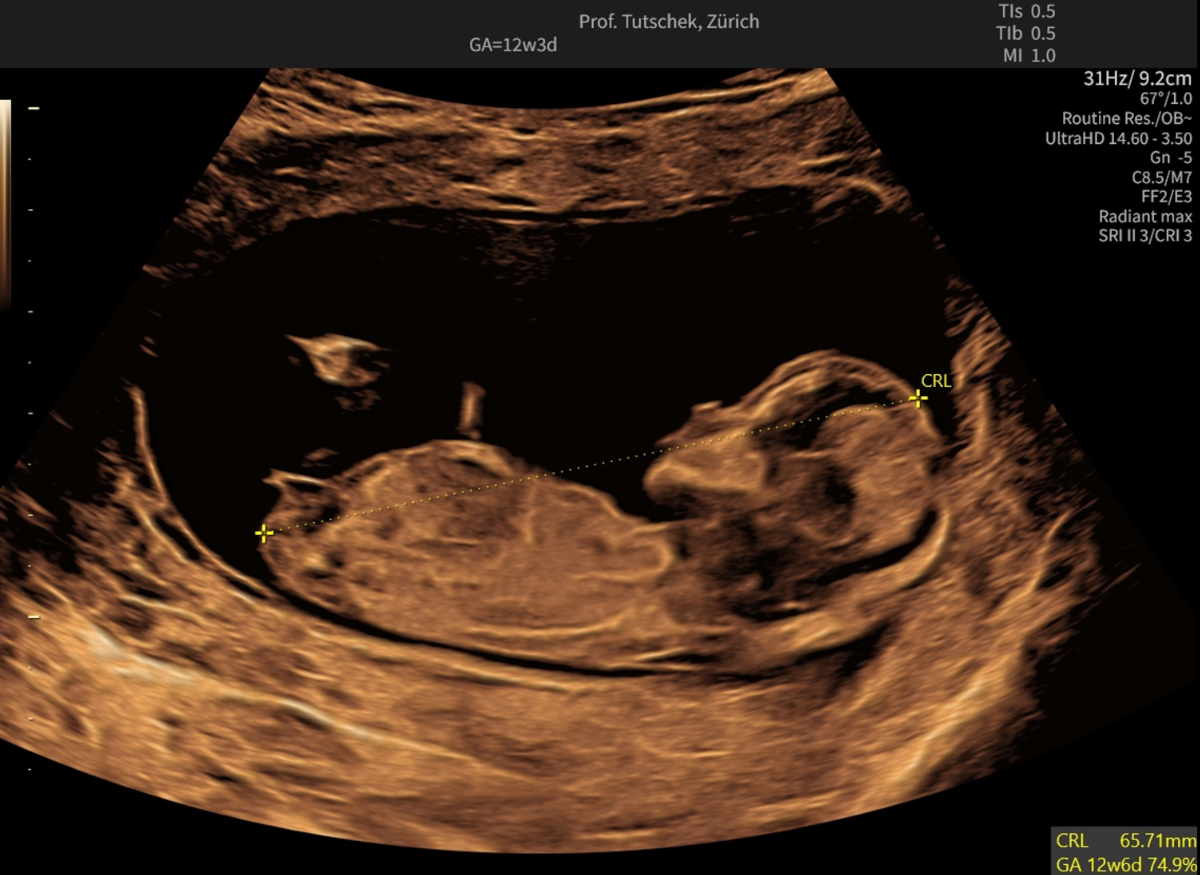
Figure 1aUltrasound measurements during the first trimester ultrasound: measurement of the crown-rump length (CRL) (65.71 mm).
DOI: https://doi.org/https://doi.org/10.57187/s.4610
Screening for fetal chromosomal anomalies has received great attention since the introduction of “combined testing” (ultrasound and maternal biomarkers) 25 years ago [1] and of “non-invasive prenatal testing” (NIPT) 17 years ago [2]. Both tests have empowered doctors to provide accurate risk assessment and individualised counselling. Switzerland was the first European country to offer non-invasive prenatal testing reimbursed by health insurance, following a structured risk assessment based on maternal characteristics, the nuchal translucency (NT) measurement and the maternal biomarkers “free-beta human chorionic gonadotropin (free beta-hCG)” and “pregnancy-associated plasma protein A” (PAPP-A).
Ultrasound (US) and blood parameters show characteristic deviations in pregnancies complicated by fetal trisomy 21, trisomy 13 and trisomy 18 as well as in many other pathological conditions. Two of three foetuses with trisomy 21 show an increased nuchal translucency (figure 1a-c).

Figure 1aUltrasound measurements during the first trimester ultrasound: measurement of the crown-rump length (CRL) (65.71 mm).
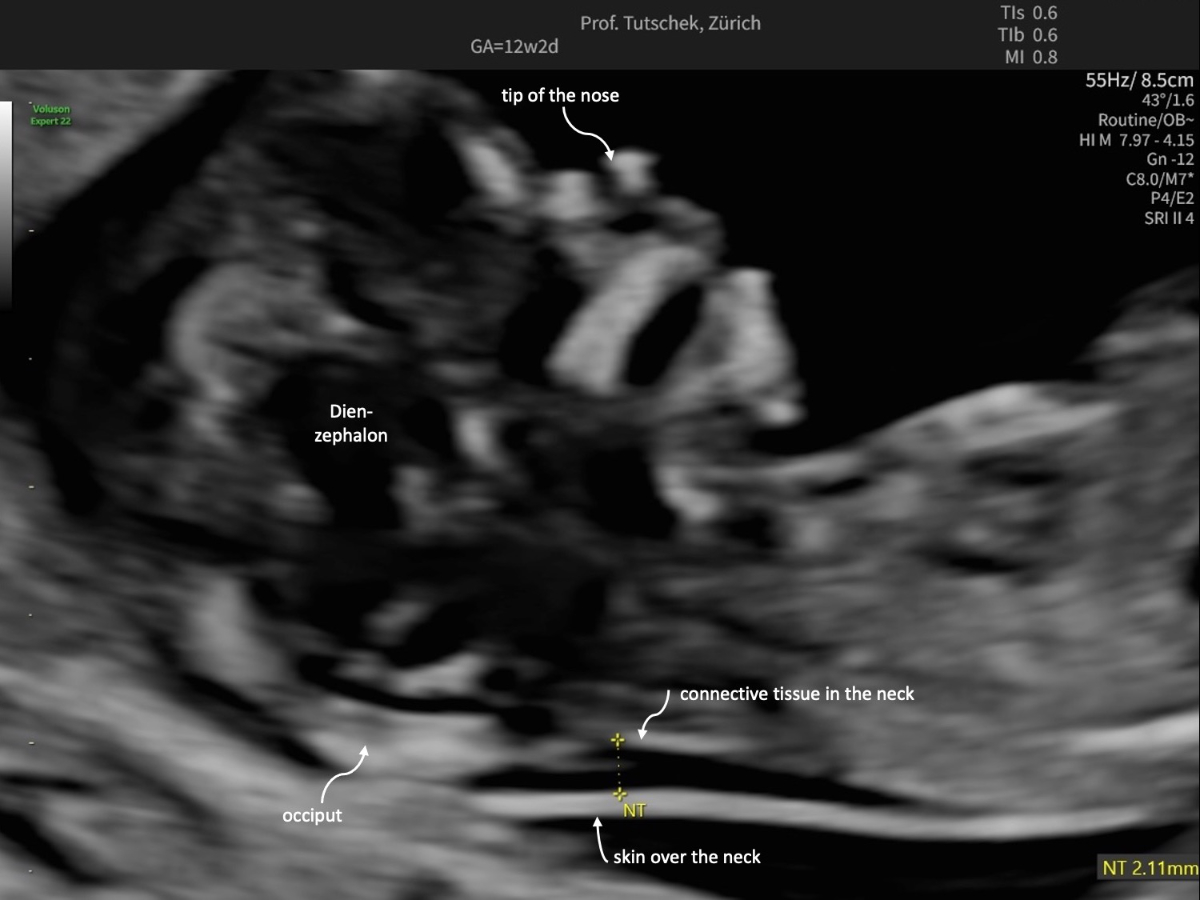
Figure 1bUltrasound measurements during the first trimester ultrasound: measurement of a normal (2.11 mm) nuchal translucency (NT).
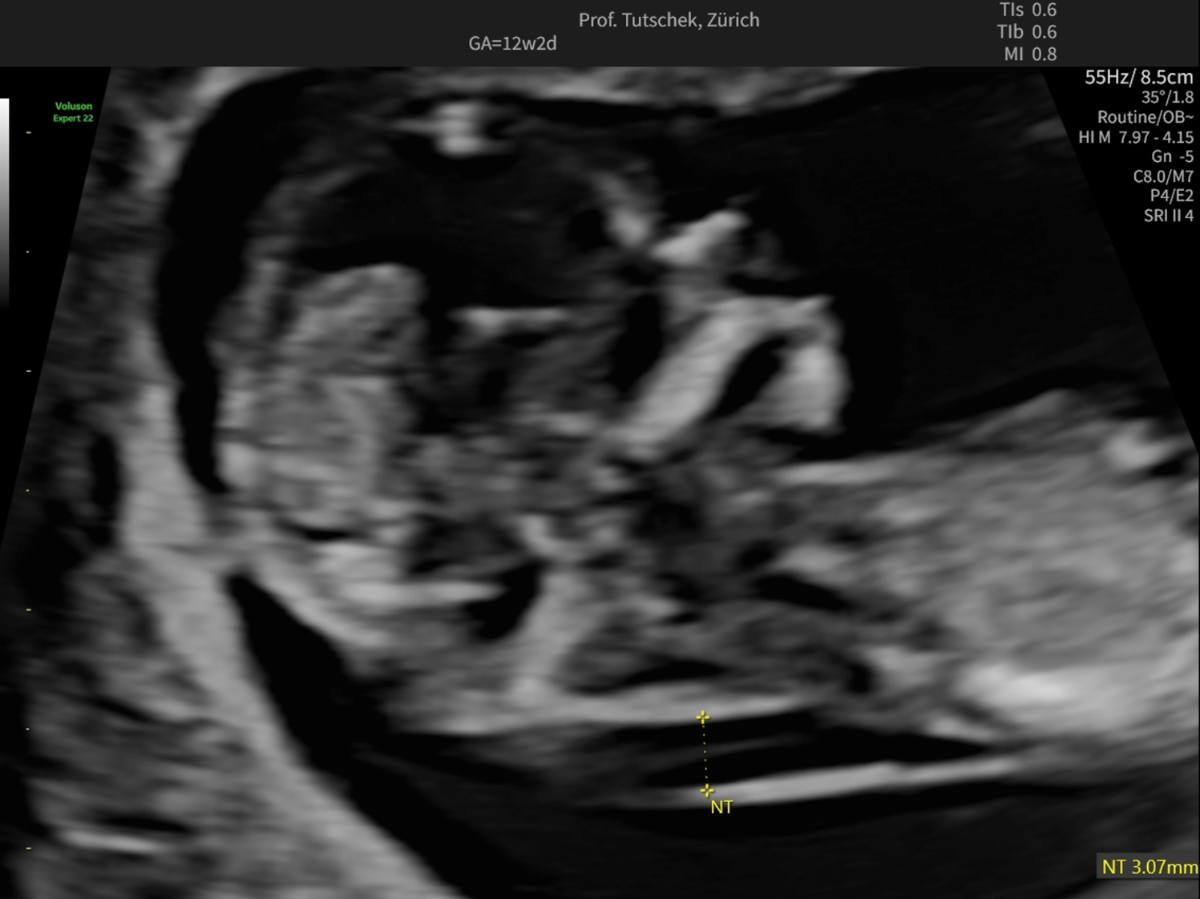
Figure 1cUltrasound measurements during the first trimester ultrasound: measurement of an increased (3.07 mm) nuchal translucency (NT).
To achieve a trisomy 21 detection rate of 85%, free beta-hCG and PAPP-A must be added in a quality-controlled fashion and using a certified algorithm. In trisomy 21, free beta-hCG is elevated and PAPP-A decreased (figure 2). The combined test (first trimester screening [FTS], called Ersttrimestertest in Switzerland) combines these two biomarkers with morphological parameters obtained by US, ideally done between 12 and 13 weeks (60–70 mm crown-rump length [CRL]).
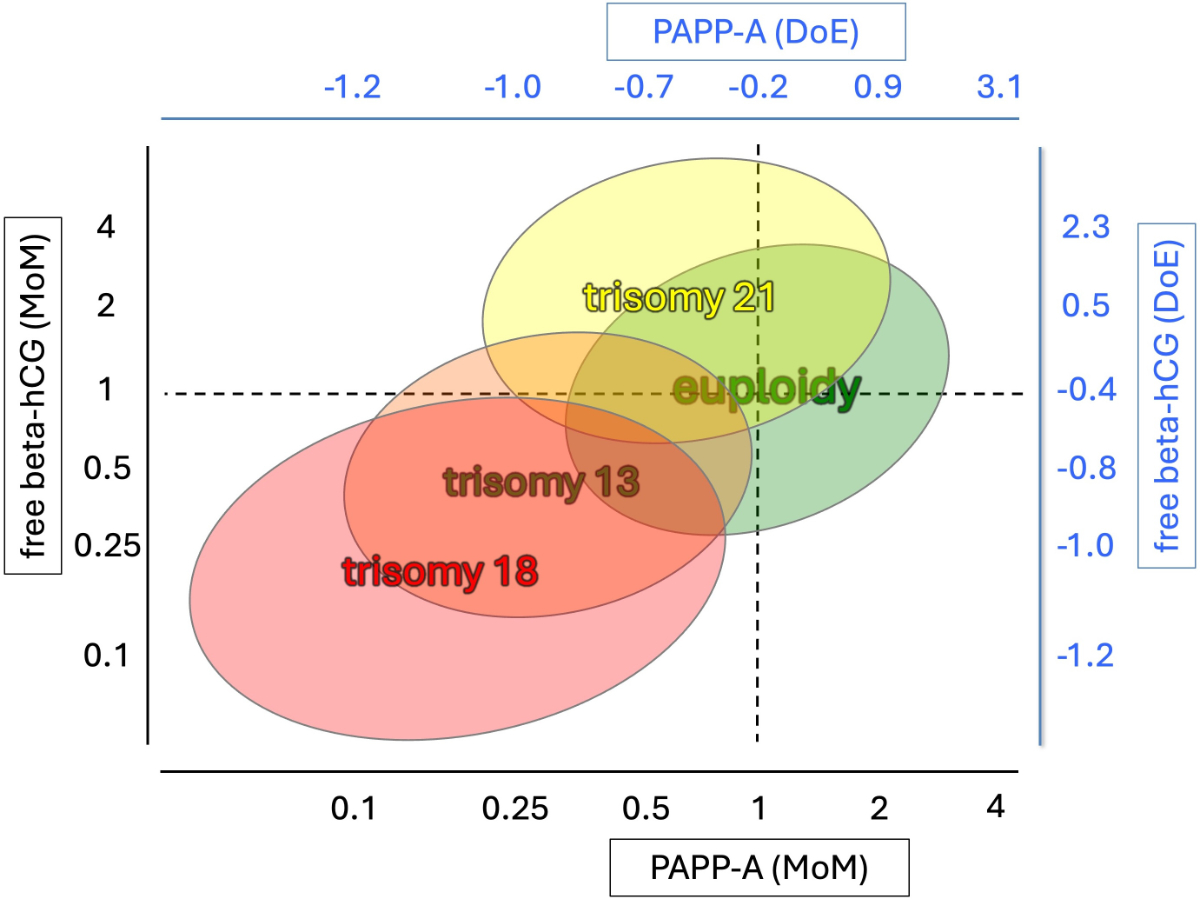
Figure 2Maternal serum pregnancy-associated plasma protein A (PAPP-A) and free-beta human chorionic gonadotropin (free beta-hCG), expressed in multiples of the median (MoM) and degrees of extremeness (DoE), in common fetal trisomies. This semi-quantitative graph has been modified from [10] by one of the authors using DoE and MoM values calculated from 60 aneuploid pregnancies (unpublished data).
First trimester screening is not limited to detecting trisomies 13/18/21; it is essential for early identification or exclusion of many major structural anomalies (see figures 3–5). According to the current Swiss guidelines [3], combined testing for trisomies should be offered only when no significant malformations have been found and when nuchal translucency is below the 95th centile. Otherwise, diagnostic procedures should be offered.
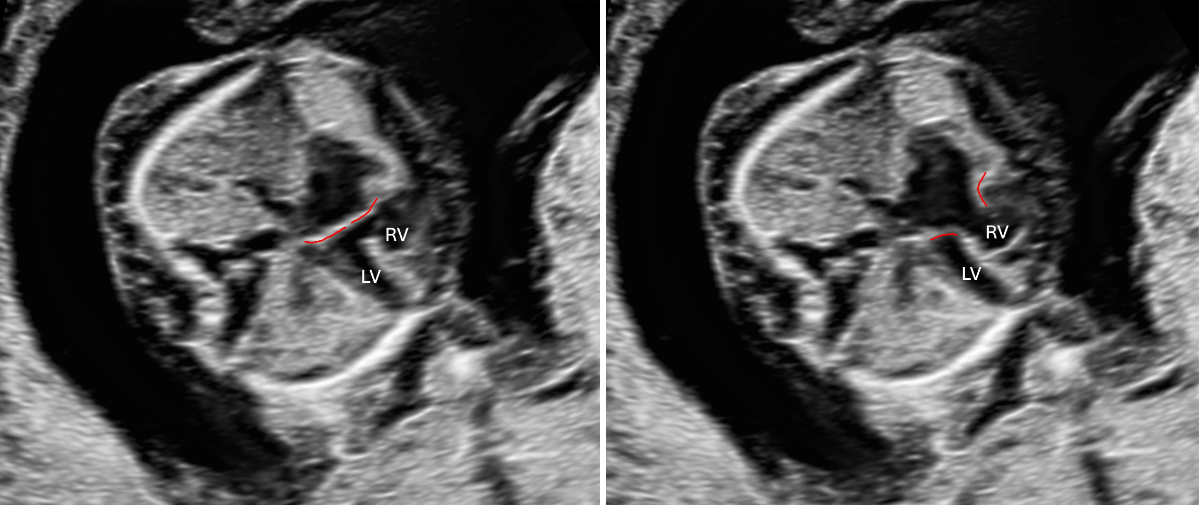
Figure 3Atrioventricular septal defect in a 13-week foetus with trisomy 21, atrioventricular valve highlighted in red. Left: AV valve closed (systole); right: AV valve open (diastole). LV: left ventricle; RV: right ventricle.
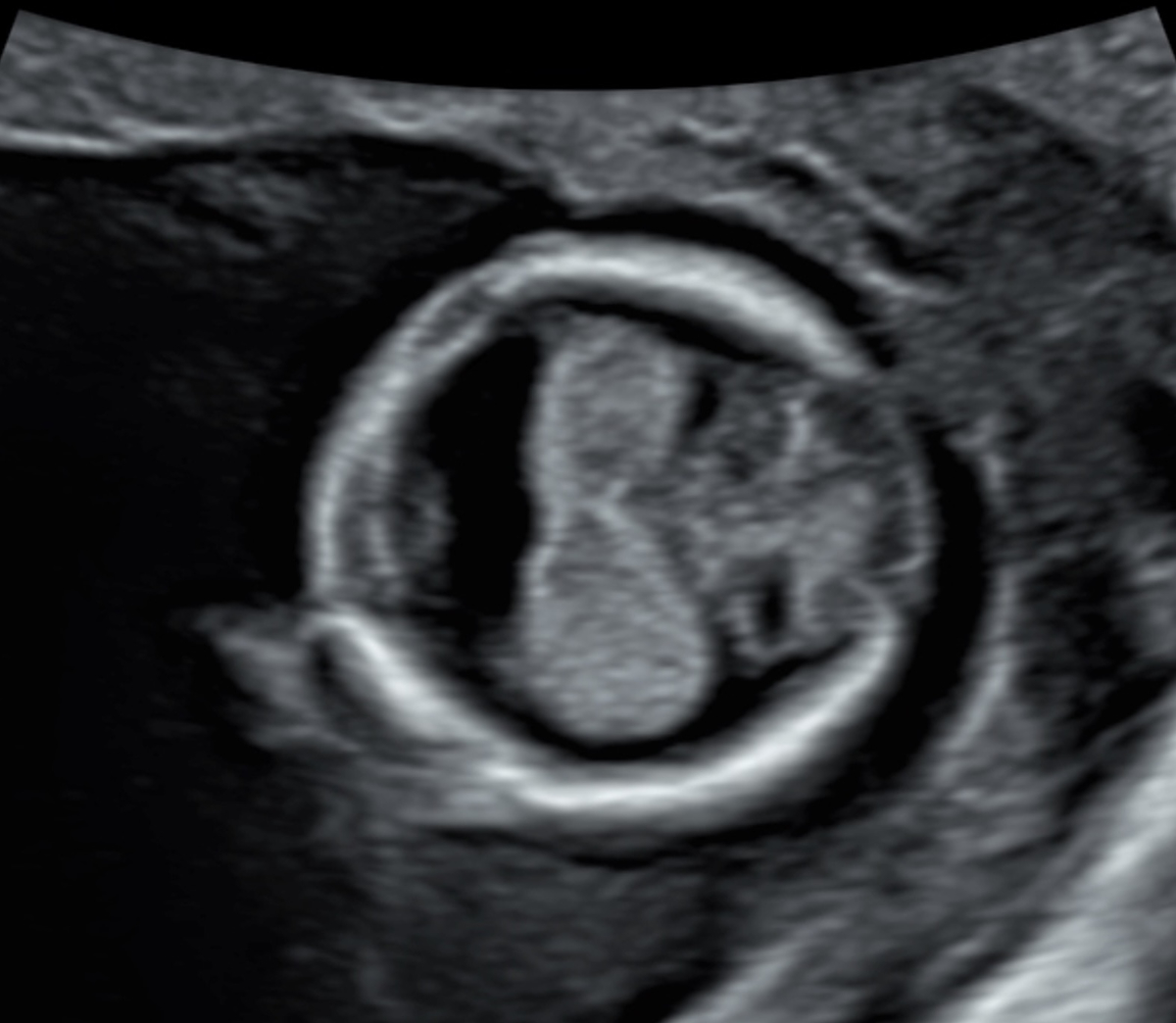
Figure 4Holoprosencephaly in a 13-week foetus: abnormal circular head shape, no midline, fused choroid plexus.
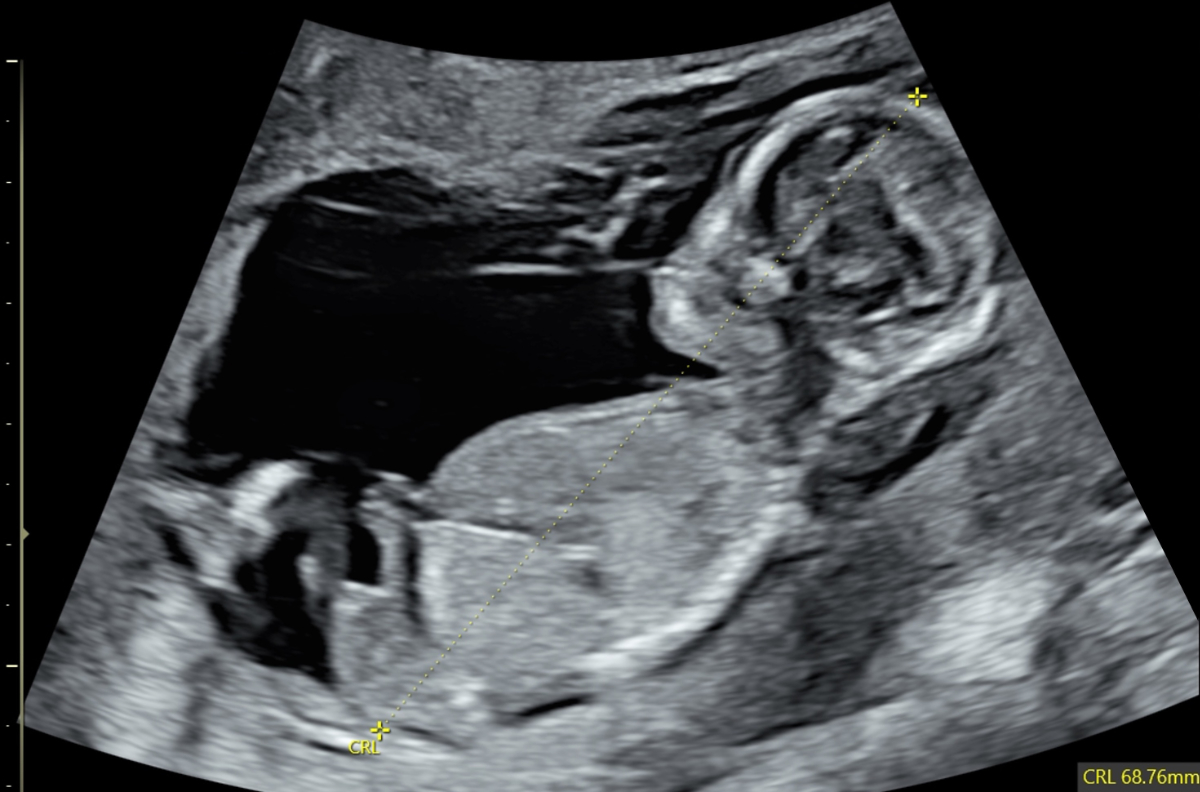
Figure 5Abnormal profile and missing nose in a 13-week foetus (CRL 68.76 mm). CRL: crown-rump length.
Unfortunately, different algorithms and nuchal translucency cut-offs exist (figure 6). This can only be understood from a historical perspective. Yet ignorance (or ignoring) of their differences leads to problems in interpretation of combined testing results. The original algorithm used in most countries worldwide has been developed and revised by the Fetal Medicine Foundation London (FMF-L). The FMF-L provides stringent quality control and audit [1, 4] (see also figure 7). A separate algorithm was developed by the Foetal Medicine Foundation Deutschland [Germany] (FMF-D) [5]: the PRC (Prenatal Risk Calculation) algorithm (lab version: Fastscreen), which has been adopted by most certified colleagues and all labs in Switzerland.
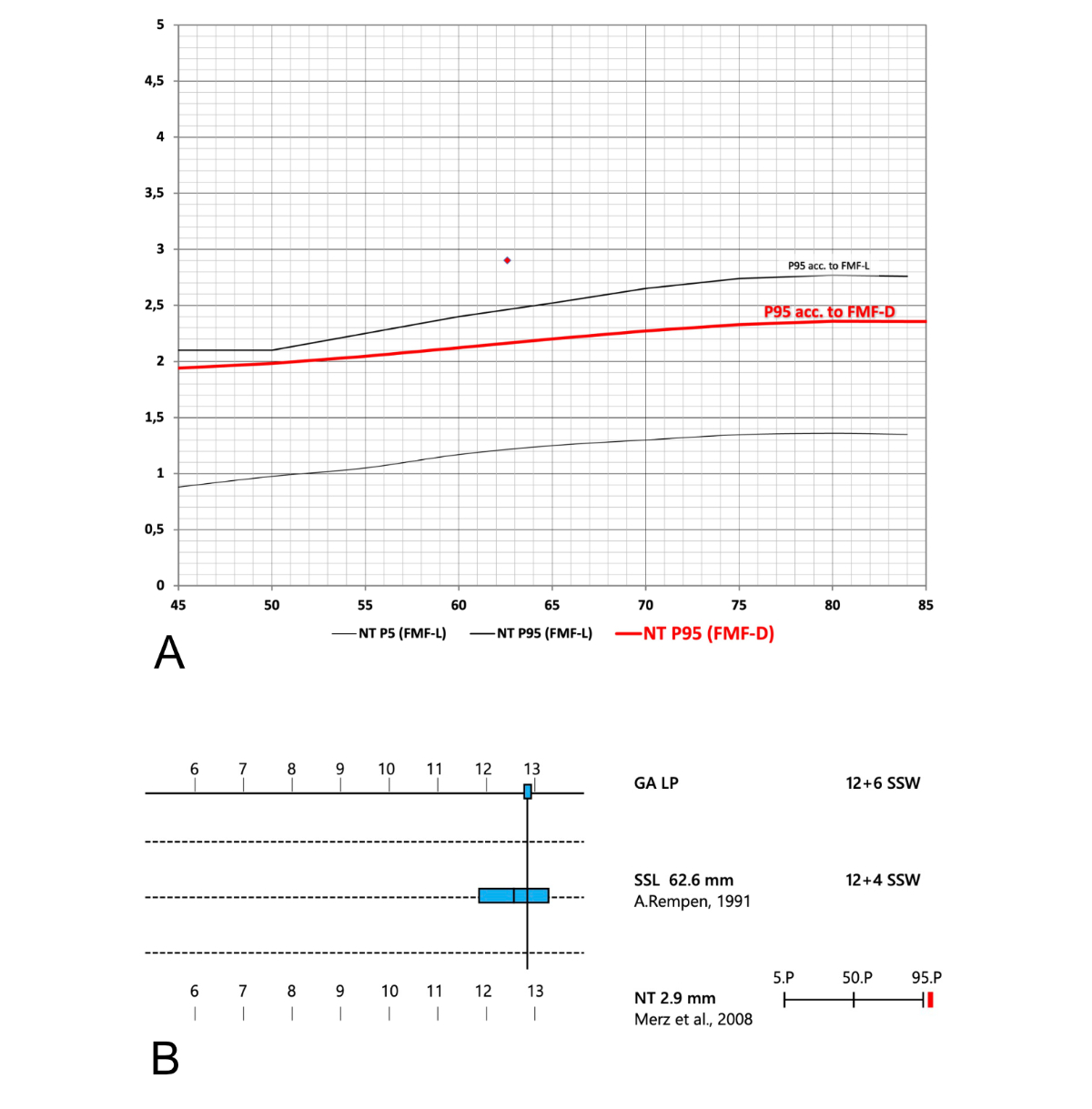
Figure 6Different cut-offs for nuchal translucency (NT) values. (A) There is a marked discrepancy between the upper end of the “normal range” (the 95th centile) according to Fetal Medicine Foundation London (FMF-L) (solid black line, curve derived from [4]) and Fetal Medicine Foundation Germany (FMF-D) (red line; curve derived from [5] and from a commercial lab report, Fastscreen, Thermo Fisher). (B) Part of a report showing a misleading graph of an increased nuchal translucency (printout of a documentation system commonly used in Switzerland). The nuchal translucency appears to be just slightly above the 95th centile. The nuchal translucency value shown in figure 6B is indicated as a red diamond on figure 6A. Only in figure 6A is the true extent of deviation of this nuchal translucency value from the normal range apparent. SSL: Scheitel-Steiss-Länge (CRL: crown-rump length); SSW: Schwangerschaftswoche (week of gestation).
FMF-L has higher cut-offs (95th centile) for nuchal translucency measurements than FMF-D (see figure 6A). FMF-L-certified examiners are subject to stricter audit and usually measure higher nuchal translucency values. The higher the nuchal translucency, the higher the risks for several conditions including chromosomal and genetic anomalies, structural heart disease and adverse pregnancy outcome in the presence of a normal karyotype [6]. Unfortunately, the software program most frequently used to document first trimester ultrasound exams in Switzerland does not show the extent of elevation of nuchal translucency above the 95th centile (figure 6B). Figure 6A shows the upper end of the normal range, i.e. the 95th centile, for FMF-L (upper black line) and for FMF-D (red line). This distinction is particularly important as, in Switzerland, a nuchal translucency ≥95th centile defines a separate group that should receive a specialised anomaly scan and counselling about diagnostic testing rather than screening only. While this cut-off (≥95th centile) is lower than many international experts would agree with, the current Swiss guideline for nuchal translucency screening [3] makes it mandatory to offer invasive testing for a nuchal translucency ≥95th centile. Online calculators allow any user (FMF-L certified or not) to use the higher FMF-L normal ranges.
One of the advantages of the FMF-L certification process, which involves a thorough audit of nuchal translucency data (see figure 7), is the quality control offered for free. The requirement of yearly submission of anonymised data and sample images for recertification may be seen as a downside by some, but, in the opinion of the authors, it is a worthwhile exercise.
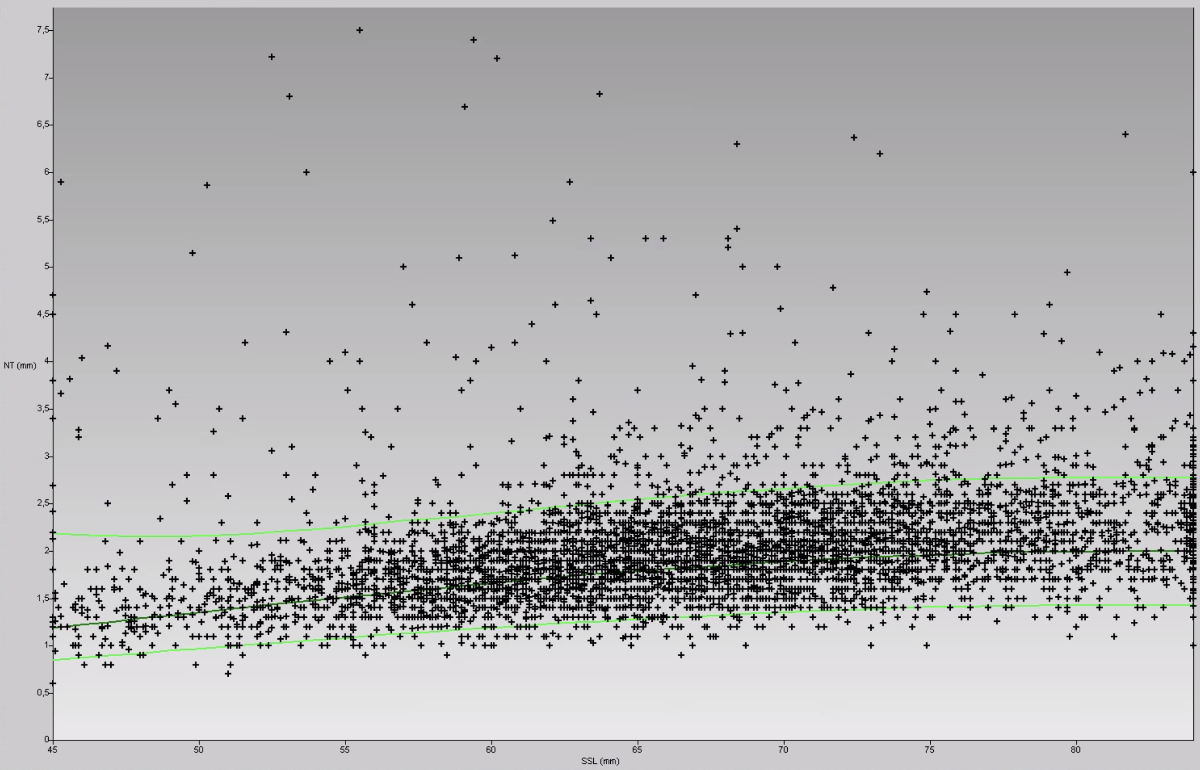
Figure 7Example of a voluntary, anonymised nuchal translucency (NT) audit, using the FMF-L algorithm and normal values (almost 5000 foetuses from a mixed screening and referral population, examined by a single operator). The X and Y axes represent crown-rump length (CRL or SSL in German) and nuchal translucency measurements, respectively. Each “+” represents the nuchal translucency measurement of one foetus. Most normal nuchal translucency measurements (in a normal population: 95%) lie below the upper green line (below the 95th centile). Data like these, together with representative nuchal translucency images, must be submitted to FMF-L to obtain the yearly renewal of a personal licence for the FMF-L risk calculation.
The common trisomies show certain patterns of the maternal blood biomarkers. These biomarkers differ by gestational age and maternal characteristics. Therefore, they must be normalised for these variables to make them comparable.
A problem with the interpretation of blood values lies in the nature of the different algorithms. The FMF-L algorithm uses “multiples of the median” (MoM) to indicate deviation of blood values from the expected median. So, for example, 2.5 MoM means that the value is 2.5 times higher than the expected median. Typically, one assumes a normal value to be between 0.5 and 2.5 MoM. The advantage of MoM is that it presents the degree of deviation on a linear scale.
The FMF-D algorithm, however, uses another unit, “degrees of extremeness” (DoE) [5], which does not represent the measured values in a linear form. A conversion into MoM is not possible. DoE has a “normal range” of −1.0 to +1.0. Unfortunately, using DoE, one cannot easily estimate how deviant a particular value is. Figure 2 shows typical deviations of biochemistry values in trisomy 13/18/21 and compares MoM and DoE.
Other aneuploidies such as Turner syndrome, triploidy or rare autosomal trisomiesoften also show abnormal blood values, most commonly low PAPP-A values [7]. A very low PAPP-A (≤0.3 MoM or roughly ≤−1.0 DoE) occasionally indicates such aneuploidies that require tests other than a non-invasive prenatal test. If in doubt, expert advice must be sought. Drawing blood for non-invasive prenatal testing but omitting a diligent US scan does not make an examiner a fetal medicine expert but leads to delayed or missed diagnoses.
The introduction of combined testing has improved the detection rate mainly for trisomy 21. Using non-invasive prenatal testing as second-line screening after combined testing has substantially reduced the false-positive rate, leading to a marked decline in invasive testing in Switzerland. Newer versions of non-invasive prenatal testing have extended its scope to chromosomes other than 13/18/21. It remains to be seen how this affects screening for rare autosomal trisomies or segmental chromosomal anomalies.
Does non-invasive prenatal testing make ultrasound screening obsolete? This question arises from the misunderstanding that first trimester screening is the search for trisomy 21. If trisomy 21 were the only condition worth screening for, non-invasive prenatal testing would be the solution as its sensitivity for trisomy 21 is at least 99%. However, trisomy 21 occurs in only 1 of 640 to 850 live births [8]. Trisomies 13/18/21 account for only about half of the aneuploidies found at birth. But congenital malformations affect 2–3% of neonates [9]. Omitting the structured anatomical scan in the first trimester reduces early detection of severe malformations and genetic conditions not covered by non-invasive prenatal testing.
No doubt non-invasive prenatal testing has revolutionised prenatal screening for aneuploidies. But there is a real risk that non-invasive prenatal testing be misunderstood – and misleadingly proposed to pregnant women – as a “diagnostic test for chromosomal problems” or, worse, a “diagnostic test for fetal health”. Used incorrectly, it can provide a false sense of security regarding genetic problems other than trisomy 21, both to the prospective parents and to a naive healthcare provider. The application of non-invasive prenatal testing makes prior diagnostic scrutiny mandatory to find relative or absolute contraindications. Any recognisable fetal malformation is an established contraindication for non-invasive prenatal testing. In the opinion of the authors, a (markedly) enlarged nuchal translucency also is a contraindication. The nuchal translucency cut-off that should prompt the offer of a diagnostic test (chorionic villus sampling or amniocentesis) is defined as the 95th centile in the current Swiss guidelines. Many international experts would probably advise to offer invasive testing from nuchal translucency values of ≥3.0 mm or ≥3.5 mm. In Switzerland, this issue is compounded by the different normal ranges used for nuchal translucency in common software and laboratory programs (see figure 6A). By far, most combined test are calculated and reported by laboratories some of whom are unaware of different nuchal translucency normal ranges or recommendations. The same is also true for the relevance of very abnormal blood analytes, mainly very low PAPP-A.
On the other hand, there is also a risk of erroneously viewing the nuchal translucency as a definitive marker that distinguishes between health and disease: A normal nuchal translucency does not guarantee fetal wellbeing and must not lead to neglect of essential components of thorough prenatal screening, diligent ultrasound examination and maternal blood biochemistry. Undue reliance on nuchal translucency, “backed up by non-invasive prenatal testing”, may result in missed diagnoses of severe structural anomalies. Detecting such conditions late in pregnancy leads to difficult decisions regarding late termination, which is associated with increased medical risks, emotional distress and, in some cases, complex legal challenges.
The first trimester screen is reimbursed by Switzerland’s basic health insurance and its full diagnostic potential is only achieved by performing a comprehensive anatomical assessment. It should not be regarded as a mere screening tool for trisomies but as a cornerstone of high-quality prenatal care. It remains essential to uphold the medical standard: to offer the appropriate exam after proper counselling and obtaining informed consent. Using a structured approach, i.e. performing a detailed ultrasound first and proceeding to non-invasive prenatal testing when indicated/not contraindicated, can also reduce healthcare costs.
First trimester screening must not be reduced to a dating scan plus blood drawing for non-invasive prenatal testing. While non-invasive prenatal testing enhances detection of the common trisomies, it cannot replace fetal anatomical assessment.
In Switzerland, the coexistence of differing algorithms (FMF-L and FMF-D) and nuchal translucency cut-offs creates confusion and inconsistent risk interpretation. This undermines quality and misleads healthcare providers and patients. Promoting certified, quality-controlled practice and harmonising standards is essential for safe, ethical and effective prenatal care.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Spencer K, Souter V, Tul N, Snijders R, Nicolaides KH. A screening program for trisomy 21 at 10-14 weeks using fetal nuchal translucency, maternal serum free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 1999 Apr;13(4):231–7. doi: https://doi.org/10.1046/j.1469-0705.1999.13040231.x
2. Lo YM, Tein MS, Lau TK, Haines CJ, Leung TN, Poon PM, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am J Hum Genet. 1998 Apr;62(4):768–75. doi: https://doi.org/10.1086/301800
3. Ochsenbein N, Burkhardt T, Raio L, Vial Y, Surbek D, Tercanli S, et al. Pränatale nicht-invasive Risikoabschätzung fetaler Aneuploidien. SGGG Expertenbrief No. 52. 2018. Available from: https://www.sggg.ch/fileadmin/user_upload/PDF/20180411_52_Praenatale_nicht-invasive_Risikoabschaetzung_fetaler_Aneuploidien_07032018.pdf
4. Wright D, Kagan KO, Molina FS, Gazzoni A, Nicolaides KH. A mixture model of nuchal translucency thickness in screening for chromosomal defects. Ultrasound Obstet Gynecol. 2008 Apr;31(4):376–83. doi: https://doi.org/10.1002/uog.5299
5. Merz E, Thode C, Alkier A, Eiben B, Hackelöer BJ, Hansmann M, et al. A new approach to calculating the risk of chromosomal abnormalities with first-trimester screening data. Ultraschall Med. 2008 Dec;29(6):639–45. doi: https://doi.org/10.1055/s-2008-1027958
6. Gadsbøll K, Brix N, Sandager P, Petersen OB, Souka AP, Nicolaides KH, et al. Increased nuchal translucency thickness and normal chromosomal microarray: danish nationwide cohort study. Ultrasound Obstet Gynecol. 2025 Apr;65(4):462–9. doi: https://doi.org/10.1002/uog.29198
7. Tørring N, Petersen OB, Becher N, Vogel I, Uldbjerg N; Danish Fetal Medicine Study Group; Danish Clinical Genetics Study Group. First trimester screening for other trisomies than trisomy 21, 18, and 13. Prenat Diagn. 2015 Jun;35(6):612–9. doi: https://doi.org/10.1002/pd.4584
8. Stallings EB, Isenburg JL, Rutkowski RE, Kirby RS, Nembhard WN, Sandidge T, et al.; National Birth Defects Prevention Network. National population-based estimates for major birth defects, 2016-2020. Birth Defects Res. 2024 Jan;116(1):e2301. doi: https://doi.org/10.1002/bdr2.2301
9. Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Adv Exp Med Biol. 2010;686:349–64. doi: https://doi.org/10.1007/978-90-481-9485-8_20
10. Kagan KO, Wright D, Valencia C, Maiz N, Nicolaides KH. Screening for trisomies 21, 18 and 13 by maternal age, fetal nuchal translucency, fetal heart rate, free beta-hCG and pregnancy-associated plasma protein-A. Hum Reprod. 2008 Sep;23(9):1968–75. doi: https://doi.org/10.1093/humrep/den224