Simultaneous adrenal gland and pancreas-kidney transplantation and associated hormonal
challenges
DOI: https://doi.org/https://doi.org/10.57187/s.4382
Fabian Rösslera,
Olivier de
Rougemonta,
Thomas Schachtnerb,
Kerstin Hübelab,
Jakob Nilssonc,
Lukas Frischknechtc,
Michael Freya,
Lorenzo
Viggiani d'Avalosa,
Jose Oberholzera,
Svenja Nöltingd,
Roger Lehmannd*,
Thomas Müllerb*
a Department of Surgery and Transplantation,
University Hospital Zurich, Zurich, Switzerland
b Department of Nephrology, University Hospital Zurich, Zurich,
Switzerland
c Department of Immunology, University Hospital Zurich, Zurich,
Switzerland
d Department of Endocrinology, University Hospital Zurich, Zurich,
Switzerland
* Equal contribution
Summary
Adrenal gland transplantation has only been
performed in rare cases, with variable results in terms
of functional activity. Consequently, there is a lack of evidence in endocrine management
and tapering hormone replacement therapy after such transplantations.
We report on a simultaneous pancreas-kidney
and adrenal gland allotransplantation in a 48-year-old female patient with type
1 diabetes and severe autoimmune adrenal insufficiency. Surgery was uneventful,
without major surgical morbidity. Pancreas and kidney graft function were excellent
from the beginning. Adrenal graft function was difficult to assess and steroid tapering
was not well tolerated and hampered clinical recovery. Despite the evidence of adequate
graft perfusion and initially even measurable levels of cortisol production, persistent
adrenal graft function was not obtained, and the patient remained on hormone replacement
therapy.
Simultaneous pancreas-kidney
and adrenal gland transplantation is technically safe, without the need for major
surgical modifications or adjustments in immunosuppression. However, it should only
be performed in combination with a kidney or pancreas-kidney transplant, which justifies
the lifelong immunosuppression. The major challenge remains the postoperative endocrine
management, with steroid tapering and adequate assessment of adrenal graft function.
Patients should be followed
by an interdisciplinary team involving endocrinologists, nephrologists and transplant
surgeons.
Introduction
Adrenal insufficiency
represents a complex endocrinological disease with multiple causes and clinical
manifestations, with the need for lifelong hormone replacement therapy. Symptoms
are various, including severe orthostatic hypotension, profound fatigue, muscle
pain and gastrointestinal disorders [1]. Laboratory findings include hypoglycaemia,
hyponatraemia and hyperkalaemia. Primary adrenal disorder, commonly caused by destructive
autoimmunity, is rare, but often associated with other glandular diseases. These
patients usually suffer from severe comorbidities, including insulin-dependent diabetes
mellitus and thyroid disorders [2]. Replacement therapy with gluco- and mineralocorticoids
is essential, with the need for additional dosages in stress situations to prevent
adrenal crisis.
Adrenal gland
transplantations have been performed since the 1960s as autologous transplantations
for the treatment of refractory Cushing’s disease following bilateral adrenalectomy
[3]. However, its effects were limited and commonly only short-term [4, 5]. Reports
on adrenal gland allotransplantation are limited. Grodstein et al. reported on the
first successful intramuscular morcellised
adrenal allograft in combination with a kidney allotransplantation in a child with
adrenal insufficiency secondary to meningococcal septicaemia
[6]. To date, only two case
reports exist on whole-organ adrenal gland allotransplantation, one simultaneous kidney-adrenal
gland and one pancreas-kidney-adrenal gland transplant [7, 8]. These transplantations
were beneficial in terms of surgical feasibility and safety, with varying results
however regarding functional activity of the transplanted adrenal grafts.
Here we report
on the first case of an adrenal gland allotransplant in Switzerland, performed in
combination with a simultaneous pancreas and kidney transplant. This case illustrates
the surgical challenges and endocrine complexity associated with diseases of the
adrenal gland and related disorders.
Case presentation
Our patient,
a 48-year-old woman, suffered from a poly-glandular autoimmune syndrome type II,
also known as Schmidt syndrome. This rare disease is associated with a primary adrenal
gland insufficiency, type 1 diabetes mellitus and thyroid disease [9]. The patient
was on substitution therapy with hydro- and fludrocortisone and thyroid hormones,
and had suffered from multiple adrenal crises in the past. Type 1 diabetes
mellitus was diagnosed at the age of 20, with unstable glycaemia and repeat severe
hypoglycaemia. HbA1c levels were above 10%, causing diabetic nephropathy over time.
At time of transplantation, serum creatinine was 234 µmol/l, equal to a glomerular
filtration
rate (GFR) of 21 ml/min/1.73 m2 according
to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 equation [10,
11].
Diabetes
was not well controlled, with recurrent episodes of diabetic ketoacidosis despite
her insulin pump, and a gradually deteriorating kidney function. Thus, the patient
fulfilled the criteria for simultaneous pancreas and kidney transplantation. Due
to the additional severe adrenal insufficiency, the decision was taken for an additional
whole-organ adrenal gland transplantation. This was possible since the need for
lifelong immunosuppression was justified by the indication for simultaneous
pancreas and kidney transplantation. Initial wait-listing was in 2013, but the patient
was kept inactive due to stable kidney function until the beginning of 2020. The
patient gave informed consent for the transplantation.
At the time
of transplantation, the patient was on morning hormone replacement therapy with
25 mg hydrocortisone (Plenadren®), 0.05 mg fludrocortisone (Astonin H®)
and 25 mg dehydroepiandrosterone (DHEA).
Surgical details
The three
grafts were from a 20-year-old male donor after brain death. The adrenal gland was
procured en bloc with the left kidney. We chose the left adrenal gland due to the
easier preservation of venous drainage via the suprarenal vein to the left renal
vein. We preserved the inferior adrenal artery as a branch from the renal artery,
and the middle adrenal artery was obtained on a common aortic patch together with
the left renal artery. The pancreas was procured en bloc with the duodenum and spleen,
as for the classic pancreas transplantation. Transport was by static cold storage
using Institute George Lopez-1 (IGL-1) preservation solution. Backtable preparation
was as for standard simultaneous pancreas and kidney transplantation, with preparation
of an iliac Y-graft to the superior mesenteric and splenic artery of the pancreas.
The kidney and adrenal gland were kept en bloc, with one common aortic patch including
the single renal artery and the middle adrenal artery. Venous drainage was by a
single renal vein, including the suprarenal vein draining into it. Access in the
recipient was through median laparotomy. First, according to our standard for
simultaneous pancreas and kidney transplantation, the whole pancreas with the duodenal
segment was implanted. Pancreatic venous drainage was via the inferior vena cava,
arterial anastomosis via the Y-graft to the common iliac artery. Exocrine drainage
was through duodeno-jejunostomy. After successful reperfusion of the pancreas, we
implanted the en bloc kidney-adrenal gland graft to the left external iliac artery
and vein. Urinary drainage was through a uretero-cystostomy and splinted with a
double-J catheter. Kidney and adrenal gland reperfusion was straightforward with
a good macroscopic result showing homogeneous pink colouration of both organs. The
patient remained haemodynamically stable during the surgery, with immediate normalisation
of blood sugar and persistent urinary output.
Surgical
time was 231 minutes, without any intraoperative complications. Cold-ischaemic time
was 7 hours and 39 min for the pancreas and 9 hours for the kidney-adrenal gland.
Anastomotic times were 31 and 32 minutes (both vein and arteries) for the pancreas
and the kidney-adrenal gland, respectively.
The immunosuppressive
regimen was the same as standardly used for simultaneous pancreas and kidney
transplantation, not modified for the additional implant of the adrenal gland. The
number of human leukocyte antigen (HLA) mismatches was 6 out of 8. Calculated panel-reactive
antibodies (cPRA) were 0% for both class I and II. Transplantation
was without any preformed donor-specific antibodies. Induction therapy included
anti-thymocyte globulin (Thymoglobulin®) and steroids. For maintenance
therapy, we used tacrolimus (Prograf®, 0.1 mg/kg body weight) in combination
with mycophenolic acid (Myfortic®, 720 mg/12 h). Rapid 5-day steroid
taper consisted of 500 mg methylprednisolone (Solumedrol®) prior to transplantation,
followed by Prednison® (100 mg on postoperative days 1 and 2, 50 mg on
days 3 and 4, and the last dosage of 25 mg on day 5).
Postoperative course
and complications
Despite immediate
pancreas and kidney graft function, the patient’s clinical recovery was delayed.
Reasons for this were episodes of inexplicable rheumatic pain, fatigue and undulating
signs of inflammation, without clear focus. After an uneventful first week, the
patient developed nonspecific pain of diffuse character. Computed tomography revealed
no abdominal pathology and showed an adequate morphological aspect and regular perfusion
of all three transplants. With persisting unclear pain and undulating signs of inflammation,
we performed relaparotomy two weeks after surgery. Reoperation was inconclusive,
without signs of infection and a regular macroscopic aspect of all three grafts.
With empirical antibiotic treatment (piperacillin/tazobactam, Tazobac®),
inflammation signs normalised fast. In the further course, however, pain characteristics
were consistent with a rheumatic disease. Pain peaks were usually in the morning
and migrating between joints, from severe left-sided hip pain to thighs and knees
on both sides, and improved with physical activity. In addition, a strong swelling
and effusion of both knees occurred. From the patient’s perspective, the most disturbing
features were fluid retention and a possible nerve compression in the area of the
renal graft, with pain radiating to the left thigh. However, repeat magnetic resonance
imaging of the hips, spine and knees did not reveal any pathology, especially no
signs of infection or gout. Nor didrepeated rheumatological and neurological
assessments. A short-time treatment with high-dose steroids (prednisone 50 mg for
3 days) led to some improvement, especially reduction of effusions, but not complete
pain relief however. We refrained from joint puncture due to the risk of infection
under immunosuppression. Due to the pain situation, the patient remained in the
hospital for 57 consecutive days.
Graft function
Pancreas
graft function was excellent from the beginning with immediate and sustained insulin
freedom. Levels of HbA1c decreased from 8.4% preoperatively to 4.2%, 5.2%, 5.2%
and 5.6% at 6, 12, 24 and 36 months after transplantation. Kidney graft function
fluctuated in the early postoperative course, with stabilised values after three
months. Kidney function improved to an estimated GFR of 41, 43, 55 and 50 ml/min/1.73
m2 at 6, 12, 24 and 36 months after transplantation.
Adrenal
graft function was difficult to assess. The patient’s replacement therapy was stopped
at the time of transplantation and the regular 5-day steroid therapy for
simultaneous pancreas and kidney transplantation was administered. However, after
the 5-day steroid taper, we were forced to restart with
hydrocortisone (25 mg/day) due to severe fatigue and orthostatic dysfunction. Fludrocortisone
(0.05 mg/day) was added from day 9. Repeated measurements of fasting cortisol levels
in the early postoperative period showed sufficient levels, suggesting an initially
active production by the adrenal graft. Adrenocorticotropic hormone (ACTH) stimulation
(Synacthen® test) showed an appropriate increase in baseline cortisol
levels on each of postoperative days 7 and 14 (figure 1).
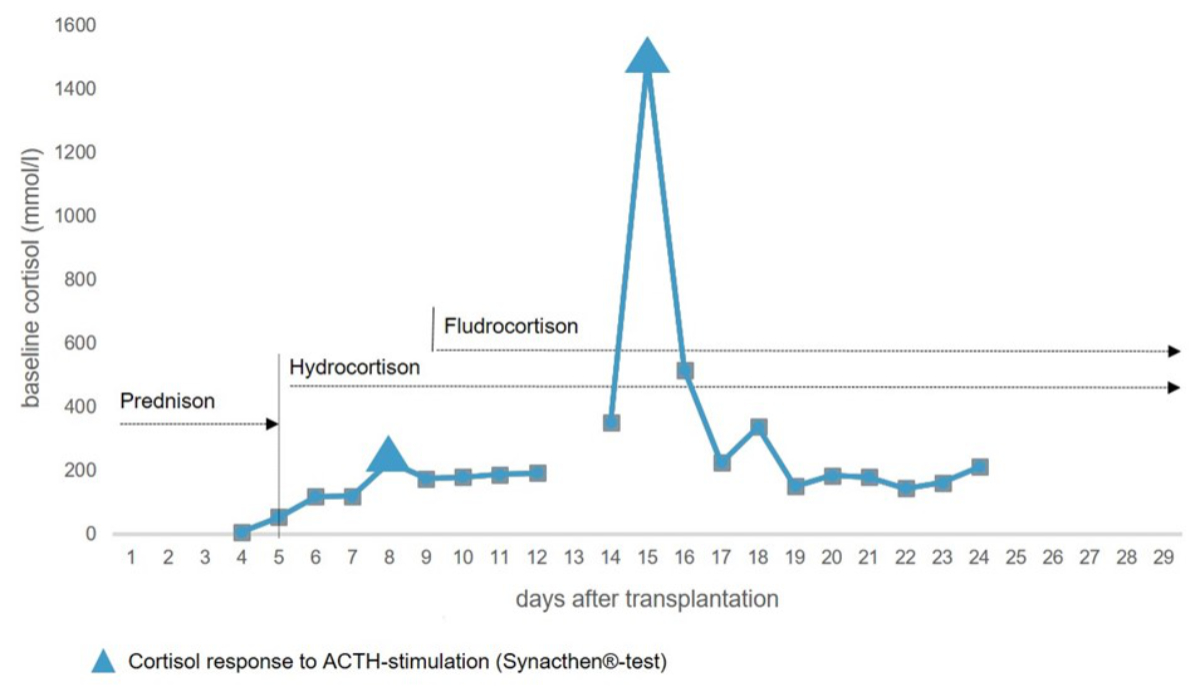
Figure 1Baseline
cortisol levels and responses to adrenocorticotropic hormone (ACTH) stimulation
within the first month after transplantation. Prednisone was administered according
to the regular 5-day steroid taper after simultaneous pancreas and kidney transplantation.
Hydrocortisone and fludrocortisone show the restart of hormone replacement therapy
after transplantation.
However,
due to repeated episodes of diarrhoea and inflammation, the patient’s cortisol turnover
was elevated and the replacement therapy had to be continued, and later even increased.
Therefore, ACTH levels remained below the minimum at two and three months after
transplantation. Aldosterone levels remained low as well. The patient was discharged
with a daily dosage of 50 mg hydrocortisone and 0.1 mg fludrocortisone. Repeated
contrast-enhanced CT scans confirmed regular perfusion of the adrenal graft (figure
2).
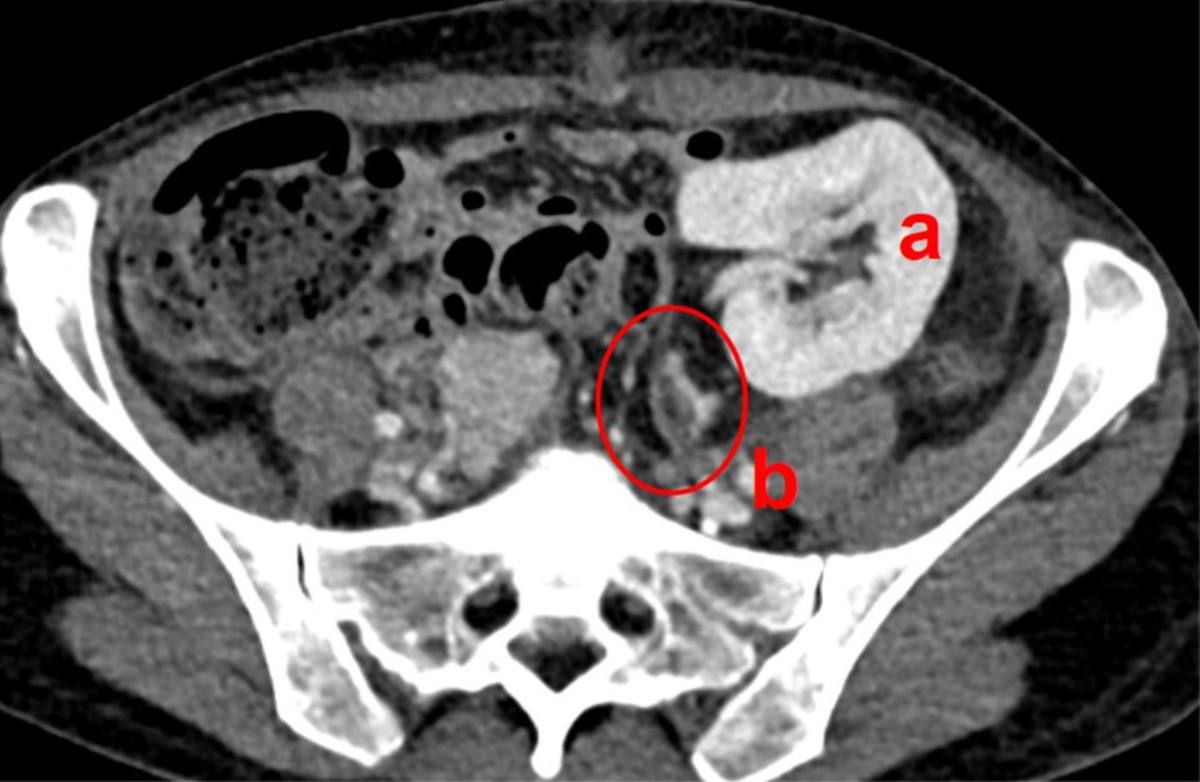
Figure 2Adrenal graft perfusion. Computed tomography on postoperative day 8 shows
regular perfusion of the kidney (a) and adrenal graft (b, encircled).
In the following
months, the patient suffered from persisting fatigue, joint pain and orthostatic
problems, hampering further reduction of replacement therapy. Repeated attempts
to lower the dosage were only partially successful due to clinical deterioration.
Fasting cortisol levels remained lower than in the immediate posttransplant course.
One year after transplantation, a positron emission tomography (PET) (122 MBq Ga-68
DOTATATE) revealed a significantly reduced adrenal Somatostatin Receptor 2 (SSTR-2)
positivity (figure 3).
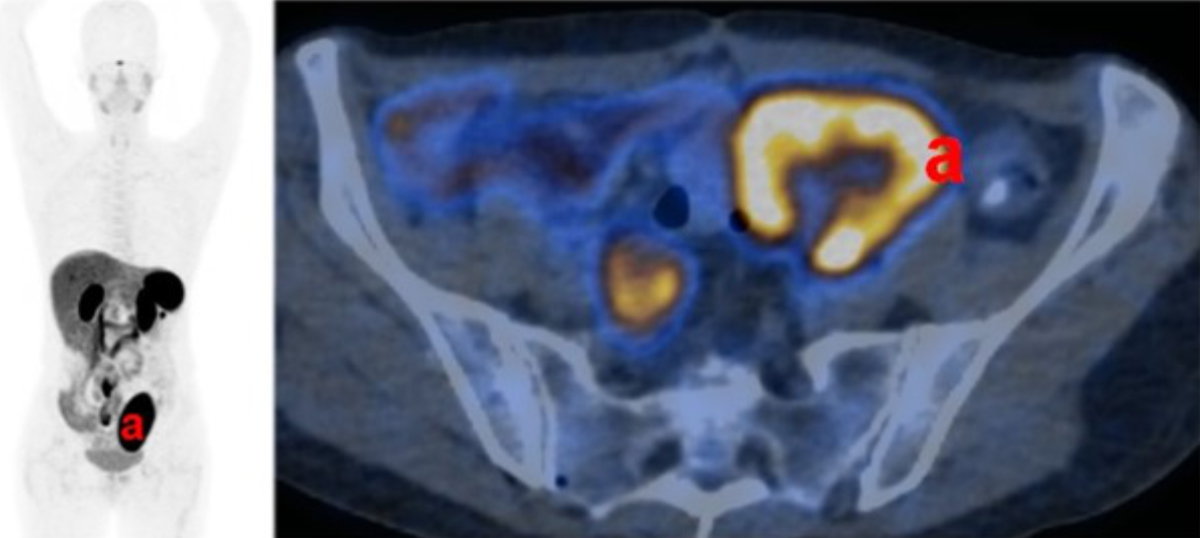
Figure 3Adrenal graft functional activity. Functional imaging (122 MBq Ga-68 DOTATATE positron
emission tomography) one year after transplantation shows strong activity in
the kidney graft (a), but no signs of activity in the adrenal graft.
At
the time of transplantation, no preformed donor-specific antibodies were present
and no de novo donor-specific antibodies appeared throughout the entire follow-up.
Adrenal gland autoantibodies were verified before transplantation, but were no longer
detectable two months after transplantation and suddenly reappeared again seven
months later. At the time of writing this manuscript, the patient remains on hormone
replacement therapy with a hydrocortisone-equivalent dose of 30 mg with stress dose
adjustment as needed and fludrocortisone (Astonin H®) 0.1–0.15 mg daily
depending on the blood pressure, electrolytes and laboratory results for renin.
At
1-year posttransplantation, a surveillance kidney allograft biopsy was performed
to assess any subclinical rejection in the kidney allograft. The kidney allograft
biopsy showed no morphological lesions related to T cell-mediated mechanisms (i0,
t1, v0, ti0, i-IFTA0, t-IFTA0). However, morphological lesions related to antibody-mediated
mechanisms were present (g1, ptc0, C4d1, cg1a, ptcml0) with microvascular inflammation
(MVI) below threshold. The biopsy was further assessed by biopsy-based transcript
diagnostics using The Molecular Microscope Diagnostic System (MMDx). The molecular
interpretation revealed mild fully-developed antibody-mediated rejection (AMR) with
R4, R5, R6 and all AMR scores of 0.21, 0.25, 0.26 and 0.73, respectively. According
to the most recent Banff 2022 classification, the biopsy findings suggest a diagnosis
of donor-specific antibodies-negative MVI.
In
response to this biopsy finding, maintenance immunosuppression was switched to a
calcineurin-inhibitor-free regimen with belatacept, mycophenolate and prednisone.
Pancreas graft venous
thrombosis
Almost 2.5
years after transplantation, the patient suffered from lower gastrointestinal bleeding.
An upper endoscopy showed evidence for variceal bleeding at the level of the graft-duodenal
anastomosis. Subsequent angiography revealed an unusual finding of a complete thrombosis
of the graft portal vein, with preserved venous outflow via newly formed varicose
veins draining into the patient’s own portal venous system (figure 4). Pancreatic
graft function remained excellent, with persistent insulin freedom and normal HbA1c.
An interdisciplinary panel opted against attempting an interventional venous recanalisation,
due to the high risk of complications. The option of a preventive graft removal
to exclude the risk of variceal bleeding was considered disproportionately risky and refused
by the patient. Within the six months after diagnosis, no further episodes of gastrointestinal
bleeding occurred under persistent graft function.
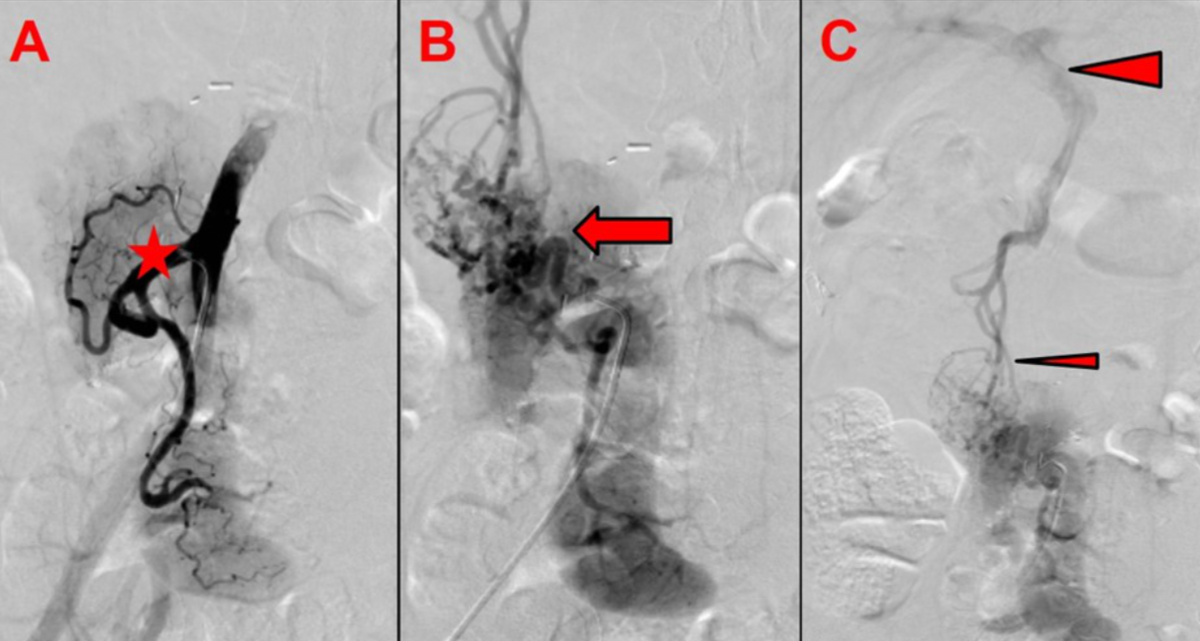
Figure 4Pancreas graft venous outflow. (A) Arterial angiography shows homogeneous
arterial perfusion of the pancreas graft with open arterial anastomosis
(asterisk: arterial Y-graft). (B) Venous angiography shows no venous outflow
via the graft portal vein and vena cava (arrow: gross variceal formations are
visible around the graft pancreas head and duodenum). (C) Venous outflow via
variceal formation draining into the patient’s own portal vein (small arrowhead:
varicose veins around the graft pancreas head and duodenum; large arrowhead:
patient’s portal vein).
Discussion
This is the
first report of a simultaneous pancreas and kidney transplant with en bloc whole
adrenal gland transplant in Switzerland and the second one worldwide. The triple
transplant was technically safe and successful, with good and persistent graft function
for the pancreas and kidney. However, independence from hormone replacement therapy
was not achieved. Despite detectable adrenal graft activity in the early postoperative
period, long-term success was limited, possibly hampered by an elevated cortisol
demand caused by unclear rheumatic pain and postoperative stress.
The success
of adrenal gland transplantation is generally poorly characterised and controversial.
Most existing reports cover autologous
transplants, in which adrenals were mainly transplanted to the thigh as an easily
accessible implant site [12]. However, most of these reports include small case
series and clinical reports, often showing conflicting results especially regarding
long-term adrenal function [4, 5]. Almost all patients still required hormone replacement
after such transplants, likely because autografts contained insufficient functional
adrenal tissue or inadequate blood supply [5]. There are only a few reports on adrenal
allotransplantation, of which only two on whole-organ adrenal gland transplantation
with varying results [7, 8]. Vouillarmet et al. reported on a simultaneous
pancreas and kidney transplant combined with adrenal gland transplant. The authors
stated satisfactory graft function one year after transplantation, documented by
a normalisation of cortisol and aldosterone baseline levels and regular uptake of
[123I]-metaiodobenzylguanidine by the adrenal graft [7]. This patient
however suffered from an acute adrenal crisis three years after transplantation,
with the need to resume hormone replacement therapy [13]. Dubernard et al. reported
on a patient receiving a simultaneous kidney and adrenal gland allograft following
bilateral nephrectomy for renal cell cancer [8]. They stated adequate responses
to ACTH stimulation early after transplantation. However, steroid-based immunosuppression
was maintained after transplantation, possibly causing secondary graft insufficiency
with the need for replacement therapy over time. Interestingly, postmortem biopsies
showed intact adrenal graft morphology.
The major
benefit of en bloc adrenal gland and kidney transplantation, with or without simultaneous
pancreas transplantation, is that it does not require major technical changes compared
to standard kidney transplantation. We chose the left adrenal gland and kidney,
to facilitate vessel preservation at procurement and anastomosis technique during
implantation. In doing so, single arterial and venous anastomoses were sufficient
for implantation, not complicating the procedure. This is in accordance with Vouillarmet
et al. [7] and Dubernard et al. [8], who also used left adrenal glands en bloc with
left kidneys. However, procurement of an adrenal gland is technically more demanding
and special attention needs to be paid to preservation of adrenal gland perfusion.
We preserved the middle and lower suprarenal arteries, whereby we only needed one
single aortic patch, including the renal artery and the middle suprarenal artery.
This is in contrast to Vouillarmet et al., where only the lower suprarenal artery
was preserved. However, there it was sufficient for adrenal perfusion as temporarily
full graft function was achieved. To point out, we performed adrenal graft implantation
without the need for technical or immunosuppressive modifications to standard
simultaneous pancreas and kidney transplantation and without additional surgical
morbidity. Reoperation two weeks after surgery was not causally related to the adrenal
graft. In general, the rate of reoperation is high after simultaneous pancreas
and kidney transplantation, reported up to 32% [14, 15]. In order to minimise technical
errors, the same specialised surgical team performed procurement and transplantation.
Graft function
of simultaneous pancreas and kidney transplantation was immediately satisfactory,
despite initial fluctuations in kidney graft function. However, adrenal graft function
was more difficult and complex to assess. In the early postoperative period, adrenal
graft activity was measurable by detection of sufficient fasting cortisol levels
and even adequate responses to ACTH stimulation. Although, very recently, a guideline
on diagnosis and therapy of glucocorticoid-induced adrenal insufficiency has been
published [16], no protocols for postoperative steroid treatment exist for this
rare type of transplantation. In addition, after many years of steroid supplementation,
the effects of dose changes vary widely among patients and are difficult to predict.
Complicating this, evidence on tapering steroid substitution in patients with Addison’s
disease is lacking. In our patient, after the regular 5-day steroid taper for
simultaneous pancreas and kidney transplantation, a complete stop of replacement
therapy was impossible due to severe steroid withdrawal symptoms. From the patient’s
perspective, the worst symptoms were severe fatigue, joint pain and effusions. Thus,
the preoperative replacement therapy with hydrocortisone and fludrocortisone was
reestablished and later even increased in dosage due to worsening symptoms. Unfortunately,
the initially detectable chemical effect of adrenal graft function never became
clinically relevant.
However,
the exact reason for adrenal graft insufficiency remained unclear. Most likely,
persistent exogenous steroid replacement caused a secondary adrenal gland insufficiency
by suppressing ACTH production, thus leading to decreased stimulation of the adrenal
graft. Strong and persistent rheumatic-like pain, combined with the postoperative
trauma, led to additional physical and mental stress, resulting in an excessive
cortisol need early after transplantation. Early adrenal graft function was apparently
not sufficient for this increased stress-related demand, making additional cortisol
dosages necessary in order to prevent clinical deterioration. This continuous exogenous
steroid intake might have caused a secondary graft insufficiency. Similarly, maintained
long-term steroid immunosuppression probably hampered Dubernard et al.’s success
after combined kidney and adrenal gland transplantation [8]. However, it is also
unclear whether or not and how long it takes for the hypothalamus-pituitary axis
to recover after discontinuation of such long-term steroid treatment. It is known
that the recovery of the hypothalamus-pituitary axis might take several months to
years in some cases after glucocorticoid-induced adrenal insufficiency [16]. Nevertheless,
in those cases, other differential diagnoses should be taken into account.
One possibility
to avoid such secondary adrenal graft insufficiency could be a steroid-free protocol.
Omitting steroids is interesting for pancreas transplantations due to the steroids’
extensive side effects, including elevated insulin resistance, hyperlipidaemia and
osteonecrosis [17]. Steroid-free regimes for simultaneous pancreas and kidney
transplantation have shown similar patient and graft survival rates and only minimal
early rejection rates compared to steroid-containing protocols [18]. In addition,
promising results have been obtained in islet cell transplantation without steroids,
with no apparent problems with rejection [19]. However, the complexity of our patient’s
primary disease and her already longstanding replacement therapy caused severe steroid
withdrawal symptoms, making it impossible to taper steroids.
The histopathological
and molecular results of the kidney allograft biopsy, however, raised suspicions
of a possible alloimmune mechanism. Despite the lack of complete concordance of
the histopathological findings in combined kidney and pancreas transplantation,
an alloimmune injury must be considered as a possible reason for the loss of function
of the adrenal gland, even if the findings for the kidney are to be regarded as
subclinical. However, in the absence of preformed and de novo donor-specific
antibodies, this histopathological finding must be interpreted cautiously, even
if biopsy-based transcript diagnostics suggest an antibody-mediated mechanism.
In addition,
we observed a reappearance of adrenal autoantibodies after transplantation. Such
an autoimmune recurrence could possibly have caused, or at least influenced, adrenal
graft loss. Adrenal gland autoantibodies were well detectable before transplantation,
together with anti-islet cell autoantibodies. However, they became undetectable
early after transplantation, but reappeared again a few months later. Such autoimmune
recurrence has been shown to be predictive of graft failure after pancreas transplantation
[20, 21]. However, data on autoimmune graft adrenalitis is lacking. Despite this,
Vouillarmet et al. reported autoimmune recurrence as a possible cause of late adrenal
graft loss after their initially successful adrenal graft allotransplant [13]. In
their case, autoantibodies were first detected one year after transplantation; graft
loss, however, did not occur until after two more years. Nevertheless, one cannot
prove autoantibodies were the cause of graft loss, as no biopsy was taken. Our case
is similar and the reappearance of autoantibodies is indeed suggestive, but we do
not have histological evidence either. Due to the lack of therapeutic benefit and
the increased risk for the patient, we also decided against a biopsy. Moreover,
there was no temporal relationship between reappearance of autoantibodies and graft
loss. Graft function was already hampered before, at a time when autoantibodies
were still undetectable.
A less likely
reason for the loss of function could have been a calcineurin inhibitor-associated
adrenocortical toxicity. Adverse effects of calcineurin inhibitors on the adrenal
cortex have been documented in animal models and in patients after kidney transplantation
[22], leading to chronic suppression of the adrenal cortex, thus hampering further
steroid reduction [23]. However, calcineurin inhibitors remain the backbone of
simultaneous pancreas and kidney transplantation maintenance regimens and
calcineurin inhibitors-withdrawal has been associated with increased rates of pancreas
rejection [24]. A technical graft failure due to thrombosis is also very unlikely,
as postoperative CT repeatedly confirmed adequate graft perfusion and the graft
appeared macroscopically normal during reoperation.
The exact
cause of adrenal graft failure thus remains unclear and difficult to assess. However,
most importantly, pancreas and kidney graft functions were persistently excellent,
and adrenal gland transplantation did not pose any additional risks for the patient.
Importantly, the function of the pancreas graft is persistent, despite the thrombosis
of the graft portal vein. Fortunately, this occlusion was chronic and slow, allowing
sufficient time for the formation of new venous bypass circuits. The venous outflow
of the pancreas graft is via the patient’s portal venous system. Any intervention
to recanalise the graft portal vein seems too risky and, as long as pancreas graft
function is preserved and venous drainage is unproblematic, all the more unnecessary.
However, the risk of variceal bleeding must be taken into account, although there
has only been one single relevant episode in the past. If bleeding recurs, we would
aim for interventional therapy as a first measure and reserve graft pancreatectomy
only for an extreme, uncontrollable case.
In the future,
we will discuss the option of a second, autologous, adrenal gland transplantation
with the patient. This would be a second attempt to treat the patient’s severe adrenal
insufficiency or to help reduce dosages of hormone replacement therapy. Different
techniques for autologous adrenal gland transplantation have been described, but
the literature on this subject is generally sparse and mainly consists of small
case series and autotransplantations [5, 6]. In our patient, we would try to limit
the surgical trauma of a reoperation, thus possibly preferring an intramuscular
implantation of a morcellised adrenal allograft, as initially described by Grodstein
et al. [6]. Their case showed excellent long-term function, with stop of hormone
replacement therapy and a graft responding well to ACTH stimulation. If another
allograft is transplanted, the current and well-tolerated immunosuppression does
not need to be adjusted or increased, but the risk of reimmunisation must be considered.
Another option would be the transplantation of a whole vascularised adrenal graft
to the epigastric artery and saphenous vein, as described for autotransplantations
in a small series by Dong et al. [5]. However, we would not recommend this due to
concerns about technical difficulties in procurement of an isolated adrenal gland
without a kidney graft in deceased donors and the subsequent high risk for complications
due to the small vessels.
Conclusion
En bloc adrenal
allotransplantation with a left kidney or combined as simultaneous pancreas and
kidney transplantation is technically safe and feasible. While procurement requires
special attention in relation to preserving vascular supply, implantation does not
require technical modifications and poses no further risk for the patient. However,
adrenal allotransplantation should only be considered in special clinical settings,
when adrenal insufficiency is combined with end-stage kidney failure requiring transplantation,
and thus justifying the need for lifelong immunosuppression. Success is
probably limited by the complex fine-tuning of hormone replacement therapy and immunological
factors, rather than technical considerations. Patients should be followed in an
interdisciplinary setting involving endocrinologists, nephrologists and transplant
surgeons.
Data sharing statement
The datasets
presented in this article are not readily available because of local restrictions.
Requests to access the datasets should be directed to the corresponding author (FR).
According to local policies, data must remain on controlled access due to patient
protection and ethical laws in Switzerland.
Informed consent
Written informed consent was obtained from the
patient for the publication of this article.
Fabian Rössler, MD
University Hospital Zurich
Department of Surgery and
Transplantation
Raemistrasse 100
CH-8091 Zurich
fabian.roessler[at]usz.ch
References
1. Husebye ES, Pearce SH, Krone NP, Kämpe O. Adrenal insufficiency. Lancet. 2021 Feb;397(10274):613–29.
10.1016/S0140-6736(21)00136-7
2. Husebye ES, Anderson MS, Kämpe O. Autoimmune Polyendocrine Syndromes. N Engl J Med.
2018 Mar;378(12):1132–41. 10.1056/NEJMra1713301
3. Barzilai D, Dickstein G, Kanter Y, Plavnick Y, Schramek A. Complete remission of Cushing’s
disease by total bilateral adrenalectomy and adrenal autotransplantation. J Clin Endocrinol
Metab. 1980 May;50(5):853–6. 10.1210/jcem-50-5-853
4. Hardy JD, Moore DO, Langford HG. Cushing’s disease today. Late follow-up of 17 adrenalectomy
patients with emphasis on eight with adrenal autotransplants. Ann Surg. 1985 May;201(5):595–603.
10.1097/00000658-198505000-00008
5. Dong D, Ji Z, Li H. Autologous Adrenal Transplantation for the Treatment of Refractory
Cushing’s Disease. Urol Int. 2019;103(3):344–9. 10.1159/000502345
6. Grodstein E, Hardy MA, Goldstein MJ. A case of human intramuscular adrenal gland transplantation
as a cure for chronic adrenal insufficiency. Am J Transplant. 2010 Feb;10(2):431–3.
10.1111/j.1600-6143.2009.02929.x
7. Vouillarmet J, Buron F, Houzard C, Carlier MC, Chauvet C, Brunet M, et al. The first
simultaneous kidney-adrenal gland-pancreas transplantation: outcome at 1 year. Am
J Transplant. 2013 Jul;13(7):1905–9. 10.1111/ajt.12296
8. Dubernard JM, Cloix P, Tajra LC, Alduglihan W, Borson F, Lefrançois N, et al. Simultaneous
adrenal gland and kidney allotransplantation after synchronous bilateral renal cell
carcinoma: a case report. Transplant Proc. 1995 Feb;27(1):1320–1.
9. Siniscalchi C, Moretti V, Cataldo S, Rocci A, Basaglia M, Tassoni MI, et al. The Schmidt
Syndrome. Acta Biomed. 2018 Jan;88(4):499–501. 10.23750/abm.v88i4.5117
10. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al.; Chronic
Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values
in the modification of diet in renal disease study equation for estimating glomerular
filtration rate. Ann Intern Med. 2006 Aug;145(4):247–54. 10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI
(Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular
filtration rate. Ann Intern Med. 2009 May;150(9):604–12. 10.7326/0003-4819-150-9-200905050-00006
12. Hardy JD, Langford HG. Adrenal Autotransplantation in Cushing’s disease. Ann N Y Acad
Sci. 1964 Nov;120(1):667–8. 10.1111/j.1749-6632.1964.tb34760.x
13. Buron F, Vouillarmet J, Thaunat O, Thivolet C, Badet L, Morelon E. Autoimmune Recurrence
as a Cause of Adrenal Gland Graft Loss? Am J Transplant. 2016 Jul;16(7):2235–6. 10.1111/ajt.13737
14. Gilabert R, Fernández-Cruz L, Real MI, Ricart MJ, Astudillo E, Montaña X. Treatment
and outcome of pancreatic venous graft thrombosis after kidney—pancreas transplantation.
Br J Surg. 2002 Mar;89(3):355–60. 10.1046/j.0007-1323.2001.02016.x
15. Troppmann C. Complications after pancreas transplantation. Curr Opin Organ Transplant.
2010 Feb;15(1):112–8. 10.1097/MOT.0b013e3283355349
16. Beuschlein F, Else T, Bancos I, Hahner S, Hamidi O, van Hulsteijn L, et al. European
Society of Endocrinology and Endocrine Society Joint Clinical Guideline: Diagnosis
and Therapy of Glucocorticoid-induced Adrenal Insufficiency. J Clin Endocrinol Metab.
2024 Jun;109(7):1657–83. 10.1210/clinem/dgae250
17. Citterio F. Steroid side effects and their impact on transplantation outcome. Transplantation.
2001 Dec;72(12 Suppl):S75–80.
18. Freise CE, Kang SM, Feng S, Posselt A, Hirose K, Hirose R, et al. Experience with
steroid-free maintenance immunosuppression in simultaneous pancreas-kidney transplantation.
Transplant Proc. 2004 May;36(4):1067–8. 10.1016/j.transproceed.2004.04.017
19. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation
in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive
regimen. N Engl J Med. 2000 Jul;343(4):230–8. doi: https://doi.org/10.1056/NEJM200007273430401
20. Occhipinti M, Lampasona V, Vistoli F, Bazzigaluppi E, Scavini M, Boggi U, et al. Zinc
transporter 8 autoantibodies increase the predictive value of islet autoantibodies
for function loss of technically successful solitary pancreas transplant. Transplantation.
2011 Sep;92(6):674–7. 10.1097/TP.0b013e31822ae65f
21. Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, et al. Recurrence
of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression,
is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes.
2010 Apr;59(4):947–57. 10.2337/db09-0498
22. Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural
history of chronic allograft nephropathy. N Engl J Med. 2003 Dec;349(24):2326–33.
10.1056/NEJMoa020009
23. Oka K, Shimodaira H, Hirano T, Sakurai E, Tamaki T, Kozaki M. Comparison of adrenal
functions in kidney transplant recipients with different long-term immunosuppressive
treatments—prednisolone and azathioprine versus prednisolone and cyclosporine. Transplantation.
1993 Sep;56(3):603–9. 10.1097/00007890-199309000-00020
24. Stock PG, Mannon RB, Armstrong B, Watson N, Ikle D, Robien MA, et al. Challenges of
calcineurin inhibitor withdrawal following combined pancreas and kidney transplantation:
results of a prospective, randomized clinical trial. Am J Transplant. 2020 Jun;20(6):1668–78.
10.1111/ajt.15817



