelated to the transplantation itself. The first heart transplant recipient was on haemodialysis, the second developed ventricular fibrillatory arrest 12 hours before the surgery requiring cardiopulmonary resuscitation and defibrillation, and the initial kidney transplant recipients also had cardiac conditions. At the current stage of clinical xenotransplantation development, managing advanced multi-organ dysfunction is an avoidable challenge and should be considered during patient selection. In comparison, the current surviving recipients have fewer serious comorbidities and low levels of sensitisation, which optimise clinical management of the xenograft.
In early 2025, Revivicor and eGenesis, two American companies developing genetically modified pigs, received FDA approval to initiate clinical trials for kidney failure. Revivicor’s trial will begin with six patients aged 55 to 70 who have ESKD and are either ineligible for allotransplantation or unlikely to receive a transplant within five years despite being on the waiting list. eGenesis plans to include three patients in its trial [25]. Following its successful kidney xenotransplantation in March 2025, the Chinese company ClonOrgan Biotechnology has also expressed interest in expanding its clinical xenotransplantation program within China.
This phase represents a critical moment for clinical xenotransplantation, as attention shifts from short-term feasibility to medium-term graft survival. With better-selected recipients and improved protocols, ongoing cases will provide essential insight into xenograft longevity in humans. Notably, the longest surviving patient has now passed the six-month mark, a milestone that will help define the future direction of the field.
Amid significant progress and growing public interest in xenotransplantation, the 18th Congress of the International Xenotransplantation Association (IXA) will be held in Geneva, Switzerland, from 30 September to 3 October 2025 [26]. The Swiss city, home to the headquarters of the World Health Organization (WHO), has long-standing ties to the field. In 2004, in the early stages of modern xenotransplantation research, the WHO adopted resolution WHA57.18 on human organ and tissue transplantation, which included pioneering recommendations on xenotransplantation. This led to the gathering of an expert panel in Geneva in 2005, where a framework for the safe and ethical implementation of clinical xenotransplantation was proposed. The following year, in collaboration with the WHO and the Transplantation Society (TTS), the International Human Xenotransplantation Inventory was established and managed by Geneva University Hospitals until 2020. Now, two decades later, hosting the IXA Congress in Geneva marks both a symbolic and practical milestone, at a time when clinical applications are beginning to show real-world potential.
Clinical xenotransplantation has entered a critical phase, moving from proof-of-concept cases to more structured and prospective upcoming trials. The progress over the past three years, particularly in kidney xenotransplantation, demonstrates the growing feasibility of this approach. With refined immunosuppressive strategies and patient selection, longer graft survival is within reach, and the focus is shifting towards assessing mid- and long-term outcomes. Ongoing trials in the USA and potentially China will provide valuable insight into the safety, durability, and scalability of xenotransplantation as a clinical solution to organ shortage. The upcoming IXA Congress in Geneva is well timed to serve as a forum for coordination and scientific exchange as xenotransplantation moves closer to clinical reality.
This work was supported by a Swiss National Science Foundation SINERGIA grant to RR and JDS (SNSF # CRSII5_198577).
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. DKCC is a consultant to eGenesis Bio of Cambridge, Massachusetts, USA. No other potential conflict of interest related to the content of this manuscript was disclosed.
1. Swisstransplant. Rapport annuel 2024. Bern, Switzerland: Swisstransplant; 2025.
2. Office fédéral de la santé publique. Premier volet de révision : introduction du consentement présumé pour le don d’organes: Office fédéral de la santé publique (OFSP); 2024 [2025-07-20]. Available from: https://www.bag.admin.ch/fr/premier-volet-de-revision-introduction-du-consentement-presume-pour-le-don-d-organes
3. Vanholder R, Domínguez-Gil B, Busic M, Cortez-Pinto H, Craig JC, Jager KJ, et al. Organ donation and transplantation: a multi-stakeholder call to action. Nat Rev Nephrol. 2021 Aug;17(8):554–68.
4. Carrier AN, Verma A, Mohiuddin M, Pascual M, Muller YD, Longchamp A, et al. Xenotransplantation: A New Era. Front Immunol. 2022;13:900594. Epub 2022/06/28. doi: . PubMed PMID: 35757701; PubMed Central PMCID: PMCPMC9218200.
5. Meier RP, Longchamp A, Mohiuddin M, Manuel O, Vrakas G, Maluf DG, et al. Recent progress and remaining hurdles toward clinical xenotransplantation. Xenotransplantation. 2021 May;28(3):e12681.
6. Meier RP, Muller YD, Balaphas A, Morel P, Pascual M, Seebach JD, et al. Xenotransplantation: back to the future? Transpl Int. 2018 May;31(5):465–77.
7. Meier RPH, Pierson RNI, Fishman JA, Buhler LH, Bottino R, Ladowski JM, et al. International Xenotransplantation Association (IXA) Position Paper on Kidney Xenotransplantation. Transplantation. 2025:. doi: . PubMed PMID: 00007890-990000000-01051.
8. Bera KD, Shah A, English MR, Harvey D, Ploeg RJ. Optimisation of the organ donor and effects on transplanted organs: a narrative review on current practice and future directions. Anaesthesia. 2020 Sep;75(9):1191–204.
9. Mou L, Pu Z, Cooper DK. Clinical Xenotransplantation of Gene-Edited Pig Organs: A Review of Experiments in Living Humans Since 2022. Medicine Bulletin. 2025;1(1):77-85. doi: https://doi.org/
10. Hu X, Hawthorne WJ, Buhler L. The International Human Xenotransplantation Inventory: Current Data and Future Directions. Transplantation. 2025:. doi: . PubMed PMID: 00007890-990000000-01048.
11. Montgomery RA, Stern JM, Lonze BE, Tatapudi VS, Mangiola M, Wu M, et al. Results of Two Cases of Pig-to-Human Kidney Xenotransplantation. N Engl J Med. 2022 May;386(20):1889–98.
12. Loupy A, Goutaudier V, Giarraputo A, Mezine F, Morgand E, Robin B, et al. Immune response after pig-to-human kidney xenotransplantation: a multimodal phenotyping study. Lancet. 2023 Sep;402(10408):1158–69.
13. Mohiuddin MM, Singh AK, Scobie L, Goerlich CE, Grazioli A, Saharia K, et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report. Lancet. 2023 Jul;402(10399):397–410.
14. Perrin S, Magill M. The Inhibition of CD40/CD154 Costimulatory Signaling in the Prevention of Renal Transplant Rejection in Nonhuman Primates: A Systematic Review and Meta Analysis. Frontiers in Immunology. 2022;Volume 13 - 2022. doi: .
15. Griffith BP, Grazioli A, Singh AK, Tully A, Galindo J, Saharia KK, et al. Transplantation of a genetically modified porcine heart into a live human. Nat Med. 2025 Feb;31(2):589–98.
16. Kawai T, Williams WW, Elias N, Fishman JA, Crisalli K, Longchamp A, et al. Xenotransplantation of a Porcine Kidney for End-Stage Kidney Disease. N Engl J Med. 2025 May;392(19):1933–40.
17. DeVries C. First-Ever Combined Heart Pump & Gene-Edited Pig Kidney Transplant Gives New Hope to Patient with Terminal Illness: NYU Langone News Hub; 2024 [20.04.2025]. Available from: https://nyulangone.org/news/first-ever-combined-heart-pump-gene-edited-pig-kidney-transplant-gives-new-hope-patient-terminal-illness
18. DeVries C. Gene-Edited Pig Kidney Gives Living Donor New Lease on Life: NYU Langone News Hub; 2024 [20.04.2025]. Available from: https://nyulangone.org/news/gene-edited-pig-kidney-gives-living-donor-new-lease-life
19. Montgomery RA. Oral presentation. American Transplant Congress (ATC) 2025; San Francisco, USA2025.
20. Massachusetts General Hospital Performs Second Groundbreaking Xenotransplant of Genetically-Edited Pig Kidney into Living Recipient [Internet]. 2025; 2025-02-07 [cited 2025-07-20]. Available from: https://www.massgeneral.org/news/press-release/mgh-performs-second-xenotransplant-of-genetically-edited-pig-kidney-into-living-recipient
21. Wang J. Duojiyin bianji zhushen yizhi renti shoushu zai xi’an huo chenggong [Multi-gene-edited pig kidney transplantation into human successfully performed in Xi'an]. Online: Xinhua News; 2025. Available from: http://sn.news.cn/20250313/b816104fba294be4ae3420d301959816/c.html. Chinese.
22. Riella LV. Oral presentation. World Transplant Congress 2025; San Francisco, USA 2025.
23. Bühler L, Awwad M, Basker M, Gojo S, Watts A, Treter S, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000 Jun;69(11):2296–304.
24. Dazhongribao. Dujia jiemi shijie shouli ganai bingren yizhi zhugan shoushu: huanzhe shuhou dishisantian zhuangtai lianghao [People's Daily. Exclusive: World’s First Pig Liver Transplant in Liver Cancer Patient – Stable on Postoperative Day 13]. Sina News. 2024 2024-05-30. Chinese.
25. Cohen J. The organ farm. Science. 2025 May;388(6750):906–12.
26. International Xenotransplantation Association. 18th Congress of the International Xenotransplantation Association Online 2025 [2025-07-20]. Available from: https://ixa2025.org/