Safety and effectiveness of left atrial appendage occlusion in patients with atrial
fibrillation and high bleeding risk: a cardinality-matched comparison with direct
oral anticoagulation on long-term stroke and bleeding rates
DOI: https://doi.org/https://doi.org/10.57187/s.4288
Thomas Gilhoferab*,
Victor Schweigerac*,
Victoria Bokemeyera,
Mario Gehlerd,
Jonathan M. Michela,
Mi Chena,
Alessandro Candrevaa,
Linn Ryberge,
Davide DiVecef,
Christian Templinf,
Barbara E. Stähliag,
Julia Stehlia,
Alexander Gotschyahi,
Philipp Jakoba,
Frank Ruschitzkaa,
Stefanie Aeschbacherj,
Philipp Krisaij,
Leo H. Bonatik,
Moa Lina Hallerlm,
Nicolas Rodondilm,
Juerg H. Beerno,
Peter Ammannd,
Giorgio Moschovitisp,
Elia Rigamontip,
Stefan Osswaldj,
David Conenq,
Fabian Nietlispachr,
Ronald Karl Binders,
Tobias Reichlint,
Michael Kühnej**,
Albert Markus Kasela**
a Department
of Cardiology, University Heart Centre, University Hospital Zurich, Zurich,
Switzerland
b Department
of Cardiology, Cantonal Hospital Winterthur, Winterthur, Switzerland
c Deutsches Herzzentrum der Charité, Campus
Virchow-Klinikum, Berlin, Germany
d Department
of Cardiology, Hospital St. Gallen, St. Gallen, Switzerland
e Department of Internal Medicine,
Hospital Zollikerberg, Zollikerberg, Switzerland
f Internal Medicine B, University
Medicine Greifswald, Greifswald, Germany
g Faculty of Medicine, University of
Zurich, Zurich, Switzerland
h Institute
of Diagnostic and Interventional Radiology, University Hospital Zurich, Zurich,
Switzerland
i Institute
for Biomedical Engineering, University and ETH Zurich, Zurich ETH-Centre, Zurich,
Switzerland
j Department
of Cardiology and Cardiovascular Research Institute Basel, University Hospital Basel,
University of Basel, Basel, Switzerland
k Research Department, Reha Rheinfelden Rheinfelden, Switzerland
l Department of General Internal Medicine,
Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
m Institute of Primary Health Care (BIHAM), University of
Bern, Bern, Switzerland
n Department of Medicine, Cantonal
Hospital Baden, Switzerland
o Laboratory
for Platelet Research, University of Zurich, Zurich, Switzerland
p Division
of Cardiology, Ente Ospedaliero Cantonale (EOC), Cardiocentro Ticino Institute,
Regional Hospital of Lugano, Lugano, Switzerland
q Population Health Research Institute, Hamilton,
Ontario, Canada
r Hirslanden Herzzentrum im Park AG, Zurich,
Switzerland
s Department
of Internal Medicine, Hospital Wels-Grieskirchen, Wels, Austria
t Department of Cardiology, Inselspital, Bern University Hospital,
University of Bern, Bern, Switzerland
* Equal contribution as first authors
** Equal contribution as last authors
Summary
STUDY AIMS: Left atrial appendage occlusion
(LAAO) is an accepted alternative stroke prevention strategy for patients with
atrial fibrillation (AF) and contraindications to oral anticoagulation despite
the lack of randomised data in this population. This study aims to compare the outcomes
of LAAO and direct
oral anticoagulation (DOAC) therapy in patients with high bleeding risk.
METHODS: This cardinality-matched analysis comprised
data from the Beat-AF and Swiss-AF cohorts (n = 3960;
enrolment from 2010 to 2014 and from 2014 to 2017, respectively), along
with the Zurich LAAO Registry (n = 535; patients included between 2010 and 2023).
The primary endpoint was a composite of stroke, cardiovascular death or major
bleeding. The individual components constituted the secondary endpoints. Time-dependent
cumulative incidence curves were constructed and a competing risk analysis was
included.
RESULTS: After matching, 478 patients with a
DOAC score ≥8 and 159 patients with previous major bleeding were compared in a
1:1 and 1:2 ratio, respectively, regarding their stroke prevention strategy
(DOAC versus LAAO). After a median follow-up time of 4.9 years (interquartile
range [IQR]: 2.2–6.1) in all patients with a DOAC score ≥8 and 4.4 years (IQR: 2.0–6.0)
in all patients with previous major bleeding, there were no significant
differences in the primary endpoint (hazard ratio
[HR]: 0.88, 95% confidence interval [CI]: 0.67–1.14, p = 0.33 and HR: 0.79, 95%
CI: 0.50–1.27, p = 0.33) and in the rates of stroke (HR: 0.74, 95% CI: 0.39–1.42,
p = 0.36 and HR: 1.09, 95% CI:
0.33–3.62, p = 0.89) and cardiovascular death (HR:
0.97, 95% CI: 0.68–1.38, p = 0.85 and HR: 0.91, 95% CI: 0.50–1.64, p = 0.74).
The rate of major bleedings was significantly lower in the LAAO group of both
cohorts (HR: 0.55, 95% CI: 0.32–0.94, p = 0.029 and
HR: 0.32, 95% CI: 0.13–0.79, p = 0.013).
CONCLUSION: In this high bleeding risk population,
LAAO was associated with similar effectiveness in preventing atrial fibrillation-related
stroke and cardiovascular death and significantly lower rates of major bleeding
compared to DOAC therapy. This strengthens the value of LAAO as an alternative stroke
prevention strategy for patients at high risk of bleeding.
Introduction
Atrial fibrillation (AF) remains a
widespread and concerning cardiac arrhythmia, affecting millions of individuals
worldwide. It is associated with a significantly increased risk of stroke,
making it a major cause of morbidity and mortality [1, 2]. To mitigate this risk,
oral anticoagulation
therapy has been the standard of care for atrial fibrillation patients at high
risk of stroke [2]. While oral
anticoagulation therapy is generally effective in preventing stroke [3], it comes
with a significant downside – the
potential for severe or life-threatening bleeding complications, in particular intracranial
haemorrhage. This inherent risk has led to a critical dilemma in the management
of atrial fibrillation patients, who are at increased risk of thromboembolic
events but also have comorbidities that heighten their likelihood of
experiencing major bleeding events.
One
viable solution to address this dilemma is left atrial appendage occlusion (LAAO).
Two randomised controlled trials (RCTs) of LAAO versus warfarin
published already ten years ago and a more recent trial of LAAO versus direct
oral anticoagulation (DOAC) have demonstrated non-inferiority of LAAO in
comparison to oral anticoagulation for preventing thromboembolic events in patients
eligible for oral anticoagulation [4–7]. On
top of that, the recently published OPTION trial also showed significantly
fewer relevant bleeding events in all-comer patients after pulmonary vein
isolation treated with LAAO as compared with DOAC [8]. LAAO even proved to have a
mortality benefit for Watchman LAAO
against vitamin K antagonists in the PROTECT-AF RCT [5] and for Amplatzer LAAO against
DOACs in two large propensity
score-matched studies [9, 10]. The
mortality benefit emerges after a few years and continues to become more
conspicuous with time, which is explainable by the accruing bleeding events in
patients with oral anticoagulation. These events occur at an increasing rate as
the patients get older and sicker. The protection against embolic events with oral
anticoagulation suffers from the typically poor compliance [9]. It is therefore not
really superior to
that associated with LAAO, which represents a mechanical vaccination against
embolic events and therefore has a 100% compliance rate [11]. All this, however, is
not reflected in current guidelines. These
guidelines suggest
considering LAAO in atrial fibrillation patients with contraindications to oral
anticoagulation [1, 2], with a IIb
recommendation in the European and a IIa recommendation in the American
guidelines. Interestingly, a contraindication to oral anticoagulation is an
exclusion criterion in almost all RCTs on LAAO including the large ongoing Champion-AF
and CATALYST trials, which are currently in the follow-up phase (ClinicalTrials.gov
ID: NCT04394546 and NCT04226547, respectively). The ASAP-TOO study, which
randomised patients with a contraindication to oral anticoagulation to
treatment with LAAO or no treatment, was prematurely discontinued due to slow
patient enrolment and it is unlikely that new randomised studies with adequate
power will be available soon [12].
The hypothesis of this study was that atrial
fibrillation patients at risk of thromboembolic events but concomitantly at high
or very high risk of DOAC-related bleeding complications may benefit from LAAO with
equally good stroke prevention compared to DOAC but with fewer bleeding complications
due to less intense antithrombotic therapy. The study aim was to demonstrate both
the effectiveness of thromboembolic protection, in terms of stroke and cardiovascular
mortality, and its safety, in terms of bleeding rates, in a patient population at
such high risk that – depending on the stroke prevention strategy – it is
usually excluded from RCTs.
Methods
Study population
Swiss-AF and Beat-AF cohorts (n = 3960)
The Beat-AF (n = 1545) and Swiss-AF (n = 2415)
studies constitute prospective, multicentre, observational cohort
investigations conducted across 14 medical facilities in Switzerland, with enrolment
spanning the years 2010 to 2014 and 2014 to 2017, respectively [13]. With the exception
of individuals
experiencing reversible forms of atrial fibrillation, those with acute illness
within the preceding 4 weeks and those unable to provide informed consent,
there were no significant exclusion criteria for participation in either study [13].
The start of participation in Beat-AF and
Swiss-AF was determined as the initial contact between the patient and the
study site. In both registries, atrial fibrillation patients received stroke
prevention measures in accordance with prevailing guidelines [1]. Beyond
this standard of care, no predefined interventions were implemented
post-inclusion in the Beat-AF and Swiss-AF registries. For the present
analysis, only patients treated with DOAC were included (n = 1230). Trained
study personnel conducted yearly outpatient visits and annual telephone
follow-ups, with systematic event adjudication.
Zurich Left Atrial Appendage Occlusion (LAAO) registry
The Zurich LAAO Registry is a combined prospective/retrospective,
single-centre registry encompassing all atrial fibrillation patients undergoing
LAAO at University Hospital Zurich. The procedural date aligned with the study
entry in the LAAO group of this comparative study, where only patients with a
suitably positioned LAA occluder at the conclusion of the procedure between June
2010 and October 2023 were considered in the current analysis. Standard
methodologies from the literature were employed for LAAO procedures at
University Hospital Zurich [14]. The procedures were performed either under general
anaesthesia with transoesophageal
echocardiography or under local anaesthesia and fluoroscopic guidance with or
without intracardiac echocardiography, depending on the physician’s preference [15].
Periprocedural adverse events were incorporated
for examination. Unsuccessful procedures were excluded, along with those
involving concomitant transcatheter aortic valve implantation or transcatheter
mitral valve edge-to-edge repair, owing to the elevated baseline risk
associated with severe valvular heart disease. Follow-up involved periodic
assessments during both inpatient and outpatient visits at University Hospital
Zurich, extending to non-cardiology visits. For patients under the care of
external physicians, family physicians were asked to complete a standardised
follow-up questionnaire. In instances where family physicians lacked
comprehensive follow-up data, direct contact with individual patients or their
relatives was made via telephone. Documentation of the source of all adverse
events was systematically compiled, and adjudication of adverse events was
undertaken by two senior interventional cardiologists.
Study design
This study encompassed participants from all three
registries, with the aim of constructing a cardinality-matched cohort to
facilitate a comparative analysis of atrial fibrillation patients with a DOAC
score of ≥8 [16] who
were either treated with DOAC or underwent LAAO for primary or secondary stroke
prevention, in a 1:1 ratio. In a second analysis, patients with a history of
major bleeding either treated with LAAO or DOAC were cardinality-matched in a
1:2 ratio. Cardinality matching represents a refinement
of propensity score matching that prioritises both balance (minimising
differences in covariates between groups) and sample size (retaining the
largest possible subset of units that satisfy a predefined level of balance).
It explicitly sets constraints on differences in covariates, ensuring that
matched groups are highly comparable. Instead of sequentially matching pairs,
it solves an optimisation problem to find the best subset of treated and
control units. By ensuring good balance across multiple covariates, it helps
mitigate confounding and reduce selection bias, making comparisons more
reliable.
Endpoints
The study specified a primary combined endpoint
of stroke, cardiovascular death or major bleeding.
As secondary endpoints, the individual
components of the primary combined endpoint were assessed. Major bleeding was
defined according to the International Society of Thrombosis and Hemostasis
criteria as either a fatal bleeding, a bleeding in a critical area or organ (e.g.
intracranial haemorrhage of any origin) or a bleeding causing a fall of 2 g/dl in
haemoglobin levels within 7 days or leading to transfusion of two or more units
of whole blood or red blood cells [17]. The
supplementary material includes the rate of clinically relevant bleeding events
(major bleeding or clinically overt non-major bleeding that either led to
hospital admission, required medical or surgical intervention or a change in
antithrombotic therapy) in patients treated with DOAC or LAAO.
Ethics
This investigation adhered to the ethical principles laid
down in the Declaration of Helsinki. The study protocols for all three cohorts
received approval from and can be accessed at the pertinent local ethics
committees (Ethikkommission Nordwest- und
Zentralschweiz, PB_2016_00793, and Kantonale Ethikkommission Zuerich,
2022-01431) or can be provided by the authors upon request. In the Swiss-AF
and Beat-AF cohorts, explicit written informed consent was obtained from every
participant. Within the Zurich LAAO Registry, individuals retrospectively
included since 2016 granted general consent, acknowledging their willingness
for their data to be utilised in research. Notably, for patients enrolled in
this registry prior to 2016, the ethics committee (Kantonale
Ethikkommission Zuerich, 2022-01431) waived the requirement to obtain informed
consent and approved the approach of contacting either the patients or their
respective family physicians as part of the follow-up process.
Statistical analysis
The distribution of
continuous variables was assessed using density
plots. Continuous variables were indicated as
median with interquartile range (IQR) and were tested for differences with the
student’s t-test or the Mann-Whitney U test, according to their distribution.
Categorical variables were summarised as counts and percentages and analysed
using Pearson’s chi-squared test or Fisher’s exact test. Long-term outcomes
were assessed by constructing cumulative incidence curves. The
proportional-hazards assumptions were verified with the use of Schoenfeld
residuals. Considering the presence of competing
risks that could be related to different risk profiles qualifying for a change
in stroke prevention strategy in one group of patients, a Fine-Gray sub
distribution hazards model was employed for the primary and secondary endpoints
using the cmprsk package in R. A two-sided p-value <0.05 was
considered statistically significant. R version 4.2 (R Foundation, Vienna,
Austria) was used for the statistical analyses and the compilation of graphs.
To compare the various treatment
strategies, cardinality matching was employed using the MatchIt package
in R. The matching covariates for the main analysis examining patients with DOAC
score ≥8
as well as for the analysis of patients with prior major bleeding were selected
based on their clinical relevance and potential to confound the association of
interest and included age, sex, hypertension, diabetes mellitus, dyslipidaemia,
the presence of coronary artery disease (CAD), a history of heart failure, a history
of stroke or transient ischaemic attack (TIA) as well as the individual CHA2DS2-VASc
and DOAC scores. The balance of matching characteristics was assessed by
estimating standardised mean differences (SMD) between groups. Operationally,
the objective was to achieve a standardised mean difference of ≤0.20 to
eliminate imbalance in a given variable between the groups. All patients in the
included registries undergo annual follow-ups. If a patient was lost to
follow-up, the last follow-up response was used only if clinical data were
available for endpoint analyses.
Results
Between 2010 and 2017, a total of 3960
patients were included in Beat-AF and Swiss-AF, while between 2010 and 2023,
473 patients received successful LAAO at University Hospital Zurich.
Comparison of patients with direct oral
anticoagulation (DOAC) score ≥8
After cardinality matching, 478 atrial
fibrillation patients with DOAC score ≥8 were included in the primary analysis (figure
1);
239 atrial fibrillation patients treated with DOAC were compared with 239
atrial fibrillation patients who underwent successful LAAO. The median age of
patients was 79.4 (74.9 to 83.0) years and 64% were
male. After cardinality matching, baseline characteristics were comparable
between groups. The median CHA2DS2-VASc score was 5 (4 to
6) in both groups (SMD: 0.0) and the median DOAC score was 9 (8.5 to 10) in the
DOAC group and 10 (8 to 10) in the LAAO group (SMD: 0.12). LAAO patients had
better left ventricular (LV) function (55% [48 to 60] in the DOAC groups and 58%
[53 to 62] in the LAAO group; SMD: 0.424) and renal
function, as measured by a clinically irrelevant but statistically significant difference
in glomerular filtration rate (53.1 [39.1–60.5] ml/min in the DOAC group and
56.0 [40.0–71.5] ml/min in the LAAO
group; SMD: 0.247). There were more patients with previous major bleeding in
the LAAO group (15% in the DOAC group versus 75% in the LAAO group; SMD: 1.521).
Detailed baseline characteristics in the cohort of patients with a DOAC score ≥8 are
summarised
in table 1.
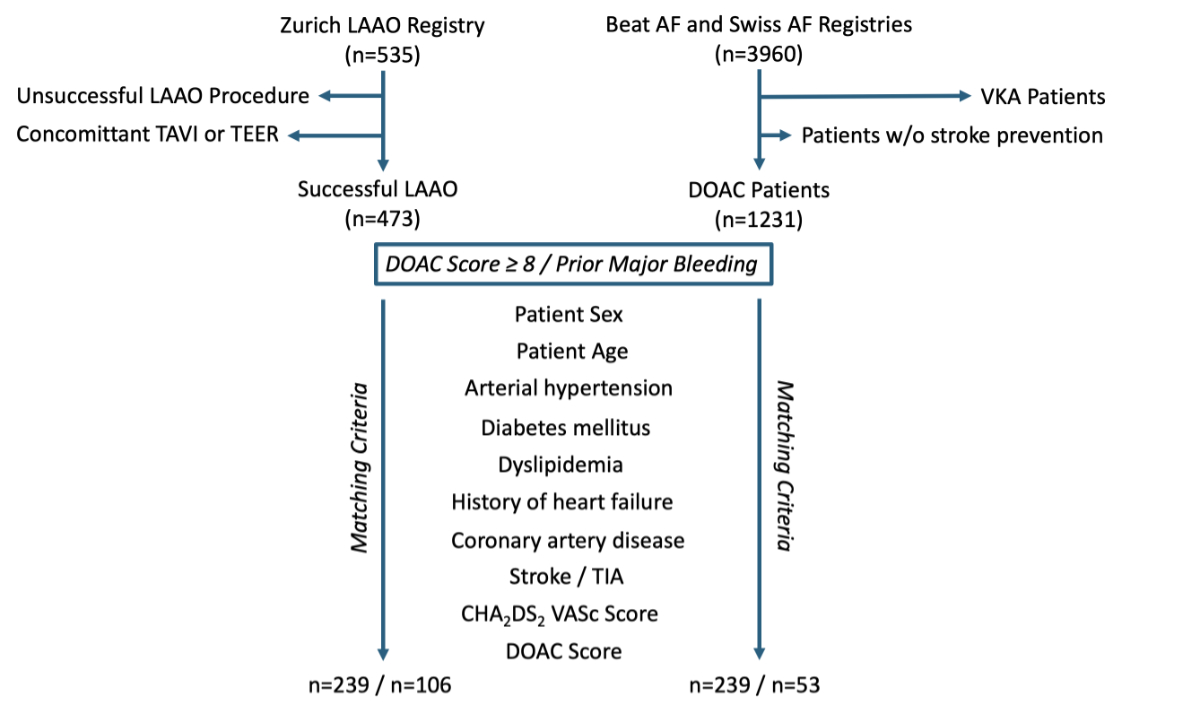
Figure 1Flowchart on patient matching of the
two groups (direct oral anticoagulation [DOAC]
score ≥8 and Prior Major Bleeding) using the MatchIt
package in R (R Foundation, Vienna, Austria). AF: atrial fibrillation; LAAO:
left atrial appendage occlusion; TAVI: transcatheter aortic valve implantation;
TEER: transcatheter edge-to-edge-repair; TIA: transient ischaemic attack; VKA:
vitamin K antagonist.
Table 1Baseline characteristics of
matched patients with a direct oral anticoagulation (DOAC) score of ≥8 who were
either treated with DOAC or with left atrial appendage occlusion (LAAO). Values
are reported in n (%) or median (IQR).
| Characteristic |
All (n = 478) |
DOAC (n = 239) |
LAAO (n = 239) |
SMD |
| Age (years) |
79.4 (74.9–83.0) |
79.3 (74.8–82.8) |
80.0 (75.5–83.0) |
0.093 |
| Male sex (%) |
307 (64.2) |
154 (64.4) |
153 (64.0) |
0.009 |
| BMI (kg/m²) |
26.3 (23.6–29.2) |
26.8 (23.7–29.7) |
26.1 (23.5–28.7) |
0.152 |
| Hypertension (%) |
401 (83.9) |
197 (82.4) |
204 (85.4) |
0.08 |
| Diabetes mellitus (%) |
135 (28.2) |
63 (26.4) |
72 (30.1) |
0.084 |
| Dyslipidaemia (%) |
245 (51.3) |
115 (48.1) |
130 (54.4) |
0.126 |
| Coronary artery disease (%) |
167 (34.9) |
78 (32.6) |
89 (37.2) |
0.097 |
| Previous myocardial infarction (%) |
85 (17.8) |
41 (17.2) |
44 (18.4) |
0.033 |
| Previous percutaneous coronary
intervention (%) |
115 (24.1) |
49 (20.5) |
66 (27.6) |
0.167 |
| Previous coronary artery bypass grafting
(%) |
44 (9.2) |
27 (11.3) |
17 (7.1) |
0.145 |
| Congestive heart failure (%) |
125 (26.2) |
62 (25.9) |
63 (26.4) |
0.01 |
| Previous stroke or transient ischaemic
attack (%) |
163 (34.1) |
79 (33.1) |
84 (35.1) |
0.044 |
| Previous systemic embolisation (%) |
23 (4.8) |
15 (6.3) |
8 (3.3) |
0.137 |
| Paroxysmal atrial fibrillation (%) |
259 (54.2) |
124 (51.9) |
135 (56.5) |
0.092 |
| Persistent
or permanent atrial fibrillation (%) |
219 (45.8) |
115 (48.1) |
104 (43.5) |
0.092 |
| Previous major bleeding (%) |
216 (45.2) |
36 (15.1) |
180 (75.3) |
1.521 |
| CHA2DS2 VASc score |
5 (4–6) |
5 (4–6) |
5 (4–6) |
0.0 |
| DOAC score |
10 (8–10) |
9 (8.5–10) |
10 (8–10) |
0.12 |
| HAS BLED score |
3 (2–4) |
2 (2–3) |
4 (3–4) |
1.8 |
| Creatinine (µmol/l) |
104 (85.0–129.0) |
108 (93.0–130.3) |
97 (78.0–125.0) |
0.332 |
| GFR (ml/min) |
54.7 (39.2–65.0) |
53.1 (39.1–60.5) |
56.0 (40.0–71.5) |
0.247 |
| LVEF (%) |
58.0 (51.3–62.0) |
55.0 (48.0–60.0) |
58.0 (53.0–62.0) |
0.424 |
| Left atrium size (mm) |
45.0 (40.0–50.0) |
44.5 (40.0–50.0) |
45.0 (40.0–51.0) |
0.064 |
Within the LAAO group, combined procedures
were performed in 27% of patients (LAAO and concomitant diagnostic angiography
in 22%, percutaneous coronary intervention [PCI] in 10%, patent foramen ovale [PFO]
closure in 5% or atrial septal defect [ASD] closure in <1%) (table S1). Atrial
fibrillation patients in the DOAC group were either started on DOAC or continued
their previously prescribed DOAC after study entry. The majority of patients (76%)
in the LAAO group received dual antiplatelet therapy for a median of 3 (1–6) months.
Lifelong single antiplatelet therapy with either aspirin or clopidogrel
monotherapy was chosen for 23% of patients after LAAO. Oral anticoagulation was
prescribed in 1% of LAAO patients for various reasons for a median of 4 (2 to 19)
months followed by single antiplatelet therapy lifelong.
Outcome of patients with a direct oral
anticoagulation (DOAC) score ≥8
After a median follow-up time of 4.9 (2.2
to 6.1) years for all patients (5.9 [4.1 to 6.3] years in the DOAC group and 2.9
[1.2 to 5.4] years in the LAAO group), there was no difference in the primary combined
endpoint of stroke, cardiovascular death or major
bleeding (118 events in the DOAC group and 88 events in the LAAO group; hazard
ratio [HR]: 0.88, 95% confidence interval [CI]: 0.67–1.14, p = 0.33; figure 2A)
between the matched cohorts of patients anticoagulated with a DOAC versus those
who underwent LAAO. While there was no significant difference in the occurrence
of stroke (20 in the DOAC group versus 11 in the LAAO group; HR: 0.74, 95% CI:
0.39–1.42, p = 0.36; figure 2B) or cardiovascular death (68 in the DOAC group
versus 56 in the LAAO group; HR: 0.97, 95% CI: 0.68–1.38, p = 0.85; figure 2C),
a significantly lower rate of major bleeding events (38 in the DOAC group
versus 21 in the LAAO group; HR: 0.55, 95% CI: 0.32–0.94, p = 0.029; figure 2D)
and a significantly lower rate of clinically relevant bleedings (79 in the DOAC
group versus 50 in the LAAO group; HR: 0.70, 95% CI: 0.50–0.99, p = 0.048;
supplementary material, figure S1) was demonstrated in the LAAO group.
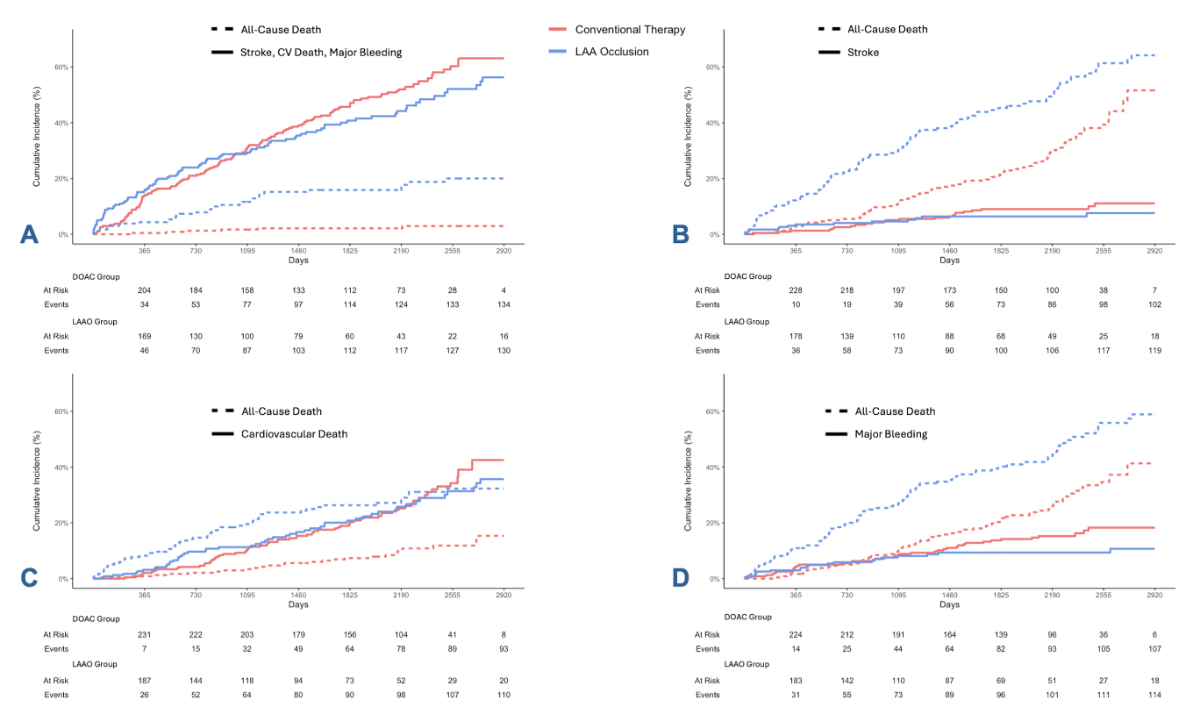
Figure 2Cumulative incidence curves on
long-term outcome of patients with a direct oral anticoagulation (DOAC) score≥8 treated
either with DOAC or with left atrial appendage occlusion (LAAO). While there
was no significant difference in the combined endpoint of stroke,
cardiovascular (CV) death and major bleeding ([A] HR:
0.88, 95% CI: 0.67–1.14, p = 0.33) and no significant
difference in the stroke rate ([B] HR: 0.74, 95% CI: 0.39–1.42,
p = 0.36) or in the rate of cardiovascular death ([C] HR: 0.97, 95% CI: 0.68–1.38,
p = 0.85),
patients with LAAO experienced significantly fewer major bleeding events during
follow-up ([D] HR: 0.55, 95% CI: 0.32–0.94, p = 0.029). LAA: left atrial appendage.
Comparison of patients with previous major
bleeding
Following the matching process using the
same matching criteria as outlined in the “Methods” section, 53 atrial
fibrillation patients with previous major bleeding managed with conventional
stroke prevention using DOAC were compared to 106 atrial fibrillation patients
with previous major bleeding who underwent successful LAAO. The median age of
patients was 74.0 (69.0 to 79.0) years and 63% were
male. Baseline characteristics including stroke risk and estimated bleeding
risk under DOAC were comparable in both groups. The median CHA2DS2-VASc
score was 4 (2 to 5) in the DOAC group and 4 (3 to 5) in the LAAO group (SMD:
0.177) and the median DOAC score was 9 (7 to 10) in both groups (SMD: 0.047). Persistent
or permanent atrial fibrillation was more commonly documented in patients in
the DOAC group (59% in the DOAC group versus 42% in the LAAO group; SMD: 0.364).
Although coronary artery disease was equally distributed among both groups (SMD:
0.102), as was previous myocardial infarction (SMD: 0.119), there were more
patients with previous percutaneous coronary intervention in the LAAO group (9%
vs 21%; SMD: 0.32) and more patients with prior coronary artery bypass grafting
(CABG) in the DOAC group (15% vs 3%; SMD: 0.44). LAAO patients had slightly
worse left ventricular function (60% [56 to 65] in the DOAC group and 58% [52
to 62] in the LAAO group; SMD: 0.392). Renal function was slightly better in
the LAAO group as measured by a glomerular filtration rate of 55.6 (44.7 to 64.6)
ml/min in the DOAC group versus 65.0 (45.0 to 83.3) ml/min in the LAAO group
(SMD: 0.419). Detailed baseline characteristics of patients with previous major
bleeding are summarised in table 2. Within the LAAO group, 22% of patients
underwent a combined procedure of LAAO and either concomitant diagnostic
angiography (19%), percutaneous coronary intervention (7%) or patent foramen
ovale closure (4%) (table S2).
Table 2Baseline characteristics of
matched patients with prior major bleeding who were either treated with direct
oral anticoagulation (DOAC) or with left atrial appendage occlusion (LAAO).
Values are reported in n (%) or median (IQR).
| Characteristic |
All (n = 159) |
DOAC (n = 53) |
LAAO (n = 106) |
SMD |
| Age (years) |
74.0 (69.0–79.0) |
75.0 (69.5–78.3) |
74.0 (69.0–79.0) |
0.038 |
| Male sex (%) |
100 (62.9) |
32 (60.4) |
68 (64.2) |
0.078 |
| BMI (kg/m²) |
26.5 (23.5–30.1) |
27.2 (23.9–30.1) |
26.4 (23.5–30.1) |
0.084 |
| Hypertension (%) |
126 (79.2) |
41 (77.4) |
85 (80.2) |
0.069 |
| Diabetes mellitus (%) |
40 (25.2) |
12 (22.6) |
28 (26.4) |
0.088 |
| Dyslipidaemia (%) |
78 (49.1) |
27 (51.0) |
52 (49.1) |
0.038 |
| Coronary artery disease (%) |
50 (31.4) |
15 (28.3) |
35 (33.0) |
0.102 |
| Previous myocardial infarction (%) |
17 (10.7) |
7 (13.2) |
10 (9.4) |
0.119 |
| Previous percutaneous coronary
intervention (%) |
27 (17.0) |
5 (9.4) |
22 (20.8) |
0.32 |
| Previous coronary artery bypass grafting
(%) |
11 (6.9) |
8 (15.1) |
3 (2.8) |
0.44 |
| Congestive heart failure (%) |
45 (28.3) |
14 (26.4) |
31 (29.2) |
0.063 |
| Previous stroke or transient ischaemic
attack (%) |
25 (15.7) |
7 (13.2) |
18 (17.0) |
0.106 |
| Previous systemic embolisation (%) |
5 (3.1) |
3 (5.7) |
2 (1.9) |
0.199 |
| Paroxysmal atrial fibrillation (%) |
85 (53.5) |
22 (41.5) |
63 (59.4) |
0.364 |
| Persistent
or permanent atrial fibrillation (%) |
74 (46.5) |
31 (58.5) |
43 (40.6) |
0.364 |
| Previous major bleeding (%) |
159 (100.0) |
53 (100.0) |
106 (100.0) |
NA |
| CHA2DS2 VASc score |
4.0 (3.0–5.0) |
4.0 (2.0–5.0) |
4.0 (3.0–5.0) |
0.177 |
| DOAC score |
9.0 (7.0–10.0) |
9.0 (7.0–10.0) |
9.0 (7.3–10.0) |
0.047 |
| HAS BLED score |
3.0 (3.0–4.0) |
3.0 (2.0–3.0) |
3.0 (3.0–4.0) |
0.9 |
| Creatinine (µmol/l) |
93 (78.0–119.0) |
104 (84.5–125.5) |
90.5 (75.3–115.0) |
0.37 |
| GFR (ml/min) |
60.8 (45.0–79.0) |
55.6 (44.7–64.6) |
65.0 (45.0–83.3) |
0.419 |
| LVEF (%) |
58.5 (51.8–62.0) |
60.0 (55.5–65.0) |
58.0 (52.0–62.0) |
0.392 |
| Left atrium size (mm) |
44.0 (40.0–49.0) |
43.0 (40.0–46.0) |
45.0 (40.5–49.5) |
0.353 |
Outcome of patients with previous major bleeding
When comparing the
matched cohorts of patients with a history of major bleeding, after a median follow-up
time of 4.4 (2.0 to 6.0) years for all
patients (5.9 [4.3 to 6.0] years in the DOAC group and 3.2 [1.5 to 6.0] years
in the LAAO group), there was no significant
difference regarding the primary composite endpoint of stroke, cardiovascular death
or major bleeding (31 events in 53 DOAC patients versus 29 events in 106 LAAO patients;
HR: 0.79, 95% CI: 0.50–1.27, p = 0.33; figure 3A). There were no significant
differences between the two groups in the occurrence of stroke (2 strokes in 53
DOAC patients and 6 strokes in 106 LAAO patients; HR: 1.09, 95% CI: 0.33–3.62,
p = 0.89; figure 3B) or cardiovascular death (17 cardiovascular deaths in 53
DOAC patients and 15 cardiovascular deaths in 106 LAAO patients; HR: 0.91, 95% CI:
0.50–1.64, p = 0.74; figure 3C). However, the LAAO group had a significantly
lower rate of major bleeding events (12 in 53 DOAC patients versus 8 in 106
LAAO patients; HR: 0.32, 95% CI: 0.13–0.79, p = 0.013; figure 3D) and a
significantly lower rate of clinically relevant bleedings (21 in 53 DOAC patients
versus 21 in 106 LAAO patients;
HR: 0.45, 95% CI: 0.24–0.83, p = 0.01; supplementary material, figure S2).
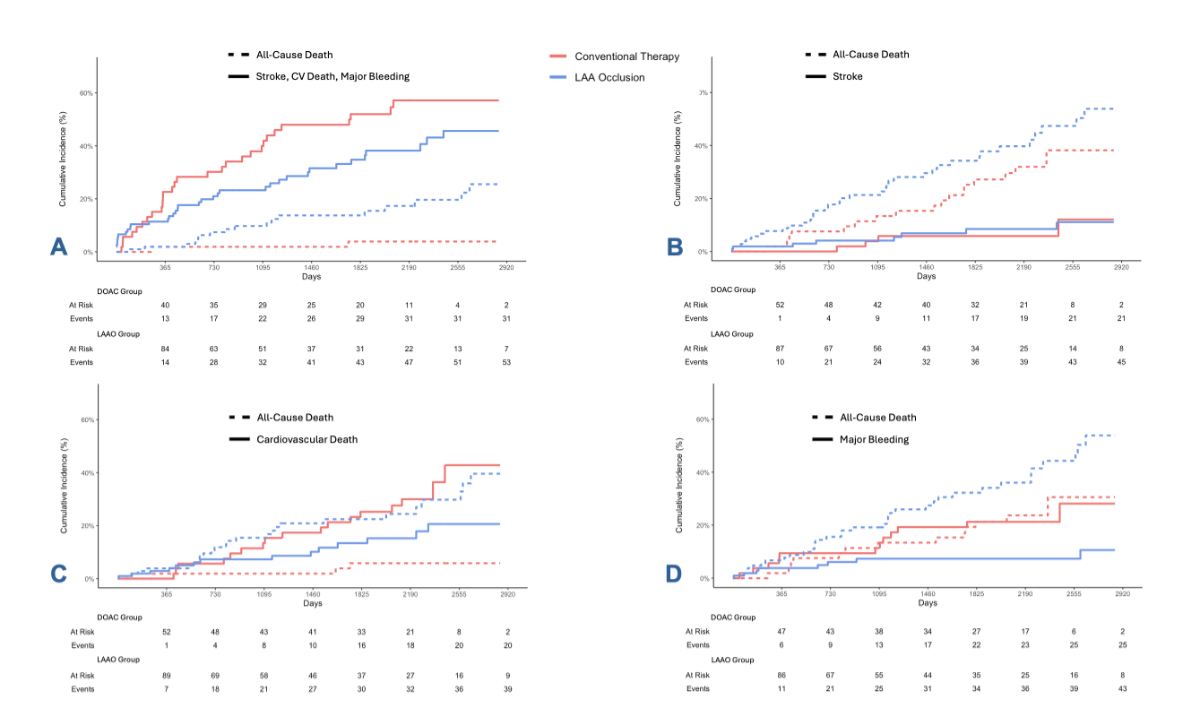
Figure 3Cumulative incidence curves on
long-term outcome of patients with previous major bleedingtreated either with direct
oral anticoagulation (DOAC)
or with left atrial appendage occlusion (LAAO). While there was no significant
difference in the combined endpoint of stroke, cardiovascular (CV) death and
major bleeding ([A] HR: 0.79, 95% CI: 0.50–1.27, p = 0.33) and no significant difference
in the stroke rate ([B] HR: 1.09, 95% CI: 0.33–3.62, p = 0.89) or
in the rate of cardiovascular death ([C] HR:
0.91, 95% CI: 0.50–1.64, p = 0.74), patients with LAAO
experienced significantly fewer major bleeding events during follow-up ([D] HR: 0.32,
95% CI: 0.13–0.79, p = 0.013).
LAA: left atrial appendage.
Discussion
This study demonstrates that atrial
fibrillation patients with an indication for stroke prevention and a high or
very high bleeding risk according to a direct oral anticoagulation (DOAC) score
of ≥8 or a
history of major bleeding have lower rates of major bleeding events and
similar rates of cardiovascular death and stroke at long-term follow-up when
treated with left atrial appendage occlusion (LAAO) as compared to a DOAC.
Current guidelines recommend to only treat
patients with LAAO if they have absolute contraindications to oral
anticoagulation [1, 18]. While the European
Society of Cardiology limits the indication for LAAO to patients with a history
of major bleeding events with an irreversible cause (ESC Class IIb
recommendation) [2], the American guidelines add
recurrent falls as another potential contraindication to oral anticoagulation
(ACC/AHA/ACCP/HRS Class IIa recommendation) [18].
Generally, the definition of contraindication to oral anticoagulation in the
literature remains blurry. The ASAP-TOO study required a shared decision by two physicians
that
a patient was deemed unsuitable for oral anticoagulation based on a history of
bleeding, blood dyscrasia and falls or other reasons to be defined as
contraindicated [12]. Similarly, consensus
papers written by LAAO experts but also by non-interventional cardiologists
recommend a more liberal indication for LAAO including atrial fibrillation
patients with recurrent bleeding events on oral anticoagulation, patients with
severely reduced renal function, patients with haemophilia, very frail patients
with an elevated risk of falls or a history of recurrent falls, and also taking
into consideration a patient’s wish to avoid oral anticoagulation [14, 19].
Bleeding risk scores have been established
to predict a patient’s risk of major bleeding events. Comparing them to the
risk of thromboembolism in atrial fibrillation patients is the challenge for
the treating physician balancing both risks and deciding on the optimal stroke
prevention strategy. As DOACs have replaced vitamin K antagonists during the
last ten years owing to their lower risk of major bleeding [20–23], the DOAC score
was recently established
for more accurate bleeding prediction in the current era [16]. A score of 8 or 9 is
assigned a high
bleeding risk (5–9.99% per year) and a maximum score of 10 a very high bleeding
risk (≥10% per
year). A history of
major bleeding on oral anticoagulation represents an important criterion in all
available bleeding risk scores and is the most common indication for LAAO in
current practice [16, 24].
As LAAO is already accepted as a valid
stroke prevention strategy, at least in patients with contraindications to oral
anticoagulation despite a lack of randomised data on that topic [1, 2], new randomised
controlled trials (RCTs)
involving such patients are hard to perform. After premature termination of
ASAP-TOO, it is unlikely that there will be an RCT enrolling patients with
contraindications to oral anticoagulation in the near future. The only published
RCT comparing LAAO to DOAC therapy showed that stroke prevention with LAAO resulted
in similar stroke rates
but significantly fewer bleeding complications after four years of follow-up [7].
While in the respective study by Osmancik
et al., patients with high bleeding risk or patients with prior clinically
relevant bleeding were included, only half of patients had a history of
previous bleeding requiring intervention or hospitalisation. The number of
patients with previous major bleeding according to the ISTH criteria is unknown in
that study but expected
to be low [17]. Furthermore, an important
part of the inclusion criteria of that study was the HAS BLED score, which
based its prognostic value for the estimation of major bleeding events on
patients treated with a vitamin K antagonist [25].
Therefore, the degree of (estimated) bleeding risk of patients included in the
study by Osmancik et al. remains somewhat speculative [7]. As matched comparisons
represent the second-highest
grade of evidence after randomised controlled data, the current study provides
important and reassuring evidence on stroke prevention using LAAO in atrial
fibrillation patients with high or very high bleeding risk. While stroke
reduction by LAAO was not significantly better than that by DOACs in both
cohorts (DOAC score ≥8 and history of major bleeds), there was a numerically smaller
stroke rate after LAAO by almost 50% and 30%, respectively. Hence, closing the left
atrial appendage for stroke prevention in atrial fibrillation may not be a must
compared to DOACs but it certainly looks attractive and should be elevated at
least to the level of DOACs in the guidelines, because of the significantly
reduced bleeding risk.
Previous propensity score-matched studies
by Gloekler et al., Nielsen-Kudsk et al., Elsheikh et al. and our group also
showed favourable results of LAAO in comparison to oral anticoagulation [9, 10, 26,
27]. However, both vitamin K
antagonists and DOAC were used in the control group of the study by Gloekler et
al. and all four studies focused primarily on patients with high stroke risk
but not specifically on patients at highest risk of bleeding [9, 10, 26, 27]. Our
first comparison between atrial
fibrillation patients treated either conventionally or with LAAO also included
patients from the Zurich LAAO Registry as well as from the Beat-AF and Swiss-AF
cohort studies [27]. Only 50% of patients
in the control group received DOAC therapy, 42% were treated with a vitamin K
antagonist and 8% did not receive any stroke prevention [27]. While the first paper
focused on secondary stroke prevention
and a patient population with highest stroke risk in general, the present paper focused
on a population with highest bleeding risk
requiring different matching criteria and only patients treated with DOACs, the
current standard of oral anticoagulation for most patients, were included in the
control group for analysis.
Besides the obvious benefits of LAAO
compared to oral anticoagulation in atrial fibrillation-related stroke
prevention representing a one-time procedure obviating the risk associated with
medication malcompliance, critical factors contributing to the relatively
limited adoption of LAAO are its potential periprocedural risks, device-related
complications and the challenge associated with antithrombotic therapy
post-LAAO [28]. The optimal regimen for
antiaggregation, the duration and individualised protocols have not been well established,
leading to uncertainty and hesitancy among clinicians. This underscores the
need for further research in this area to define recommendations for post-LAAO antithrombotic
therapy. Based on the curves in figures 2D and 3D, the present study did not
show any significant rise in bleeding events during the first three months
following LAAO, the time when the vast majority of LAAO patients was on dual
antiplatelet therapy. This adds to the encouraging literature about dual
antiplatelet therapy being safe in patients with previous bleeding events under
oral anticoagulation [29]. Alternative
antithrombotic medication protocols like half-dose DOAC have been tested with
promising results [30]. Single
antiplatelet therapy following LAAO has been used in a few cases in our
registry and also worldwide. Data on the routine implementation of single
antiplatelet therapy, however, are lacking, although from a pathophysiological
perspective single antiplatelet therapy could have its justification and could
potentially minimise bleeding rates even more. An RCT comparing the different
protocols will be needed to clarify the optimal antithrombotic strategy post-LAAO.
Medication-based alternatives to DOAC and
LAAO for atrial fibrillation patients with elevated bleeding risk, namely
factor XI inhibitors, are being studied but despite their promising theoretical
pharmacological effects, the OCEANIC-AF study (NCT05643573), the first RCT
comparing this novel anticoagulation agent to DOAC, was prematurely terminated
due to inferior efficacy with regards to thromboembolic protection [31].
Limitations and strengths
This is a non-randomised comparison.
Despite matching, there is residual confounding probably due to a selection
bias, reflected by the significantly higher all-cause mortality rates in the
LAAO group compared to the DOAC group (represented by the dotted lines in figures
2 and 3). This shows that patients currently referred for LAAO may represent an
extremely high-risk group, often due to comorbidities that also increase their
risk of bleeding events which could be supported by the observation of many
more cancer-related deaths and more deaths from infection or sepsis in the LAAO
group (list of non-cardiovascular mortality causes in supplementary material).
Risk scores like the CHA2DS2 VASc and the DOAC scores are
imperfect matching parameters [16, 32].
Although they help in estimating the likelihood of a certain event, they do not
represent measurable characteristics. To compensate for this, a large number of
measurable baseline characteristics was chosen for the matching process.
However, despite adequate matching, the real bleeding risk, at least in the
analysis of patients with a high DOAC score, is likely to be higher in the LAAO
group as it included many more patients with a history of major bleeding.
Although outdated in the current DOAC era and therefore not a matching
criterion, the HAS BLED score, a more traditional risk score estimating the
risk of major bleeding events in atrial fibrillation patients when treated with
a vitamin K antagonist, is significantly higher in both LAAO groups [25]. This, however,
highlights the potential
of LAAO in such high-risk populations as the bleeding rates at follow-up are still
significantly lower among the patients treated with LAAO in both analyses.
Although a success rate of LAAO of around 98% is reported in the current
literature [33], which corresponds to
results in the Zurich LAAO Registry [15],
only successful LAAO procedures were included in the current study which
represents another limitation.
Strengths of the study include the
observational design allowing a broader and more inclusive patient population,
thus offering valuable insights into the real-world utilisation of LAAO, and
the long-term follow-up. Nevertheless, based on the nature of this study it
needs to be highlighted that retrospective studies can only provide
hypothesis-generating results and are not intended to provide definitive
evidence.
Conclusion
In patients with atrial fibrillation and a
high bleeding risk and in patients with a history of major bleeding,
percutaneous LAAO may provide similar stroke prevention and a reduced risk of
bleeding on long-term follow-up compared to DOAC therapy. Acknowledging the still-lacking
RCTs to confirm these hypotheses-generating
data, to LAAO as
first-line stroke protection in patients with atrial fibrillation, at least in
patients with a life expectancy of 5 years or more.
Data sharing statement
As this study contains large raw patient data of three
different cohorts including multiple parameters not relevant to this manuscript,
the authors were granted access to the requested information necessary for the
production of the current analysis from the responsible study board. Access to
the deidentified patient data from all three cohorts used for matching can be
granted by the corresponding author upon request.
Dr med. univ. Thomas Gilhofer
Department of Cardiology
Cantonal
Hospital Winterthur
Brauerstrasse 15
CH-8400 Winterthur
thomas.gilhofer[at]ksw.ch
References
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al.;
ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management
of atrial fibrillation developed in collaboration with the European Association for
Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of
atrial fibrillation of the European Society of Cardiology (ESC) Developed with the
special contribution of the European Heart Rhythm Association (EHRA) of the ESC [Erratum
in: Eur Heart J. 2021 Oct 21;42] [40] [:4194. doi: 10.1093/eurheartj/ehab648. PMID:
32860505]. Eur Heart J. 2021 Feb;42(5):373–498. 10.1093/eurheartj/ehaa945 10.1093/eurheartj/ehaa612
2. Van Gelder IC, Rienstra M, Bunting KV, Casado-Arroyo R, Caso V, Crijns HJ, et al.;
ESC Scientific Document Group. 2024 ESC Guidelines for the management of atrial fibrillation
developed in collaboration with the European Association for Cardio-Thoracic Surgery
(EACTS) [Erratum in: Eur Heart J. 2025 Jul 07:ehaf306. doi: 10.1093/eurheartj/ehaf306.
PMID: 39210723]. Eur Heart J. 2024 Sep;45(36):3314–414. 10.1093/eurheartj/ehae176 10.1093/eurheartj/ehae176
3. Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke
in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999 Oct;131(7):492–501.
10.7326/0003-4819-131-7-199910050-00003
4. Holmes DR Jr, Doshi SK, Kar S, Price MJ, Sanchez JM, Sievert H, et al. Left Atrial
Appendage Closure as an Alternative to Warfarin for Stroke Prevention in Atrial Fibrillation:
A Patient-Level Meta-Analysis. J Am Coll Cardiol. 2015 Jun;65(24):2614–23. 10.1016/j.jacc.2015.04.025
5. Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P, et al.; PROTECT
AF Steering Committee and Investigators. Percutaneous left atrial appendage closure
vs warfarin for atrial fibrillation: a randomized clinical trial [Erratum in: JAMA.
2015 Mar 10;313] [10] [:1061. PMID: 25399274]. JAMA. 2014 Nov;312(19):1988–98. 10.1001/jama.2014.15192
6. Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective
randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients
with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial [Erratum
in: J Am Coll Cardiol. 2014 Sep 16;64] [11] [:1186. PMID: 24998121]. J Am Coll Cardiol.
2014 Jul;64(1):1–12. 10.1016/j.jacc.2014.04.029
7. Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, et al.; PRAGUE-17 Trial
Investigators. 4-Year Outcomes After Left Atrial Appendage Closure Versus Nonwarfarin
Oral Anticoagulation for Atrial Fibrillation. J Am Coll Cardiol. 2022 Jan;79(1):1–14.
10.1016/j.jacc.2021.10.023
8. Wazni OM, Saliba WI, Nair DG, Marijon E, Schmidt B, Hounshell T, et al.; OPTION Trial
Investigators. Left Atrial Appendage Closure after Ablation for Atrial Fibrillation.
N Engl J Med. 2025 Apr;392(13):1277–87. 10.1056/NEJMoa2408308
9. Nielsen-Kudsk JE, Korsholm K, Damgaard D, Valentin JB, Diener HC, Camm AJ, et al. Clinical
Outcomes Associated With Left Atrial Appendage Occlusion Versus Direct Oral Anticoagulation
in Atrial Fibrillation. JACC Cardiovasc Interv. 2021 Jan;14(1):69–78. 10.1016/j.jcin.2020.09.051
10. Gloekler S, Fürholz M, de Marchi S, Kleinecke C, Streit SR, Buffle E, et al. Left
atrial appendage closure versus medical therapy in patients with atrial fibrillation:
the APPLY study. EuroIntervention. 2020 Oct;16(9):e767–74. 10.4244/EIJ-D-20-00201
11. Nietlispach F, Moarof I, Taramasso M, Maisano F, Meier B. Left atrial appendage occlusion.
EuroIntervention. 2017;13(AA):AA78-AA84. doi: 10.4244/EIJ-D-17-00412. PMID: 28942389.
12. Holmes DR, Reddy VY, Buchbinder M, Stein K, Elletson M, Bergmann MW, et al. The Assessment
of the Watchman Device in Patients Unsuitable for Oral Anticoagulation (ASAP-TOO)
trial. Am Heart J. 2017 Jul;189:68–74. 10.1016/j.ahj.2017.03.007
13. Conen D, Rodondi N, Mueller A, Beer J, Auricchio A, Ammann P, et al. Design of the
Swiss Atrial Fibrillation Cohort Study (Swiss-AF): structural brain damage and cognitive
decline among patients with atrial fibrillation. Swiss Med Wkly. 2017 Jul;147(2728):w14467.
10.4414/smw.2017.14467
14. Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GY, et al.; ESC Scientific
Document Group. EHRA/EAPCI expert consensus statement on catheter-based left atrial
appendage occlusion - an update. Europace. 2020 Feb;22(2):184. 10.1093/europace/euz258
15. Gilhofer TS, Schweiger V, Gehler M, Bokemeyer V, Chen M, Candreva A, et al. Long-term
outcomes after echocardiography versus fluoroscopy-guided left atrial appendage closure:
is there still a role for a simplified approach? Catheter Cardiovasc Interv. 2024 Aug;104(2):343–55.
10.1002/ccd.31126
16. Aggarwal R, Ruff CT, Virdone S, Perreault S, Kakkar AK, Palazzolo MG, et al. Development
and Validation of the DOAC Score: A Novel Bleeding Risk Prediction Tool for Patients
With Atrial Fibrillation on Direct-Acting Oral Anticoagulants. Circulation. 2023 Sep;148(12):936–46.
10.1161/CIRCULATIONAHA.123.064556
17. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific
and Standardization Committee of the International Society on Thrombosis and Haemostasis.
Definition of major bleeding in clinical investigations of antihemostatic medicinal
products in non-surgical patients. J Thromb Haemost. 2005 Apr;3(4):692–4. 10.1111/j.1538-7836.2005.01204.x
18. Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, et al.; Peer
Review Committee Members. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management
of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart
Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024 Jan;149(1):e1–156.
10.1161/CIR.0000000000001263 doi: https://doi.org/10.1161/CIR.0000000000001193
19. Potpara T, Grygier M, Häusler KG, Nielsen-Kudsk JE, Berti S, Genovesi S, et al. Practical
guide on left atrial appendage closure for the non-implanting physician: an international
consensus paper. Europace. 2024 Mar;26(4):euae035. 10.1093/europace/euae142 doi: https://doi.org/10.1093/europace/euae035
20. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al.; RE-LY
Steering Committee and Investigators. Dabigatran versus warfarin in patients with
atrial fibrillation. N Engl J Med. 2009 Sep;361(12):1139–51. doi: https://doi.org/10.1056/NEJMoa0905561
21. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al.; AVERROES
Steering Committee and Investigators. Apixaban in patients with atrial fibrillation [Erratum
in: N Engl J Med. 2010 Nov 4;363] [19] [:1877. PMID: 19717844]. N Engl J Med. 2011 Mar;364(9):806–17.
10.1056/NEJMoa0905561 doi: https://doi.org/10.1056/NEJMoa1007432
22. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al.; ARISTOTLE
Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation.
N Engl J Med. 2011 Sep;365(11):981–92. 10.1056/NEJMoa1107039
23. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al.; ROCKET AF Investigators.
Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011 Sep;365(10):883–91.
10.1056/NEJMoa1009638
24. Chang G, Xie Q, Ma L, Hu K, Zhang Z, Mu G, et al. Accuracy of HAS-BLED and other bleeding
risk assessment tools in predicting major bleeding events in atrial fibrillation:
A network meta-analysis. J Thromb Haemost. 2020 Apr;18(4):791–801. 10.1111/jth.14692
25. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly
score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation:
the Euro Heart Survey. Chest. 2010 Nov;138(5):1093–100. 10.1378/chest.10-0134
26. Elsheikh S, Alobaida M, Bucci T, Buckley BJ, Gupta D, Irving G, et al. Left Atrial
Appendage Occlusion versus Direct Oral Anticoagulants in the Prevention of Ischaemic
Stroke in Patients with Atrial Fibrillation. Cerebrovasc Dis. 2025;54(1):81–88. 10.1159/000536546
27. Gilhofer T, Bokemeyer V, Schweiger V, Gehler M, Michel J, Chen M, et al. Long-Term
Outcome of Patients with Atrial Fibrillation and High Risk of Stroke Treated with
Oral Anticoagulation or Left Atrial Appendage Occlusion: A Cardinality Matched Analysis.
Cardiology. 2024 Oct:1–15. 10.1159/000541907; Epub ahead of print.
28. Kramer A, Patti G, Nielsen-Kudsk JE, Berti S, Korsholm K. Left Atrial Appendage Occlusion
and Post-procedural Antithrombotic Management. J Clin Med. 2024 Jan;13(3):803. 10.3390/jcm13030803
29. Gilhofer TS, Nestelberger T, Kang M, Inohara T, Alfadhel M, McAlister C, et al. Stroke
Prevention With Left Atrial Appendage Closure in Patients With Atrial Fibrillation
and Prior Intracranial Hemorrhage. CJC Open. 2023 Mar;5(6):404–11. 10.1016/j.cjco.2023.03.004
30. Della Rocca DG, Magnocavallo M, Di Biase L, Mohanty S, Trivedi C, Tarantino N, et
al. Half-Dose Direct Oral Anticoagulation Versus Standard Antithrombotic Therapy After
Left Atrial Appendage Occlusion. JACC Cardiovasc Interv. 2021 Nov;14(21):2353–64.
10.1016/j.jcin.2021.07.031
31. Piccini JP, Patel MR, Steffel J, Ferdinand K, Van Gelder IC, Russo AM, et al.; OCEANIC-AF
Steering Committee and Investigators. Asundexian versus Apixaban in Patients with
Atrial Fibrillation. N Engl J Med. 2025 Jan;392(1):23–32. 10.1056/NEJMoa2407105
32. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification
for predicting stroke and thromboembolism in atrial fibrillation using a novel risk
factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010 Feb;137(2):263–72.
10.1378/chest.09-1584
33. Landmesser U, Skurk C, Tzikas A, Falk V, Reddy VY, Windecker S. Left atrial appendage
closure for stroke prevention in atrial fibrillation: current status and perspectives.
Eur Heart J. 2024 Aug;45(32):2914–32. 10.1093/eurheartj/ehae398
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.4288.


