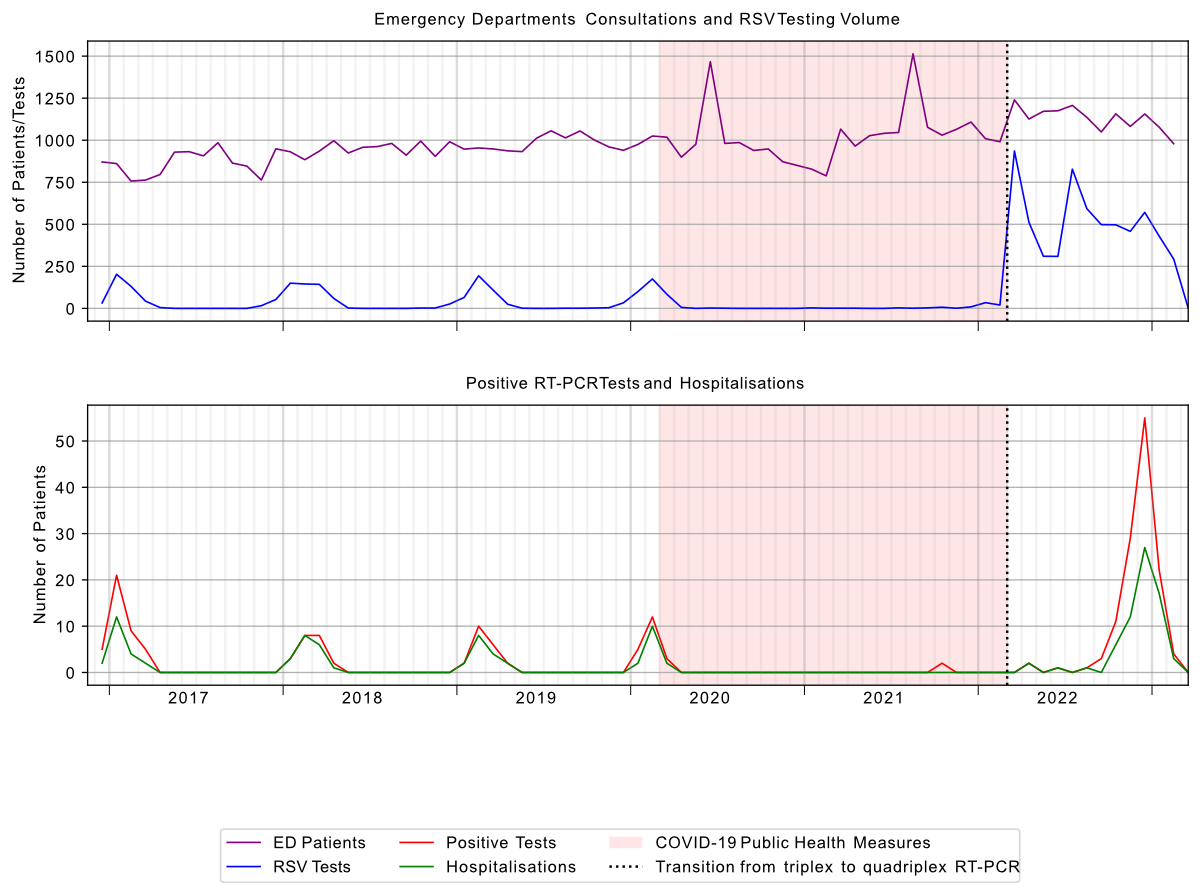
Figure 1Spital Emmental RSV testing and emergency department (ED) patient volume December 2016 – March 2023. RSV: respiratory syncytial virus; RT-PCR: reverse transcription–polymerase chain reaction.
DOI: https://doi.org/https://doi.org/10.57187/s.4324
Respiratory syncytial virus (RSV) is a common respiratory pathogen within the Paramyxoviridae family. It is a single-stranded negative-sense RNA virus and exists as two antigenic subtypes. RSV transmission occurs through respiratory droplets with an incubation period of two to eight days, typically causing upper and lower respiratory tract infections. Peak seasonal incidence occurs during winter months.
While RSV-associated morbidity has historically been considered primarily a problem of childhood, it is increasingly recognised as an important cause of adult respiratory disease [1], particularly in immunocompromised individuals and in adults over 65 years of age [2]. Risk factors include predisposing lung conditions such as asthma or chronic lung disease. RSV infection can also exacerbate pre-existing health conditions such as asthma, chronic obstructive pulmonary disease and congestive heart failure, and cardiac arrhythmia and myocardial infarction may occur. Notably, RSV represents one of the leading causes of mortality in haematopoietic stem cell transplant recipients [2]. Treatment for RSV infection is generally supportive. For severely immunocompromised adults, ribavirin and palivizumab [3] have been used as off-label therapeutic options [4]. Protective vaccines for adults have become available, with recent Centre for Disease Control and Prevention (CDC) recommendations for a single vaccination for adults aged 75 and over or aged 60–74 at risk of developing severe RSV infection [5] and newly published Swiss recommendations for prenatal maternal vaccination, and adults aged 75 and over or aged 60–74 at risk of developing severe RSV infection [6].
This study aims to analyse the clinical and epidemiological characteristics of RSV-infected patients, both hospitalised and ambulatory, who presented to our institution between 2016 and 2023. We specifically investigated predisposing factors, patient demographics, treatment approaches and clinical outcomes. Furthermore, we examined temporal patterns and incidence of RSV infection during periods of COVID-19-related public health measures.
This retrospective cohort study was conducted at Spital Emmental, a regional hospital with 180 beds in Switzerland serving a population of approximately 110,000, between 26 December 2016 and 6 February 2023. Data were collected from patients undergoing nasopharyngeal reverse transcription–polymerase chain reaction (RT-PCR) following our hospital’s internal standard of care for syndromic testing of patients with respiratory symptoms. Patients over the age of 16 who were being treated at Spital Emmental were included in the analysis. A study protocol was not preregistered. In 2016, the RT-PCR tests were initially performed with a focus on influenza and its treatment with neuraminidase inhibitors based on symptoms of respiratory infections. The initial testing algorithm was restricted to hospitalised patients who qualified for therapy, those with progressive respiratory symptoms or those requiring higher levels of care. Following the emergence of SARS-CoV-2 in 2020, the syndromic testing strategy was modified to align with national guidance, prioritising SARS-CoV-2 RT-PCR testing, which was initially performed by an external reference laboratory, for epidemiological surveillance and to guide isolation measures. The availability of quadrivalent RT-PCR tests in March 2022 enabled in-house testing for the detection of SARS-CoV-2 infection and subsequent cohort isolation. Clinical data were systematically extracted from electronic medical records using a standardised data collection template. Structured data elements included RT-PCR results, patient demographics (age and sex) and hospitalisation duration. Non-numerical characteristics including presenting symptoms (using predefined categories based on common RSV manifestations), comorbidities, therapeutic interventions, complications and outcomes including discharge destination and mortality (defined as non-survival to hospital discharge in inpatients) were collated in Microsoft Excel (Office 2019). The primary investigator reviewed all admission documentation and emergency department notes, with a second investigator independently verifying a random sample of cases to ensure consistency and accuracy of data capture.
All cases diagnosed 48 hours after admission were defined as nosocomial, by analogy with the Infectious Diseases Society of America (ISDA) definition for hospital-acquired pneumonia [7]. For pandemic-related analyses, we specifically examined the period between 13 March 2020 and 17 February 2022, which corresponded with the Swiss Federal Office of Public Health’s COVID-19 control measures.
Analyses were performed using Python 3.10.12 with NumPy 1.26.4, Pandas 2.2.2 and Matplotlib 3.8.0 under PSF/BSD License and implemented using Jupyter Notebooks. Patients without an emergency department clinical record (reflecting external referral for testing without a consultation) were excluded from our analysis.
Initial testing of nasopharyngeal samples employed a trivalent RT-PCR assay (GeneXpert Influenza A/B and RSV) until 8 March 2022, after which a quadrivalent Xpert Xpress assay (SARS-CoV-2, Influenza A/B, RSV) was implemented. The Positive Percentage Agreement (PPA) and the Negative Percentage Agreement (NPA) are described as 100% [8]. The PCR cycle threshold for test positivity was locally set at 40.
During the study period, 8135 RT-PCR tests were performed, with 231 (2.8%) positive for RSV. Two cases were documented with positive results for another pathogen in addition to RSV: co-infection with influenza in both cases. Emergency department presentations increased over the study period. The monthly distribution of positive tests was compatible with typical winter seasonality of RSV infections (figure 1). The cumulative number of cases in female and male patients was similar (122 and 109, respectively). The mean age was 69 years (range 17–96 years). Of all patients with a positive RSV RT-PCR, 69% required hospitalisation, 21% received outpatient treatment and 10% tested positive during an existing hospital admission.

Figure 1Spital Emmental RSV testing and emergency department (ED) patient volume December 2016 – March 2023. RSV: respiratory syncytial virus; RT-PCR: reverse transcription–polymerase chain reaction.
Of the 231 RSV-positive cases, complete clinical data were available for 194 patients and are summarised in table 1; 37 cases were excluded from further analysis due to lack of available clinical information (figure 2), representing external referral for a laboratory test without clinical assessment in our emergency department. In this cohort, the most common symptoms at presentation were cough or sore throat (73%), followed by dyspnoea (44%) and fever (39%). The prevalence of symptoms fluctuated throughout the study period (figure 3). A chest X-ray was performed in 155 (80%) of patients. A chest CT scan was performed in 37 cases. Pulmonary infiltrates were documented in 39% (n = 76) of these diagnostic images.
Table 1Patient characteristics of patients with positive RSV RT-PCR, Spital Emmental 2016–2023.
| Total | Outpatient | Inpatient | |||
| (n = 194) | (n = 37) | (n = 157) | |||
| Demographics | Female | 98 (51%) | 19 (51%) | 79 (50%) | |
| ≤20 years | 5 (3%) | 4 (11%) | 1 (1%) | ||
| 21–40 years | 14 (7%) | 9 (24%) | 5 (3%) | ||
| 41–60 years | 27 (14%) | 12 (32%) | 15 (10%) | ||
| 61–80 years | 74 (38%) | 9 (24%) | 65 (41%) | ||
| >80 years | 74 (38%) | 3 (8%) | 71 (45%) | ||
| Comorbidities | Cardiac disease | 90 (46%) | 6 (16%) | 84 (54%) | |
| Diabetes mellitus | 43 (22%) | 0 (0%) | 43 (27%) | ||
| Chronic kidney disease | 51 (26%) | 3 (8%) | 48 (31%) | ||
| Anaemia | 72 (37%) | 4 (11%) | 68 (43%) | ||
| Malignancy | 42 (22%) | 4 (11%) | 38 (24%) | ||
| Immunosuppression | 16 (8%) | 5 (14%) | 11 (7%) | ||
| Pulmonary disease | 85 (44%) | 8 (22%) | 77 (49%) | ||
| Imaging | Chest imaging performed | 172 (89%) | 24 (65%) | 148 (94%) | |
| Chest X-ray | 155 (80%) | 23 (62%) | 132 (84%) | ||
| Chest CT | 37 (19%) | 2 (5%) | 35 (22%) | ||
| Treatment | Systemic steroids | 77 (40%) | 5 (14%) | 72 (46%) | |
| Antibiotic therapy | 105 (54%) | 5 (14%) | 100 (64%) | ||
| Level of care | Intensive care unit | 10 (5%) | – | 10 (6%) | |
| Intermediate care | 4 (2%) | – | 4 (3%) | ||
| Outcome | In-hospital mortality | 9 (5%) | – | 9 (6%) | |
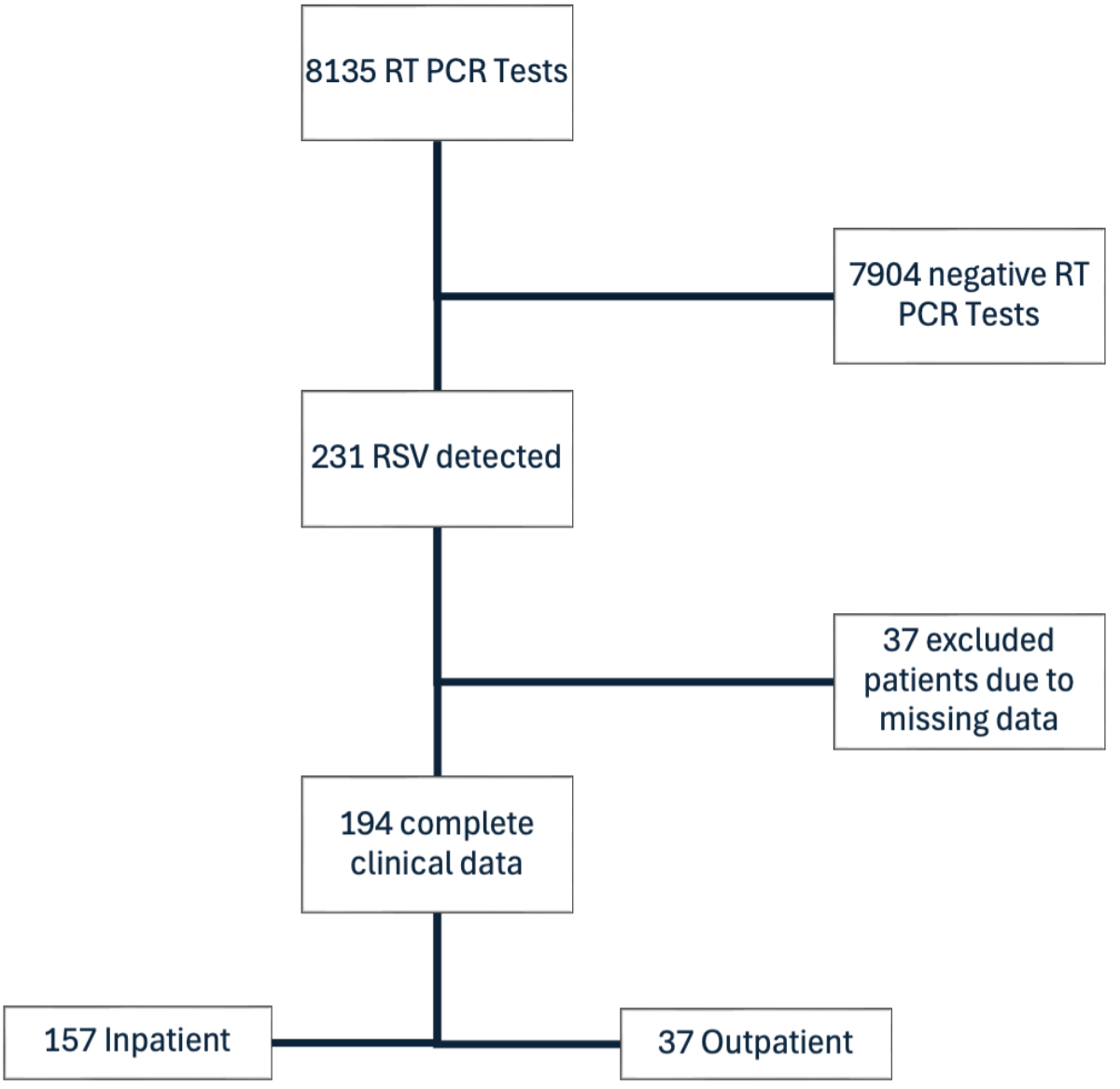
Figure 2A STROBE flowchart of RSV testing at Spital Emmental 2016–2023: patient selection and analysis. RSV: respiratory syncytial virus; RT-PCR: reverse transcription–polymerase chain reaction.
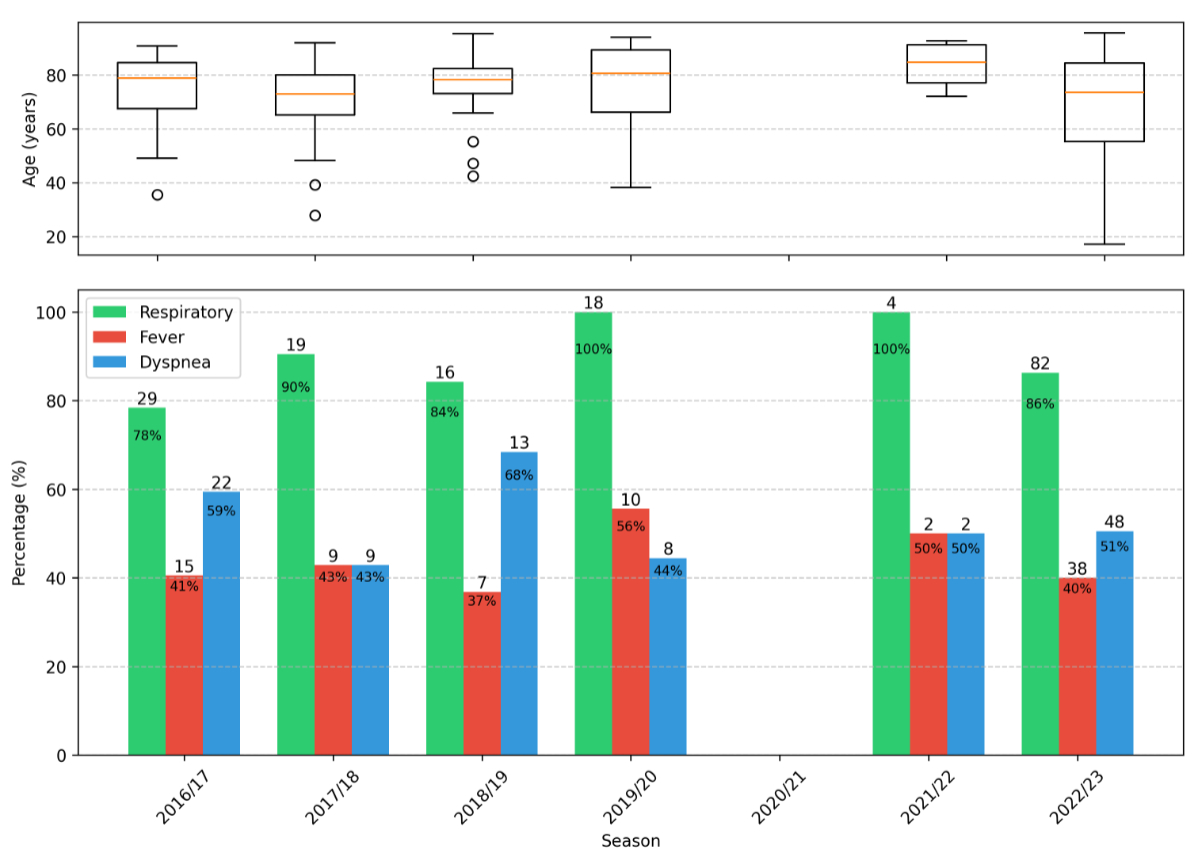
Figure 3Seasonal variation of symptoms and age distribution of 194 patients with positive reverse transcription–polymerase chain reaction (RT-PCR) tests at Spital Emmental 2016–2023. Age boxplots display: median (orange central line); interquartile range (IQR) Q1–Q3 (box); whiskers extending to most extreme points within 1.5 × IQR of Q1 and Q3; and individual points for outliers beyond these bounds.
A subgroup analysis of the 157 patients who were hospitalised following a positive RSV test at admission was performed. This hospitalised cohort showed equal sex distribution with a mean age of 77 years. The majority (86%) of the patients admitted to hospital were over 60 years of age. Nineteen cases (12%) were classified as nosocomial infections, with a mean time to detection of 4 days post-admission. The predominant comorbidities were congestive heart failure (54%), anaemia (43%) and pulmonary disease (49%) (table 1).
Radiological assessment was performed in 147 (94%) of the hospitalised patients, comprising 132 chest X-rays and 35 CT scans. Therapeutic interventions included antibiotics in 100 patients (64%) and corticosteroids in 72 patients (46%) (table 1). Critical care was required in 14 cases (9%): 10 patients (6%) needed intensive care and 4 (3%) intermediate care. Respiratory support was provided via non-invasive ventilation in 5 patients and intubation with positive pressure ventilation in 5 patients.
The mean hospital stay was 8 days (range 1–38 days). Of the hospitalised cohort, 66% were discharged home, 15% transferred to nursing homes, 11% to rehabilitation facilities and the outcomes of the remaining 2% are unknown due to hospital transfers at discharge (figure 4). In-hospital mortality was 6%.
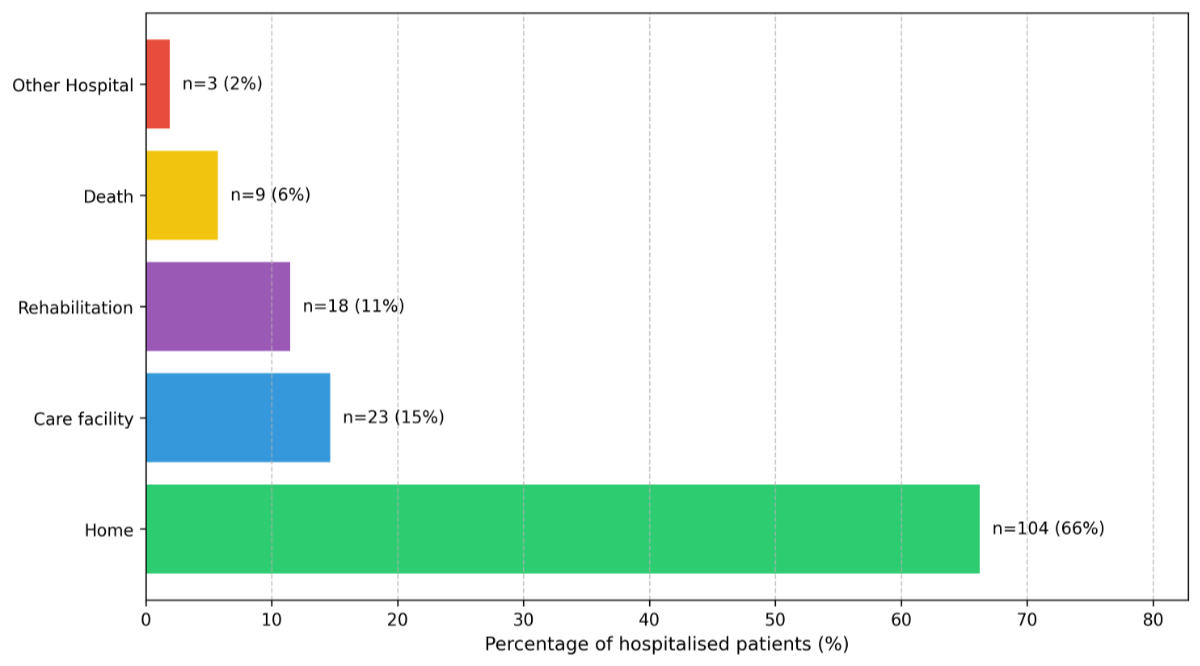
Figure 4Discharge outcomes of 157 hospitalised respiratory syncytial virus (RSV) patients.
Of 8135 RT-PCR tests performed, 1766 (22%) were conducted before the COVID-19 pandemic, 125 (1%) during the pandemic period and 6244 (77%) after pandemic measures were lifted. Among 231 RSV-positive cases, 99 occurred pre-pandemic, 4 during the pandemic and 128 after pandemic measures were lifted.
Seasonal patterns were observed across the study period. Pre-pandemic RSV infections were most frequently documented between January and March. In the first seasonal peak following lifting of COVID-19 restrictions, cases were most frequently documented between November and January (figure 5). During the pandemic period itself, only four cases were documented (two each in March and December), insufficient for descriptive temporal analysis (figure 1).
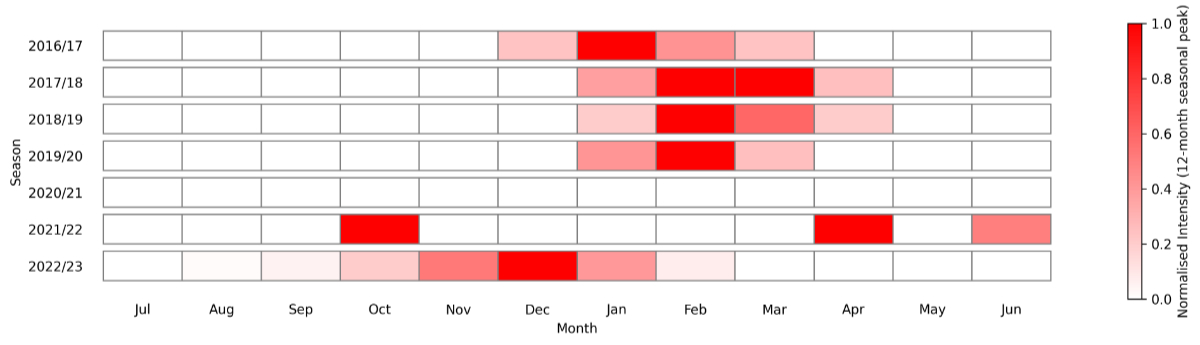
Figure 5Temporal pattern of maximal seasonal intensity* of positive RSV RT-PCR tests at Spital Emmental 2016–2023. * Normalised intensity calculated as monthly number of positive tests relative to the 12 month (July–June) seasonal monthly peak value. RSV: respiratory syncytial virus; RT-PCR: reverse transcription–polymerase chain reaction.
On 8 March 2022, testing methodology changed from trivalent to quadrivalent RT-PCR. During the period from December 2016 to March 2022 (five seasons), 1845 RT-PCR tests were performed, with 103 positive RSV results (6%). In the subsequent period to February 2023 (two seasons), 6290 tests were performed, yielding 128 positive results (2%). In a typical pre-pandemic season (2018/19), 3.7% of emergency department patients were tested. During the 2021/22 and 2023/23 seasons following cessation of COVID-19 pandemic measures and implementation of the local assay, 15.8% and 42.0% of patients, respectively, were tested. In the three consecutive seasons from 2017/18 to 2019/20, the rates of hospitalisation were 86%, 80% and 70%, respectively. After the change of assay, 53% of patients were hospitalised during the following seasonal peak.
In our cohort, we observed a high mean age (70 years overall, 77 years in hospitalised patients), consistent with the literature describing the increased risk of RSV infection among elderly populations [9]. The hospitalised patients in our study frequently presented with comorbidities such as pulmonary disease and congestive heart failure, conditions previously identified as risk factors for RSV complications [2].
Our data demonstrate the significant healthcare resource utilisation associated with RSV infection. The mean hospital stay was 8 days, with frequent use of antibiotics and corticosteroids, possibly reflecting a suspicion for bacterial superinfection and exacerbation of underlying pulmonary conditions. A prolonged hospital stay was also documented in a prospective cohort study in a French tertiary hospital [9]. The need for post-acute care was substantial, with approximately one quarter of patients requiring rehabilitation or nursing home placement. Our observed in-hospital mortality of 6% is in keeping with findings from a US systematic review examining RSV outcomes in adults aged over 65 years or those with comorbidities [2]. Studies have examined specific risk factors for severe RSV disease. Patients with cardiovascular disease have been shown to have increased rates of ICU admission and mortality compared to those without cardiovascular disease [3]. In a separate Swiss analysis comparing RSV with influenza, ICU admission rates were 16.5% and 9.4% for median ages of 78 and 74, respectively [9].
Comparative data on nosocomial RSV infections in adults are limited, possibly reflecting historical testing practices rather than true infection rates, an example of a bias in the form of base-rate neglect. In our institution, prior to the widespread adoption of multiplex PCR testing, RSV testing was typically restricted to severe cases requiring intermediate or intensive care, potentially leading to under-recognition of nosocomial transmission.
The transition from targeted RSV testing to multiplex respiratory virus detection reflects evolving diagnostic capabilities. Our experience with the change from trivalent to quadrivalent PCR testing, accelerated by pandemic-related changes in testing strategies, is an example of this shift.
RSV testing raises both diagnostic and therapeutic issues. While the clinical presentation overlaps with other respiratory viruses, identifying RSV may be relevant for infection control and in high-risk patients, and could be hypothesised to influence antibiotic prescribing patterns.
Our findings reflect this complexity. Although patients with radiological infiltrates frequently received antibiotics, some patients with a positive RT-PCR test without a pulmonary infiltrate also received antibiotics, demonstrating that clinical decisions, such as those concerning antibiotic use, also occur independently of viral detection. Evidence regarding the effect of routine RSV testing on these kinds of therapeutic decision shows mixed results. An Austrian study found that introduction of multiplex RT-PCR increased RSV detection, particularly among older patients with comorbidities [10]. A Norwegian study of community-acquired pneumonia suggested a potential benefit of routine testing leading to faster specific treatment [11]. However, other research has found no association between testing and clinical interventions such as antibiotic use or hospitalisation [12]. The current value of routine RSV testing thus remains uncertain. A systematic evaluation of the relevant factors could however enable the development of an evidence-based testing algorithm. We suggest that research should examine how testing strategies could incorporate clinical and epidemiological factors including age, immune status and comorbidities, seasonality, local infection control requirements and healthcare resource usage implications. An optimal testing approach would also change if an RSV-specific therapy were available, as this would shift the risk-benefit balance of respiratory syndrome-based screening.
During the period of COVID-19-related public health interventions, there was a marked change in the incidence of positive RSV RT-PCR tests in our cohort. In other international cohorts, with consistent prospective surveillance, testing demonstrated a dramatic reduction of RSV activity in the 2020/21 winter season [13]. When the restrictions were lifted, RSV infections showed renewed activity with an unusually early seasonal pattern. These changes were observed beyond Switzerland, with similar findings reported in other European countries and the USA [14, 15].
Several factors have been hypothesised to have contributed to these changes, in particular the focus on hygiene measures (such as masks, hand hygiene and social distancing), or reduced exposure to RSV during pregnancy and early childhood [13]. The increased use of RT-PCR testing for respiratory symptoms may also have changed behaviour. On first inspection, the typical winter seasonality of RSV infection in our cohort also appears to have been disrupted, with an almost complete absence of documented positive tests in the winter of 2020/21, but this ignores the marked reduction in testing (figure 1). This is most likely explained by a change in testing behaviour due to the rapid implementation of a separate RT-PCR for SARS-CoV-2 which was performed externally in a reference laboratory during the early phases of the pandemic in 2020 with concurrent reduction in the use of the in-house point-of-care trivalent RT-PCR test. Testing behaviour then changed again due to the implementation of an in-house point-of-care quadrivalent test in March 2022 and the cessation of sending nasopharyngeal swabs externally for testing.
The change in our institutional testing strategy reflects a form of surveillance bias. This non-random type of information bias has been described as referring to the idea that “The more you look, the more you find”[16], but in our specific local scenario for RSV in winter 2020/21, reduced testing has potentially masked local epidemiological RSV patterns which might align with those observed in larger systematic surveillance networks, provocatively reformulated as “The less systematically you look, the less you know”.
A second phenomenon of atypical interseasonal resurgences has also been described [13]. Continued Swiss paediatric surveillance for RSV showed a high level of regional variability in this period of interseasonal activity prior to an early and strong winter season in 2022/23 [17]. Sporadic positive tests were documented in April and June of 2022 during a dramatic increase in our testing volume, but these cases may represent isolated infections rather than true interseasonal activity, especially given the very low positivity rate during this period (2 positives from 510 tests in April and 1 positive from 308 tests in June 2022).
A third phenomenon of early and intense winter peaks following lifting of restrictions has been described. We also observed early and intense peak RSV activity in late 2022, with both the highest number of positive tests and hospitalisations in our dataset occurring in December 2022 (figure 5).
Analysis of Denmark’s unusually large winter RSV wave in 2021/22 showed that increased testing was only able to explain 70% of the difference to the pre-pandemic period [18]. Immunity debt, where epidemiological patterns may shift due to a change in pathogen-specific population immunity (due to diminishing levels of antibiotics, birth of immunologically naive infants and genetic mutations in the pathogen), has been proposed as a mechanism to explain such post-pandemic peaks [19].
The interpretation of this local peak is complicated by the changes in our testing practices, particularly the substantial increase in testing volume following implementation of quadriplex RT-PCR. The lack of standardised testing criteria across the entire study period and absence of denominator population data limits our ability to differentiate between enhanced case detection and true changes in disease incidence and to make causal assumptions. The unprecedented local peak in hospitalisations nevertheless represents a significant peak in healthcare resource utilisation during this time period.
Our study has several important methodological limitations that must be considered.
First, the retrospective design inherently limited the ability to standardise data collection and may have introduced selection bias, particularly in symptom documentation and clinical decision-making. Missing data resulted in the exclusion of 37 cases (16% of identified cases), potentially affecting the representativeness of our findings.
Second, changes in testing methodology and testing criteria during the study period introduced detection bias. The transition from targeted RSV testing to broader respiratory virus screening, coupled with varying institutional testing practices during the COVID-19 pandemic, precludes detailed temporal comparisons of incidence rates.
Third, the single centre nature of our data may limit external validity, especially given regional variations in both RSV epidemiology and institutional practices. The lack of a defined catchment population prevents the calculation of true incidence rates and limits our ability to make population-level inferences.
Fourthly, the lack of a control group and standardised outcome measures limits our ability to adjust for confounding in our analyses. This is particularly important when interpreting patterns of hospitalisation and clinical characteristics over different time periods in our cohort.
This retrospective analysis provides a granular view of how local changes in diagnostic workflows can affect data interpretation at the level of a single regional hospital. Our experience with the transition from targeted RSV testing to multiplex respiratory virus detection, coupled with pandemic-related operational changes, reveals nuances in testing patterns that might be obscured in larger population-level studies. While our data captured significant RSV-associated in-hospital mortality and resource use in adults, including substantial rates of hospitalisation and critical care requirements, the interpretation of these findings is inherently linked to changes in testing practices. In view of the effect of RSV on healthcare resources and patient outcomes, the recent introduction of preventive vaccination strategies represents a promising development in the management of respiratory syncytial virus. Our experience suggests that when testing strategies and operational changes are carefully considered, regional hospitals may be able contribute meaningful epidemiological data to complement established traditional primary care-based respiratory virus surveillance systems.
The data and code that support the findings of this study are available from the corresponding author upon reasonable request given granular single-patient level data contained within the dataset.
This study received no funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Staadegaard L, Caini S, Wangchuk S, Thapa B, de Almeida WA, de Carvalho FC, et al. The Global Epidemiology of RSV in Community and Hospitalized Care: Findings From 15 Countries. Open Forum Infect Dis. 2021 Mar;8(7):ofab159. doi: https://doi.org/10.1093/ofid/ofab159
2. Colosia A, Costello J, McQuarrie K, Kato K, Bertzos K. Systematic literature review of the signs and symptoms of respiratory syncytial virus. Influenza Other Respir Viruses. 2023 Feb;17(2):e13100. doi: https://doi.org/10.1111/irv.13100
3. Woodruff RC, Melgar M, Pham H, Sperling LS, Loustalot F, Kirley PD, et al.; Respiratory Syncytial Virus Hospitalization Surveillance Network (RSV-NET). Acute Cardiac Events in Hospitalized Older Adults With Respiratory Syncytial Virus Infection. JAMA Intern Med. 2024 Jun;184(6):602–11. doi: https://doi.org/10.1001/jamainternmed.2024.0212
4. Simões EAF, DeVincenzo JP, Boeckh M, Bont L, Crowe JE, Griffiths P, et al. Challenges and opportunities in developing respiratory syncytial virus therapeutics. J Infect Dis. 2015 Mar 15;211 Suppl 1(Suppl 1):S1–20.
5. CDC. RSV Vaccine Guidance for Older Adults. Respiratory Syncytial Virus Infection (RSV). 2024. Available from: https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/older-adults.html
6. Bundesamt für Gesundheit. Impfempfehlungen gegen Erkrankungen mit dem Respiratorischen Synzytial-Virus (RSV). Respiratorisches-Synzytial-Virus (RSV). BAG Bulletin 47. 2024. Available from: https://www.bag.admin.ch/bag/de/home/krankheiten/krankheiten-im-ueberblick/rsv.html
7. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep;63(5):e61–111. doi: https://doi.org/10.1093/cid/ciw353
8. Cepheid. Xpert Xpress CoV-2/Flu/RSV plus Package Insert. Available from: https://www.cepheid.com/en-CH/tests/respiratory/xpert-xpress-cov-2-flu-rsv-plus.html
9. Chorazka M, Flury D, Herzog K, Albrich WC, Vuichard-Gysin D. Clinical outcomes of adults hospitalized for laboratory confirmed respiratory syncytial virus or influenza virus infection. PLoS One. 2021 Jul;16(7):e0253161. doi: https://doi.org/10.1371/journal.pone.0253161
10. Schubert L, Steininger J, Lötsch F, Herdina AN, Redlberger-Fritz M, Tobudic S, et al. Surveillance of respiratory syncytial virus infections in adults, Austria, 2017 to 2019. Sci Rep. 2021 Apr;11(1):8939. doi: https://doi.org/10.1038/s41598-021-88537-5
11. Markussen DL, Serigstad S, Ritz C, Knoop ST, Ebbesen MH, Faurholt-Jepsen D, et al. Diagnostic Stewardship in Community-Acquired Pneumonia With Syndromic Molecular Testing: A Randomized Clinical Trial. JAMA Netw Open. 2024 Mar;7(3):e240830. doi: https://doi.org/10.1001/jamanetworkopen.2024.0830
12. Schober T, Wong K, DeLisle G, Caya C, Brendish NJ, Clark TW, et al. Clinical Outcomes of Rapid Respiratory Virus Testing in Emergency Departments: A Systematic Review and Meta-Analysis. JAMA Intern Med. 2024 May;184(5):528–36. doi: https://doi.org/10.1001/jamainternmed.2024.0037
13. Chuang YC, Lin KP, Wang LA, Yeh TK, Liu PY. The Impact of the COVID-19 Pandemic on Respiratory Syncytial Virus Infection: A Narrative Review. Infect Drug Resist. 2023 Jan;16:661–75. doi: https://doi.org/10.2147/IDR.S396434
14. Chow EJ, Uyeki TM, Chu HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. 2023 Mar;21(3):195–210.
15. Hayes Vidal-Quadras C, Mrabet Deraoui I, Muehlethaler V. Impact of the COVID-19 pandemic on the epidemiology of bronchiolitis at Hôpital du Jura in Delémont, Switzerland: a retrospective observational study. Swiss Med Wkly. 2024 Jul;154(7):3768. doi: https://doi.org/10.57187/s.3768
16. Haut ER, Pronovost PJ. Surveillance bias in outcomes reporting. JAMA. 2011 Jun;305(23):2462–3. doi: https://doi.org/10.1001/jama.2011.822
17. Meyer Sauteur PM, Plebani M, Trück J, Wagner N, Agyeman PK, Meyer Sauteur PM, et al.; RSV EpiCH investigators. Ongoing disruption of RSV epidemiology in children in Switzerland. Lancet Reg Health Eur. 2024 Sep;45:101050. doi: https://doi.org/10.1016/j.lanepe.2024.101050
18. Nygaard U, Hartling UB, Nielsen J, Vestergaard LS, Dungu KH, Nielsen JS, et al. Hospital admissions and need for mechanical ventilation in children with respiratory syncytial virus before and during the COVID-19 pandemic: a Danish nationwide cohort study. Lancet Child Adolesc Health. 2023 Mar;7(3):171–9. doi: https://doi.org/10.1016/S2352-4642(22)00371-6
19. Munro AP, House T. Cycles of susceptibility: Immunity debt explains altered infectious disease dynamics post-pandemic. Clin Infect Dis. 2024; Available from: https://hal.science/hal-04731541