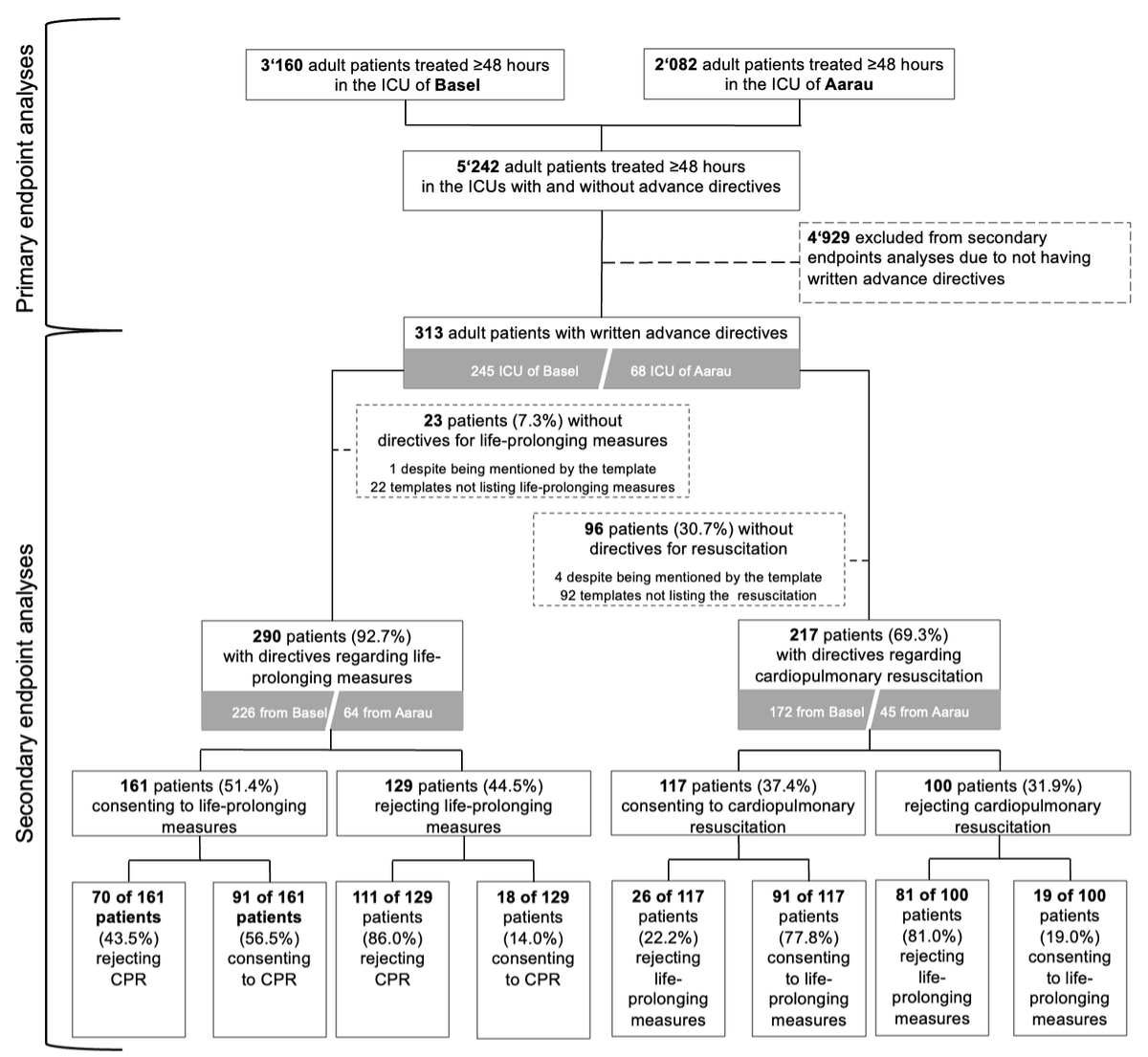
Figure 1Flowchart. CPR: cardiopulmonary resuscitation; ICU: intensive care unit. For the definition of life-prolonging measures, please refer to the Methods section.
DOI: https://doi.org/https://doi.org/10.57187/s.4625
Advance directives are legal instruments empowering individuals to specify healthcare preferences in case of incapacitation [1] (Swiss Academy of Medical Sciences: https://www.samw.ch). Despite their importance, the consistent use and screening for directives by healthcare professionals is insufficient. A review of 17 studies involving 149,413 patients and 1210 healthcare professionals found varying prevalence rates of advance directives in critically ill patients in intensive care units (ICUs), ranging from 2.6% in Northern and Southern Europe to 49% in North America, with consistently low screening rates (<10%) among healthcare providers [2]. When identified, advance directives are associated with increased do-not-resuscitate orders, care limitations, shorter ICU stays and reduced costs. However, challenges in implementing directives include inconsistent wording, variable adherence and complex patient preferences [2]. For instance, older adults in Switzerland have shown conflicting preferences, such as preferring cardiopulmonary resuscitation (CPR) while rejecting life-prolonging treatments such as mechanical ventilation [3]. These challenges result in physicians following directives by withholding resuscitation for up to 25% of patients [2]. While some studies suggest that advance directives influence treatment decisions, evidence remains inconclusive and hampered by methodological issues [4–9]. Some studies suggest that patients with directives are less likely to receive life-sustaining treatments or die in hospitals [10–12], but a clear association with directives has yet to be demonstrated. A recent review reported that care adjustments are based on directives in 71% of cases in neurocritical ill adults [13]. However, the quality of evidence remains weak, with a high risk of bias [13].
Advance directives in elderly or frail patients may facilitate discussions with families, potentially enhancing ethical practices [14]. Limited evidence, however, suggests that directives influence ICU decisions on life-prolonging measures, with increased communication and ethical practices linked to better patient-centred outcomes [14]. The low prevalence of directives is concerning, and studies promoting their use and translation are lacking [13, 15]. The latter is critical as advances in modern intensive care medicine led to new and unforeseen situations with a substantial amount of patients surviving with severe disabilities [16–19]. Disabled ICU survivors, in addition, can be a significant psychological and economic burden for their relatives and families [16, 19]. Therefore, high-quality evidence regarding the use and translation of directives into clinical ICU practice is urgently needed.
Thus, given the evolving landscape of critical care and the increasing complexity of treatment decisions, the primary aim of the present study was to investigate the prevalence of advance directives regarding CPR and life-prolonging measures in ICU patients of two Swiss ICUs. To contextualise how advance directives are created, interpreted and translated into clinical practice and which patient profiles influence whether and how patients choose to formulate advance directives and what aspects they include, secondary objectives included analysing their content, clinical translation, and associated patient characteristics and clinical outcomes.
This retrospective observational bicentric cohort study was performed at the ICUs of the Cantonal Hospital of Aarau and the University Hospital of Basel, both Swiss tertiary (academic) medical care centres. The ICU-specific characteristics of the two multidisciplinary ICUs during the study period were as follows:
In Aarau, the number of beds was between 20 and 30; the most frequent main diagnoses were cardiovascular, followed by gastrointestinal, metabolic, trauma, respiratory and neurological; the median NEMS (nine equivalents of nursing manpower use score) for the first 24 hours was 9 (interquartile range [IQR]: 1–18); the median length of stay was 0.9 days (0.6–1.9); and all-cause mortality was 3.1%.
In Basel, the number of beds was between 30 and 35; the most frequent main diagnoses were cardiovascular, followed by neurological, respiratory, gastrointestinal, metabolic and trauma; the median NEMS for the first 24 hours was 18 (IQR: 18–27); the median length of stay was 1.3 days (IQR: 0.9–2.6); and all-cause mortality was 6.1%.
The ethics committee of Northwest and Central Switzerland (https://www.eknz.ch/) approved the study (ID2020-00584), and the requirement for patientconsent was waived. Data were collected for the ongoing bicentric ADVISE (Advance Directive Implementation and Scientific Evaluation) study (https://clinicaltrials.gov ID NCT04348318). STROBE guidelines were followed for study conduct, data acquisition, analysis and reporting [20]. Also, the study was conducted following the ethical principles laid down in the 1964 Declaration of Helsinki [21] and its later amendments. Information regarding the local Swiss jurisdiction can be obtained from the supplemental digital content in the appendix [22, 23].
From January 2020 to December 2022 (deviating from the original study protocol and its amendments aiming to assess data from 2011 to 2022), the data of all consecutive adult patients (≥18 years) who were treated for ≥48 hours in ICUs of either centre were collected from the digital medical patient records and entered into a predefined online assessment interface within the database organiser REDCap version 14.1.2 (Research Electronic Data Capture) hosted by the University Hospital of Basel and developed by Vanderbilt University. REDCap is a secure, web-based software platform designed for data collection and management, featuring audit trails, user-level access controls and compliance with HIPAA and GDPR [24].
In addition to demographic data, the following clinical data were collected: ICU admission characteristics, principal diagnoses and severity of illness (as described in the following section) at ICU admission, level of consciousness at ICU admission, treatment characteristics, including noninvasive or mechanical ventilation, mechanical haemodynamic support, administration of vasopressors, antibiotics, opioids, artificial nutrition, blood products, and insertion of catheters and/or drains during intensive care. Patient records were further screened for notes regarding treatment adjustments during the ICU stay, including withdrawal/discontinuation of life-prolonging treatment and change of CPR status. The time elapsed from ICU admission to change of CPR status was noted. In addition, notes and discussions about withholding life-prolonging measures were assessed. Furthermore, we assessed inpatient outcomes, such as death and return to premorbid function at discharge and complications during ICU care, including infections and severe arterial hypotension requiring the use of vasopressors, as reported in patient records.
Illness severity was graded at admission to the ICU by using theAcute Physiology And Chronic Health Evaluation (APACHE) II score [25, 26], the Charlson Comorbidity Index [27] and the Good Outcome Following Attempted Resuscitation (GO-FAR) score [28, 29]. In brief, the GO-FAR score is a validated scoring system used in hospitalised patients to assess their likelihood of surviving a cardiac arrest with a good neurological outcome, defined as independent daily functioning with minimal to no disability [28, 29]. The score is based on various factors that can affect a patient’s prognosis after a cardiac arrest, such as their age, underlying medical conditions and the initial rhythm of their heart, with lower scores indicating a better chance of survival with a good neurological outcome [28].
The following aspects of the patients’ advance directives were assessed and entered into the database:
Screening of all advance directives and data extraction were performed by four reviewers (SMB, PSCK, YE, DV), with continuous consultations to guarantee data robustness. In cases where interpretation of the directive content was unclear or ambiguous, RS (the study supervisor) was consulted. These cases were then discussed in a joint review process to reach consensus.
The primary endpoint was the prevalence of directives regarding CPR and life-prolonging measures in patients of two Swiss ICUs. Although detailed data analysis was limited to ICU patients with documented advance directives, we chose to report prevalence data relative to the entire ICU population during the study period. For the authors, this approach was justified by the current paucity of data on the use of advance directives in Swiss ICUs, making it essential to contextualise our findings by illustrating the overall frequency of advance directives in this setting.
Secondary endpoints were the clinical translation of directives, differences between patients consenting and not consenting to life-prolonging measures, their clinical characteristics, clinical translation, patient in-hospital outcomes including death, return to premorbid function and median GO-FAR scores. Clinical translation of directives was measured by treatment adaptations during intensive care, including withholding and/or withdrawal of life-sustaining measures, change of CPR status, time elapsed from ICU admission to treatment adaptations, and time elapsed from ICU admission to change of CPR status. These secondary endpoints were critical in contextualising how patients’ directives are created, interpreted and translated into clinical practice and which patient profiles influence whether and how patients choose to formulate directives and what content they include.
For descriptive statistics regarding baseline and admission characteristics and characteristics of the directives’ clinical translation, continuous variables were summarised using medians and interquartile ranges (IQRs). In contrast, categorical variables were summarised using counts and percentages. Further, patients were categorised as consenting or not to life-prolonging measures and consenting or not to CPR according to their directives. Among these groups, univariable comparisons of proportions were performed by the chi-squared test or Fisher’s exact test. For comparisons of continuous variables, first the Shapiro-Wilk test was used to distinguish between normally and non-normally distributed variables. Second, normally distributed variables were analysed with the Student’s t-test, whereas variables violating the normal distribution were analysed with the Mann-Whitney U test. The significance level for univariable comparisons of baseline and admission characteristics was adjusted using the Bonferroni correction for multiple comparisons and set at a significance level at a two-sided p-value ≤0.01 due to the Bonferroni correction.
Multivariable analyses were performed to identify characteristics independently associated with not consenting to life-prolonging measures and CPR using logistic regression, with significant associations being defined at a two-sided p-value ≤0.05. Variables differing significantly in our univariable comparisons between patients consenting and not consenting to life-prolonging treatment or CPR were included in our multivariable models. As withdrawal/withholding of life-prolonging treatment due to presumed poor prognosis by the treating physicians may confound patients’ consent to life-prolonging measures or CPR, we decided to include this variable into the multivariable models independent of its performance in the univariable comparisons. The Hosmer-Lemeshow chi-squared goodness-of-fit tests were performed for multivariable logistic regression models, which provide summary measures of calibration based upon a comparison of observed and estimated outcomes [30].
Statistical analysis was performed using Stata version 16.1 (StataCorp LLC, College Station, TX, USA).
No custom software libraries or unpublished code were used beyond built-in REDCap and Stata functionalities. The analytical workflow relied on standard Stata procedures, and no novel code was developed. As a result, no analytical code repository or DOI is available.
Among 5242 ICU patients treated for ≥48 hours in the two ICUs (3160 in Basel; 2082 in Aarau), advance directives were retrospectively identified in 313 patients (6.0%; 245 in Basel; 68 in Aarau).
The proportion of patients with and without directives regarding life-prolonging treatment and CPR and the proportion of patients consenting and not consenting to these measures are presented in the flowchart (figure 1). Of the 313 patients with available advance directives, 290 (92.7%) provided directives regarding life-prolonging measures and 217 (69.3%) provided directives regarding CPR. Of the 161 patients consenting to life-prolonging measures overall, 91 (56.5%) also consented to CPR. However, 18 of 129 patients not consenting to life-prolonging measures (14.0%) consented to CPR despite explicitly refusing life-prolonging measures and 26 of 117 patients consenting to CPR (22.2%) did not wish to receive life-prolonging measures (figure 1).

Figure 1Flowchart. CPR: cardiopulmonary resuscitation; ICU: intensive care unit. For the definition of life-prolonging measures, please refer to the Methods section.
86.3% of advance directives were completed using standardised templates, 91.4% designated a healthcare proxy and the median time between advance directive completion and ICU admission was two years (IQR: 0–4 years).
Appendix table S1 presents the demographics, clinical baseline and admission characteristics, and illness severity scores of all critically ill patients with written advance directives. Patients with advance directives were elderly (median age: 72 years, IQR: 65–79) and had a high burden of comorbidities (median Charlson Comorbidity Index: 5, IQR: 4–7). 131 patients (41.9%) were previously treated in intensive care units and 73 patients (23.3%) were care-dependent prior to hospital admission.
The median Glasgow Coma Scale (GCS) score at ICU admission was 8 (IQR: 3–14) and only 98 patients (31.3%) had a GCS ≥14, indicating the potential ability to communicate their will independently. Thus, 215 (68.7%) had a lower GCS score, with 157 patients (48.6%) being comatose (GCS ≤8) at ICU admission.
Univariable comparisons of demographics and baseline characteristics between critically ill ICU patients consenting and not consenting to life-prolonging measures (n = 290) and cardiopulmonary resuscitation (n = 217) are presented in table 1 (upper half). Comparisons revealed that women declined life-prolonging measures more frequently than men despite having the same median age (72 years [IQR: 66–78] for men and 72 years [IQR: 65–78] for women). Patients previously treated in an ICU were more likely to consent to life-prolonging measures. Further comparisons revealed no differences regarding care dependency, civil status or confession after Bonferroni correction for multiple comparisons.
Table 1Univariable comparisons of demographics and baseline characteristics between critically ill patients treated in the ICU for >48 hours and consenting and not consenting to life-prolonging measures (n = 290) and cardiopulmonary resuscitation (n = 217) according to their advance directives.
| Characteristics | Patients consenting to life-prolonging measures (n=161) | Patients not consenting to life-prolonging measures (n=129) | p-value* | ||
| Demographics and baseline characteristics | n / median | % / IQR | n / median | % / IQR | |
| …Age (years; median, IQR) | 71 | 65-78 | 72 | 67-80 | 0.109 |
| …Female sex (n, %) | 52 | 32.3 | 61 | 47.3 | 0.009 |
| Baseline characteristics | |||||
| …Care dependency before admission (n, %) | 39 | 24.2 | 30 | 23.3 | 0.847 |
| …Previous ICU stay (n, %) | 81 | 50.3 | 42 | 32.6 | 0.002 |
| Civil status (n, %) | |||||
| …Married | 82 | 50.9 | 57 | 44.2 | 0.836 |
| …In partnership | 12 | 7.5 | 13 | 10.1 | |
| …Divorced | 10 | 6.2 | 8 | 6.2 | |
| …Single | 4 | 2.5 | 2 | 1.6 | |
| …Widowed | 11 | 6.8 | 11 | 8.5 | |
| …Unknown / Not stated | 42 | 26.1 | 38 | 29.5 | |
| Confession (n, %) | |||||
| …None | 64 | 39.8 | 48 | 37.2 | 0.276 |
| …Roman Catholic | 46 | 28.6 | 34 | 26.4 | |
| …Reformed | 40 | 24.8 | 34 | 26.4 | |
| …Other | 4 | 2.5 | 10 | 7.8 | |
| …Unknown / Not stated | 7 | 4.4 | 3 | 2.3 | |
| Characteristics | Patients consenting to CPR (n=117) | Patients not consenting to CPR (n=100) | p-value* | ||
| Demographics | n / median | % / IQR | n / median | % / IQR | |
| …Age (years; median, IQR) | 69 | 62–77 | 75 | 68–82 | <0.001 |
| …Female sex (n, %) | 35 | 29.9 | 52 | 52.0 | 0.001 |
| Baseline characteristics | |||||
| …Care dependency before admission (n, %) | 23 | 19.7 | 26 | 26.0 | 0.265 |
| …Previous ICU stay (n, %) | 51 | 43.6 | 36 | 36.0 | 0.255 |
| Civil status (n, %) | |||||
| …Married | 61 | 52.1 | 40 | 40.0 | 0.148 |
| …In partnership | 12 | 10.3 | 8 | 8.0 | |
| …Divorced | 8 | 6.8 | 5 | 5.0 | |
| …Single | 2 | 1.7 | 2 | 2.0 | |
| …Widowed | 6 | 5.1 | 14 | 14.0 | |
| …Unknown / Not stated | 28 | 23.9 | 31 | 31.0 | |
| Confession (n, %) | |||||
| …None | 50 | 42.7 | 43 | 43.0 | 0.038 |
| …Roman Catholic | 31 | 26.5 | 21 | 21.0 | |
| …Reformed | 27 | 23.1 | 26 | 26.0 | |
| …Other | 2 | 1.7 | 9 | 9.0 | |
| …Unknown / Not stated | 7 | 6.0 | 1 | 1.0 | |
CPR: cardiopulmonary resuscitation; ICU: intensive care unit; IQR: interquartile range.
* Statistical significance set at a two-sided p-value ≤0.009 after Bonferroni correction for multiple comparisons. Bolding indicates significance.
Univariable comparisons between patients consenting and not consenting to CPR (n = 217) revealed that patients not consenting to CPR were older and more frequently women (table 1, lower half).
Regarding CPR directives, no other differences in baseline or admission characteristics were identified between patients wanting and not wanting to be resuscitated after Bonferroni correction for multiple comparisons.
Table 2 presents multivariable models, including all demographic and clinical baseline characteristics significantly differing between patients consenting and not consenting to life-prolonging measures and CPR. These analyses revealed that female sex was independently associated with not consenting to life-prolonging measures and CPR.
Table 2Uni- and multivariable analyses regarding characteristics associated with patients not consenting to life-prolonging measures and CPR.
| Characteristics | Univariable analyses | Multivariable analyses | ||||
| Characteristics associated with not consenting to life-prolonging measures | uaOR | 95% CI | p-value | aOR | 95% CI | p-value* |
| …Female sex (vs male sex) | 1.88 | 1.17–3.03 | 0.010 | 1.66 | 1.02–2.72 | 0.043 |
| …Previous ICU stay (vs previous stay) | 0.48 | 0.29–0.77 | 0.003 | 0.51 | 0.31–0.83 | 0.007 |
| …Withdrawal/withholding life-prolonging measures due to presumed poor prognosis (vs no withdrawal/withholding) | 0.28 | 0.11–0.70 | 0.007 | 0.31 | 0.12–0.79 | 0.015 |
| Characteristics associated with not consenting to CPR | uaOR | 95% CI | p-value | aOR | 95% CI | p-value** |
| …Age (per increasing number of years) | 1.04 | 1.01–0.06 | 0.008 | 1.04 | 1.01–1.06 | 0.008 |
| …Female sex (vs male sex) | 2.54 | 1.45–4.43 | 0.001 | 2.56 | 1.44–4.55 | 0.001 |
| …Withdrawal/withholding life-prolonging measures due to presumed poor prognosis (vs no withdrawal/withholding) | 0.41 | 0.17–1.04 | 0.060 | 0.41 | 0.16–1.05 | 0.062 |
aOR: adjusted odds ratio; CI: confidence interval; CPR: cardiopulmonary resuscitation; ICU: intensive care unit; uaOR: unadjusted odds ratio.
* Hosmer-Lemeshow goodness-of-fit test, chi-squared: 0.70; p = 0.706. Bolding indicates significance.
** Hosmer-Lemeshow goodness-of-fit test, chi-squared: 2.77; p = 0.948. Bolding indicates significance.
Characteristics of the clinical translation and treatment adaptation of patients’ advance directives among all patients (n = 313) are outlined in table 3, left columns.
Table 3Characteristics of the clinical translation and treatment adaptation of patients’ advance directives among all patients (n = 313) and their univariable comparisons between patients consenting and not consenting to life-prolonging measures (n = 290).
| Characteristics | Patients with advance directives (total cohort n = 313, incl. 23 patients providing no information in this context) | Patients consenting to life-prolonging measures (n = 161) | Patients not consenting to life-prolonging measures (n = 129) | ||||
| Treatment adaptation during intensive care | n / median | % / IQR | n / median | % / IQR | n / median | % / IQR | p-value* |
| …Overall (not mutually exclusive; n, %) | 104 | 33.2 | 52 | 32.3 | 42 | 32.6 | 0.963 |
| ……Withholding of life-sustaining measures (n, %) | 76 | 24.3 | 42 | 26.1 | 27 | 20.9 | 0.305 |
| ……Withdrawal of life-sustaining measures (n, %) | 63 | 20.1 | 35 | 21.7 | 22 | 17.1 | 0.318 |
| ……Time from ICU admission to withdrawal of life-sustaining measures (days; median, IQR) | 6 | 3–11 | 6 | 3–13 | 5 | 2–8 | 0.072 |
| ……CPR status changed (n, %) | 63 | 20.1 | 32 | 19.9 | 24 | 18.6 | 0.785 |
| ……Time from ICU admission to CPR status change (days; median, IQR) | 3 | 1–8 | 6 | 2–9 | 1 | 0–5 | 0.003 |
| Noted reasons for treatment adaptation during intensive care (n, %) | n | % of subgroup | n | % of subgroup | n | % of subgroup | p-value* |
| …Of all patients with treatment adaptations (of 104 patients with treatment adaptations) | n | % of 104 | n | % of 52 | n | % of 42 | |
| ……Translation of patients’ directives | 53 | 51.0 | 20 | 38.5 | 27 | 64.3 | 0.013 |
| ……Presumed poor prognosis irrespective of directives | 33 | 31.7 | 24 | 46.2 | 6 | 14.3 | 0.002 |
| ……Treatment adaptation following reasoning of third parties (i.e., relatives, healthcare agents) | 4 | 3.9 | 3 | 5.8 | 1 | 2.4 | |
| ……No reasoning indicated in patient records | 14 | 13.5 | 5 | 9.6 | 8 | 19.0 | |
| …Reasons for withdrawal of life-sustaining measures of all patients (of 63 patients with withdrawal) | n | % of 63 | n | % of 35 | n | % of 22 | |
| ……Translation of patients’ directives | 37 | 58.7 | 15 | 42.9 | 18 | 81.8 | 0.006 |
| ……Presumed poor prognosis irrespective of directives | 23 | 36.5 | 18 | 51.4 | 3 | 13.6 | 0.005 |
| ……Treatment adaptation following reasoning of third parties (i.e. relatives, healthcare agents) | 3 | 4.8 | 2 | 5.7 | 1 | 4.6 | |
| ……No reasoning indicated in patient records | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| …Reasons for withholding of life-sustaining measures (of 76 patients with treatment adaptations) | n | % of 76 | n | % of 42 | n | % of 27 | |
| ……Translation of patients’ directives | 39 | 51.3 | 16 | 38.1 | 19 | 70.4 | 0.009 |
| ……Presumed poor prognosis irrespective of directives | 30 | 39.5 | 23 | 54.8 | 4 | 14.8 | 0.001 |
| ……Treatment adaptation following reasoning of third parties (i.e. relatives, healthcare agents) | 2 | 2.6 | 2 | 4.8 | 0 | 0.0 | |
| ……No reasoning indicated in patient records | 5 | 6.6 | 1 | 2.4 | 4 | 14.8 | |
| …Reasons for CPR status change (of 63 patients with CPR status change) | n | % of 63 | n | % of 32 | n | % of 24 | |
| ……Translation of patients’ directives | 31 | 49.2 | 12 | 37.5 | 16 | 66.7 | 0.031 |
| ……Presumed poor prognosis irrespective of directives | 21 | 33.3 | 16 | 50.0 | 2 | 8.3 | 0.001 |
| ……Treatment adaptation following reasoning of third parties (i.e. relatives, healthcare agents) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| ……No reasoning indicated in patient records | 11 | 17.5 | 4 | 12.5 | 6 | 25.0 | |
| In-hospital outcomes | |||||||
| …Length of intensive care unit stay (days; median, IQR) | 6 | 3–11 | 6 | 4–11 | 5 | 3–9 | 0.240 |
| …Length of hospital stay (days; median, IQR) | 17 | 11–31 | 18 | 12–32 | 17 | 10–31 | 0.113 |
| …In-hospital death (n, %) | 104 | 33.2 | 47 | 29.2 | 49 | 38.0 | 0.114 |
| …Return to premorbid function (n, %) | 63 | 20.1 | 40 | 24.8 | 19 | 14.7 | 0.033 |
CPR: cardiopulmonary resuscitation; ICU: intensive care unit; IQR: interquartile range.
* Statistical significance set at a two-sided p-value ≤0.01 after the Bonferroni correction for multiple comparisons. Bolding indicates significance.
Overall, treatment modifications documented in physician notes revealed that withdrawal/withholding of life-sustaining therapies and/or changes in CPR status occurred in over one-third of patients. The median time from ICU admission to treatment change was 3 days (IQR: 3–11) for CPR status and 6 days (IQR: 1–8) for other life-sustaining measures. Notably, 51.0% of treatment adaptations aligned with patients’ directives, while 31.7% were based on presumed poor prognosis independent of directives or followed reasoning by surrogate decision-makers in 3.9% of cases. The reasons explicitly for withdrawal and/or withholding of life-sustaining measures and the change of CPR status revealed the same reasons with similar proportions as outlined in table 3.
Univariate analysis (n = 290) assessed the association between patient consent to life-prolonging measures and treatment adaptation according to documented directives as outlined in table 3, right columns. Analyses regarding the median time from ICU admission to CPR status change revealed that CPR status was changed sooner in patients not consenting to life-prolonging measures compared to those consenting.
Further details regarding withdrawal and withholding of specific life-sustaining measures among critically ill ICU patients consenting or not consenting to life-prolonging measures according to their advance directives (n = 290) are presented in appendix table S2. While the descriptive analyses revealed no differences regarding withdrawal or withholding of intensive care, CPR and treatment of infections, the number of patients not consenting to life-prolonging measures was higher regarding withdrawal or withholding of haemodynamic, respiratory and renal support, surgery, artificial nutrition and the administration of blood products.
In-hospital outcomes revealed no significant difference between patients consenting and not consenting to life-prolonging measures after the Bonferroni correction for multiple comparisons (table 3). Analyses regarding the median GO-FAR scores of survivors and non-survivors, as well as patients with and without return to premorbid baseline of critically ill patients with advance directives treated in the ICU for >48 hours consenting or not consenting to life-prolonging measures are shown in figures 2A and 2B. These analyses revealed no significant differences in median GO-FAR scores between survivors and non-survivors, regardless of their prior consent to life-prolonging measures. Similarly, surviving and non-surviving patients who did not consent to life-prolonging measures had comparable median GO-FAR scores.
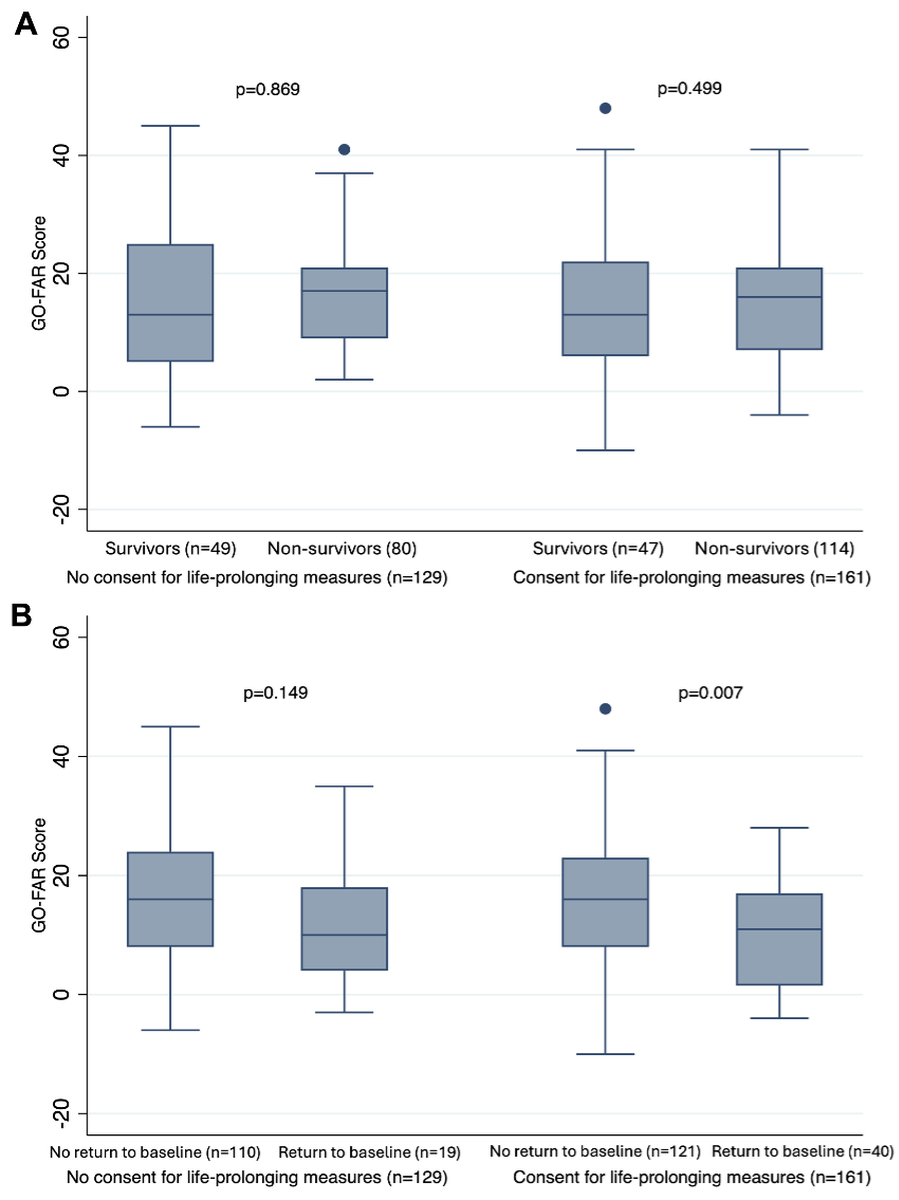
Figure 2Median GO-FAR scores of (A) survivors and non-survivors as well as (B) patients with and without return to premorbid baseline of critically ill patients with advance directives treated in the ICU for >48 hours who consented or did not consent to life-prolonging measures. Box plot measures: centre line (box midline) represents the median GO-FAR score of each subgroup; box edges (top and bottom of the box) represent the 25th percentile (lower quartile) and 75th percentile (upper quartile) – this is the interquartile range (IQR); whiskers (extending from the box) to the smallest and largest values are within 1.5 × IQR from the lower and upper quartiles, respectively. They capture the main range of the data; dots outside the whiskers represent outliers, i.e. individual patients with GO-FAR scores beyond 1.5 × IQR from the box edges. GO-FAR: Good Outcome Following Attempted Resuscitation (range: −15 to 76) [41]. GO-FAR score and corresponding risk group (survival to discharge with minimal neurological disability*): GO-FAR score: ≥24, very low survival (<1%); GO-FAR score: 14 to 23, low survival (1–3%); GO-FAR score: −5 to 13, average survival (3–15%); GO-FAR score: −15 to −6, above-average survival (>15%). * Patient is conscious, alert and able to work but might have mild neurological or psychological deficits, such as mild dysphagia or minor cranial nerve abnormalities [28].
In contrast, analyses of the GO-FAR scores for patients who consented to life-prolonging measures revealed higher scores in patients not reverting to their premorbid function (median score: 16 [IQR: 8–23] vs 11 [IQR: 1.5–17]; p = 0.007).
This bicentric Swiss observational study investigated the prevalence, clinical translation and associated outcomes of advance directives in critically ill intensive care unit (ICU) patients. Our primary finding is that only 6% of patients admitted for ≥48 hours had a documented advance directive, a concerningly low proportion in a population at high risk of decisional incapacity. This low proportion of advance directives reinforces the urgent need for earlier and more widespread advance care planning, particularly as over two-thirds of patients with advance directives had a Glasgow Coma Scale (GCS) score <14 at ICU admission and were likely unable to express their will – underscoring the importance of preemptive documentation as previously discussed [2].
These results are consistent with previous studies, including a large prospective multicentre cohort involving 12,870 ICU patients across 199 ICUs in 36 countries, which reported an overall prevalence of advance directives of 13% in Central Europe, and even lower (3–4%) in Northern/Southern Europe, Asia, Latin America, Australia/New Zealand and Africa [14]. In Switzerland, national survey data estimate that only up to one-quarter of the population has a written advance directive, and only a minority are recognised upon hospital admission [3, 31]. The low prevalence of advance directives in our sample likely reflects this national reality, particularly among severely ill patients where decisional incapacity is common.
In contrast, the prevalence of advance directives in the US is higher, presumably due to the widespread use of central registries [14, 32]. In Switzerland, according to a national survey, only one-third of healthcare professionals are commonly involved in cardiopulmonary resuscitation (CPR), approximetely two-thrids posses advance directives, two-thirds would refuse mechanical ventilation for themselves, and approximetely 60% would not want to be resuscitated [33].
Our analysis of the content of advance directives revealed substantial internal inconsistencies that suggest important challenges in patients’ understanding of intensive care and end-of-life decision-making. While 93% of patients with advance directives addressed life-prolonging treatments, only 69% specified preferences regarding CPR. Moreover, a notable proportion of patients expressed contradictory wishes, such as 14% of those declining life-prolonging treatments still requesting CPR, and 22% of CPR-consenting patients refusing other life-sustaining interventions.
These findings are hypothesis-generating and suggest that, despite widespread use of standardised advance directive templates (86%) and healthcare proxy designations (91%), many patients may not fully grasp the medical implications of their decisions. This mirrors findings of the large prospective multicentre cohort work by Feldman et al. mentioned above [14], where patients frequently chose CPR but declined other interventions that would typically be part of post-resuscitation ICU care. Similar inconsistencies were also reported in a Swiss general population survey [3], in which many individuals who favoured CPR also rejected intubation and mechanical ventilation – despite the reality that CPR usually involves both.
These inconsistencies likely reflect misunderstandings about the goals and outcomes of intensive care and CPR, as well as a potential overestimation of recovery potential from cardiac arrest [3]. They also point to structural limitations in the current advance directives templates, which may oversimplify complex clinical realities or fail to guide patients in articulating preferences that are internally coherent.
While these descriptive findings cannot establish causation, they provide a strong rationale for future prospective studies evaluating how educational interventions or guided discussions may improve the quality, clarity and consistency of advance directives. Another approach would be refining templates to improve clarity and facilitate physician adherence to patient wishes. In particular, adding a section about the minimum quality of life acceptable for the individual to continue living might allow physicians to refrain from life-sustaining therapies when the probability of achieving the predefined minimum quality of life is minimal. As proposed in the “care planning umbrella model” by Hickman et al., caregivers, patients and relatives should decide together if a proposed treatment will result in the desired quality of life the patient has pre-specified [34]. Previous studies regarding interventions to increase the quality and recognition of advance directives revealed that the most effective approach is multimodal, using informative material and repeated conversations between healthcare professionals and patients [35].
Another hypothesis-generating finding in our study is the observed sex difference in treatment preferences. In our study, female sex was independently associated with declining both life-prolonging interventions and CPR, even after adjustment for age, comorbidities and previous ICU admission. This pattern is consistent with prior literature showing that women are more likely to limit intensive interventions [36] and that female sex is a risk factor for care limitation in the ICU [37, 38].
Our retrospective data cannot identify the underlying causes of this association, but potential explanations include sociocultural and generational influences, such as greater social isolation among elderly women or different perceptions of quality of life. These findings should be further explored in future studies using qualitative or prospective designs. Sociocultural factors may plausibly influence decision-making, especially among older cohorts. Women in these groups may face limited social support and isolation due to their longer life expectancy, often outliving their male counterparts. Additionally, men may still encounter more significant challenges in assuming caregiving responsibilities for their wives at home compared to the reverse. The latter, however, represents another hypothesis that deserves further attention in future studies.
Conversely, patients with prior ICU experience were more likely to consent to life-prolonging treatments. This may suggest that personal familiarity with intensive care fosters trust, rather than deterring patients from future ICU-level interventions. This finding, while exploratory, supports the idea that patient experience is a key factor influencing end-of-life preferences and may be valuable to address in advance care planning conversations.
Despite the relatively low prevalence and inconsistent content of advance directives, we observed that in 51.0% of cases, documented treatment adaptations aligned with the advance directives, while 31.7% were guided by presumed prognosis and 3.9% by surrogate decision-makers – proportions consistent with findings from the international study by Feldman et al. [14]. This suggests that advance directives, when available and interpretable, are often considered in clinical decision-making.
That said, treatment changes, including withdrawal or withholding of interventions and changes in CPR status, occurred early, typically within the first 3 to 6 days of ICU admission, and CPR preferences were acted on more quickly when patients had declined life-prolonging measures. These patterns imply that clear directives may expedite decision-making, although the retrospective design limits causal inferences.
The variation in adherence to advance directives and reliance on surrogate decision-making further supports the need for more structured advance care planning that allows for interpretation aligned with patients’ values and prognosis. Again, these are hypothesis-generating insights that require confirmation in prospective designs.
Importantly, no significant differences in in-hospital mortality or functional recovery were observed between patients consenting or not consenting to life-prolonging measures. This is a reassuring signal that respecting patient autonomy – even when it entails forgoing intensive interventions – does not necessarily compromise survival or recovery. These findings are, however, exploratory and limited by potential confounding and selection bias.
Among patients consenting to life-prolonging measures, those who failed to return to premorbid function had significantly higher GO-FAR scores, indicating a worse baseline prognosis (median 16 vs 11; p = 0.007). This difference may reflect that patients who are open to aggressive care sometimes carry an inherently higher risk of poor recovery. While these associations do not imply causality, they suggest a potential role for prognostic tools like GO-FAR to inform advance care planning and align expectations between patients and clinicians.
The bicentric observational design limits the generalisability. However, many characteristics of our population are similar to those of other adult ICU cohort studies, such as age [39, 40], sex category [40], admission diagnosis [39] and illness severity [40]. Due to the retrospective nature of our study and inconsistent documentation in patient records, we could not attain comprehensive data regarding the exact time the treating healthcare professionals recognised directives. Therefore, we cannot exclude that patients for whom directives were recognised but not documented by the treating healthcare workers might have been missed. However, as the availability of advance directives is regarded as a significant advantage in patient-centred treatment, it seems unlikely that many such scenarios have not been documented. Another shortcoming of the retrospective design is the lack of proof that the decision to withdraw and/or withhold care was exclusively based on the patients’ directives. Limited by the study’s retrospective nature, we could not investigate the underlying reasons for the absence of patient directives. Hence, we could not distinguish between patients who never completed an advance directive and those for whom existing directives were not located or identified. As our study aimed to assess the prevalence of directives, this potential shortcoming did not affect our results.
Another confounder might be the fusion of the two separate ICUs into one at the medical care centre in Aarau in 2021 and concurrent team composition adaptations. The retrospective design did not allow any assessments regarding the connection between the patients’ quality of life and advance directives. However, the latter was not the aim of our study and could be overcome with future prospective studies. Lastly, the exclusion of patients without directives did not allow us to examine potential differences in patient characteristics between individuals with and without advance directives. The latter, however, was not the aim of the present study.
The prevalence of patients admitted to intensive care with advance directives is low, and substantial obstacles must be overcome to translate advance directives into clinical practice.
While the retrospective design limits causal conclusions, the hypothesis-generating findings of our secondary endpoint analyses suggest that current advance directive practices may not fully support informed, value-based decision-making at the end of life. Our data reveal inconsistent or contradictory content of advance directives that highlight a need for better preemptive communication and documentation of patient wishes. Honouring patients’ choices to refuse life-prolonging treatments does not seem to reduce their chances of survival or recovery. This highlights the ethical responsibility to improve advance care planning – by providing better systems, clearer tools and more effective education to support informed decision-making.
The datasets generated and analysed during the current study are not publicly available due to data protection regulations and institutional policy governing the use of sensitive patient information. However, deidentified study data (including associated data dictionaries) may be made available upon reasonable request to the principal investigator (RS), contingent upon approval by the corresponding institutional ethics committee and in compliance with applicable data privacy laws.
Requests must specify the intended use and type of analyses proposed. Data sharing will be limited to qualified researchers for purposes of scientific replication or secondary analyses that are aligned with the original research aims. Data access will be granted on a case-by-case basis under a formal data use agreement, and no data will be shared for commercial use.
Additional study documentation (e.g. study protocol, analysis code) may also be shared upon request where applicable. Data will remain available for such requests for a period of 5 years following publication of the study.
For all inquiries, please contact the principal investigator (i.e. corresponding author).
The corresponding author had full access to the complete dataset, and assumes responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions: Raoul Sutter, Sira M. Baumann, Dominik Vock and Simon A. Amacher planned and designed the study. Raoul Sutter, Sira M. Baumann, Paulina S.C. Kliem, Yasmin Erne and Simon A. Amacher acquired and interpreted the data. Raoul Sutter analysed the data and wrote the manuscript. Sira M. Baumann, Simon A. Amacher, Yasmin Erne, Pascale Grzonka, Sebastian Berger, Sabina Hunziker, Martin Lohri, Caroline E. Gebhard, Mathias Nebiker, Luca Cioccari and Raoul Sutter interpreted the data, revised the manuscript and substantially contributed to the initial draft. All authors approved the final submitted version.
Use of artificial intelligence-assisted technologies: The grammar-checking software Grammarly (Grammarly, San Francisco, USA) was used to proofread and increase readability. The large language model ChatGPT-4omni (OpenAI, San Francisco, USA) was used to paraphrase and summarise some of the manuscript’s content. After using these applications, the authors edited the content as needed and verified its accuracy. The corresponding author takes full responsibility.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Sira M. Baumann reports no disclosures. – Dominik Vock reports no disclosures. – Paulina S.C. Kliem reports no disclosures. – Simon A. Amacher reports no disclosures. Unrelated to the present work, he received grants from the Mach-Gaensslen Foundation Switzerland and the Nora van Meeuwen-Haefliger Foundation of the University of Basel, Switzerland. – Yasmin Erne reports no disclosures. – Pascale Grzonka reports no disclosures. – Sebastian Berger reports no disclosures. – Martin Lohri reports no disclosures. – Sabina Hunziker reports no disclosures. Unrelated to the present work, she is supported by the Swiss National Foundation (SNF) (Ref 10001C_192850/1 and 10531C_182422), the Gottfried Julia Bangerter-Rhyner Foundation (8472/HEG-DSV) and the Swiss Society of General Internal Medicine (SSGIM). – Caroline E. Gebhard reports no disclosures. – Mathias Nebiker reports no disclosures. – Luca Cioccari reports no disclosures. Unrelated to the present work, he received grants from the Research Fund of the Clinical Trials Unit, University of Bern, and from the Foundation for Research in Anaesthesia and Intensive Care, University Hospital of Bern, and from the Research Council of the Cantonal Hospital of Aarau, Switzerland as well as educational grants from Hamilton Medical and Fresenius Medical Care and speaker honoraria from OrphaSwiss. All are unrelated to the present work. – Raoul Sutter reports no disclosures. Unrelated to the present work, he received research grants from the Swiss National Foundation (No 320030_169379), the Research Fund of the University of Basel, the Scientific Society Basel and the Gottfried Julia Bangerter-Rhyner Foundation. He received personal grants from UCB Pharma and holds stocks in Alcon, Novartis, Roche and Johnson & Johnson.
1. Bosshard G, Zellweger U, Bopp M, Schmid M, Hurst SA, Puhan MA, et al. Medical End-of-Life Practices in Switzerland: A Comparison of 2001 and 2013. JAMA Intern Med. 2016 Apr;176(4):555–6.
2. Baumann SM, Kruse NJ, Kliem PS, Amacher SA, Hunziker S, Dittrich TD, et al. Translation of patients’ advance directives in intensive care units: are we there yet? J Intensive Care. 2023 Nov;11(1):53.
3. Gross S, Amacher SA, Rochowski A, Reiser S, Becker C, Beck K, et al. “Do-not-resuscitate” preferences of the general Swiss population: results from a national survey. Resusc Plus. 2023 Apr;14:100383.
4. Teno JM, Lynn J, Phillips RS, Murphy D, Youngner SJ, Bellamy P, et al.; Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Do formal advance directives affect resuscitation decisions and the use of resources for seriously ill patients? SUPPORT Investigators [doi]. J Clin Ethics. 1994;5(1):23–30.
5. Smedira NG, Evans BH, Grais LS, Cohen NH, Lo B, Cooke M, et al. Withholding and withdrawal of life support from the critically ill. N Engl J Med. 1990 Feb;322(5):309–15.
6. Schneiderman LJ, Kronick R, Kaplan RM, Anderson JP, Langer RD. Effects of offering advance directives on medical treatments and costs. Ann Intern Med. 1992 Oct;117(7):599–606.
7. Lo B, Saika G, Strull W, Thomas E, Showstack J. ‘Do not resuscitate’ decisions. A prospective study at three teaching hospitals [doi]. Arch Intern Med. 1985 Jun;145(6):1115–7.
8. Goodman MD, Tarnoff M, Slotman GJ. Effect of advance directives on the management of elderly critically ill patients. Crit Care Med. 1998 Apr;26(4):701–4.
9. Danis M, Southerland LI, Garrett JM, Smith JL, Hielema F, Pickard CG, et al. A prospective study of advance directives for life-sustaining care. N Engl J Med. 1991 Mar;324(13):882–8.
10.Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality of end-of-life care: a national study. J Am Geriatr Soc. 2007 Feb;55(2):189–94.
11. Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010 Apr;362(13):1211–8.
12. Degenholtz HB, Rhee Y, Arnold RM. Brief communication: the relationship between having a living will and dying in place. Ann Intern Med. 2004 Jul;141(2):113–7.
13. Sutter R, Meyer-Zehnder B, Baumann SM, Marsch S, Pargger H. Advance Directives in the Neurocritically Ill: A Systematic Review [doi]. Crit Care Med. 2020 Aug;48(8):1188–95.
14. Feldman C, Sprung CL, Mentzelopoulos SD, Pohrt A, Hartog CS, Danbury C, et al.; Ethicus-2 Study Group. Global Comparison of Communication of End-of-Life Decisions in the ICU. Chest. 2022 Nov;162(5):1074–85.
15. Sprung CL, Truog RD, Curtis JR, Joynt GM, Baras M, Michalsen A, et al. Seeking worldwide professional consensus on the principles of end-of-life care for the critically ill. The Consensus for Worldwide End-of-Life Practice for Patients in Intensive Care Units (WELPICUS) study. Am J Respir Crit Care Med. 2014 Oct;190(8):855–66.
16. Herridge MS, Azoulay É. Outcomes after Critical Illness. N Engl J Med. 2023 Mar;388(10):913–24.
17. Rousseau AF, Prescott HC, Brett SJ, Weiss B, Azoulay E, Creteur J, et al. Long-term outcomes after critical illness: recent insights. Crit Care. 2021 Mar;25(1):108.
18. Geense WW, Zegers M, Peters MA, Ewalds E, Simons KS, Vermeulen H, et al. New Physical, Mental, and Cognitive Problems 1 Year after ICU Admission: A Prospective Multicenter Study. Am J Respir Crit Care Med. 2021 Jun;203(12):1512–21.
19. Schwitzer E, Jensen KS, Brinkman L, DeFrancia L, VanVleet J, Baqi E, et al. Survival ≠ Recovery: A Narrative Review of Post-Intensive Care Syndrome. CHEST Crit Care. 2023;1(1):100003.
20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct;370(9596):1453–7.
21. Rickham PP. Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki [doi]. BMJ. 1964 Jul;2(5402):177.
22. Richtlinien Patientenverfügung SA. Available at: https://www.samw.ch/de/Ethik/Themen-A-bis-Z/Patientenverfuegung.html
23. Swiss Medical Association - Advance directive. Available at: https://www.fmh.ch/dienstleistungen/recht/patientenverfuegung.cfm
24. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81.
25. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system [doi]. Crit Care Med. 1985 Oct;13(10):818–29. doi: https://doi.org/10.1097/00003246-198510000-00009
26. Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system [doi]. Crit Care Med. 1981 Aug;9(8):591–7. doi: https://doi.org/10.1097/00003246-198108000-00008
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation [doi]. J Chronic Dis. 1987;40(5):373–83. doi: https://doi.org/10.1016/0021-9681(87)90171-8
28. Ebell MH, Jang W, Shen Y, Geocadin RG; Get With the Guidelines–Resuscitation Investigators. Development and validation of the Good Outcome Following Attempted Resuscitation (GO-FAR) score to predict neurologically intact survival after in-hospital cardiopulmonary resuscitation. JAMA Intern Med. 2013 Nov;173(20):1872–8.
29. Amacher SA, Blatter R, Briel M, Appenzeller-Herzog C, Bohren C, Becker C, et al. Predicting neurological outcome in adult patients with cardiac arrest: systematic review and meta-analysis of prediction model performance. Crit Care. 2022 Dec;26(1):382.
30. Hosmer DW, Lemesbow S. A goodness of-fit test for the multiple logistic regression model [doi]. Commun Stat Theory Methods. 1980;9(10):1043–69. doi: https://doi.org/10.1080/03610928008827941
31. Baumann SM, Amacher SA, Erne Y, Grzonka P, Berger S, Hunziker S, et al. Advance directives in the intensive care unit: an eight-year vanguard cohort study. J Crit Care. 2025 Feb;85:154918.
32. Baumann SM, Kruse NJ, Kliem PS, Amacher SA, Hunziker S, Dittrich TD, et al. Translation of patients’ advance directives in intensive care units: are we there yet? J Intensive Care. 2023 Nov;11(1):53.
33. Amacher SA, Gross S, Becker C, Arpagaus A, Urben T, Gaab J, et al. Misconceptions and do-not-resuscitate preferences of healthcare professionals commonly involved in cardiopulmonary resuscitations: A national survey. Resusc Plus. 2024 Feb;17:100575.
34. Hickman SE, Lum HD, Walling AM, Savoy A, Sudore RL. The care planning umbrella: the evolution of advance care planning. J Am Geriatr Soc. 2023 Jul;71(7):2350–6.
35. Tamayo-Velázquez MI, Simón-Lorda P, Villegas-Portero R, Higueras-Callejón C, García-Gutiérrez JF, Martínez-Pecino F, et al. Interventions to promote the use of advance directives: an overview of systematic reviews. Patient Educ Couns. 2010 Jul;80(1):10–20.
36. Kaufmann M, Perren A, Cerutti B, Dysli C, Rothen HU; Swiss Society of Intensive Care Medicine. Severity-Adjusted ICU Mortality Only Tells Half the Truth-The Impact of Treatment Limitation in a Nationwide Database. Crit Care Med. 2020 Dec;48(12):e1242–50.
37. Block L, Petzold M, Syrous AN, Lindqvist B, Odenstedt Hergès H, Naredi S. Age, SAPS 3 and female sex are associated with decisions to withdraw or withhold intensive care. Acta Anaesthesiol Scand. 2019 Oct;63(9):1210–5.
38. Amacher SA, Zimmermann T, Gebert P, Grzonka P, Berger S, Lohri M, et al.; Swiss ICU Trial Group. Sex disparities in ICU care and outcomes after cardiac arrest: a Swiss nationwide analysis. Crit Care. 2025 Jan;29(1):42.
39. Kruser JM, Aaby DA, Stevenson DG, Pun BT, Balas MC, Barnes-Daly MA, et al. Assessment of Variability in End-of-Life Care Delivery in Intensive Care Units in the United States. JAMA Netw Open. 2019 Dec;2(12):e1917344–1917344.
40. Hartog CS, Peschel I, Schwarzkopf D, Curtis JR, Westermann I, Kabisch B, et al. Are written advance directives helpful to guide end-of-life therapy in the intensive care unit? A retrospective matched-cohort study. J Crit Care. 2014 Feb;29(1):128–33.
41. Ebell MH, Jang W, Shen Y, Geocadin RG; Get With the Guidelines–Resuscitation Investigators. Development and validation of the Good Outcome Following Attempted Resuscitation (GO-FAR) score to predict neurologically intact survival after in-hospital cardiopulmonary resuscitation. JAMA Intern Med. 2013 Nov;173(20):1872–8.
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.4625.