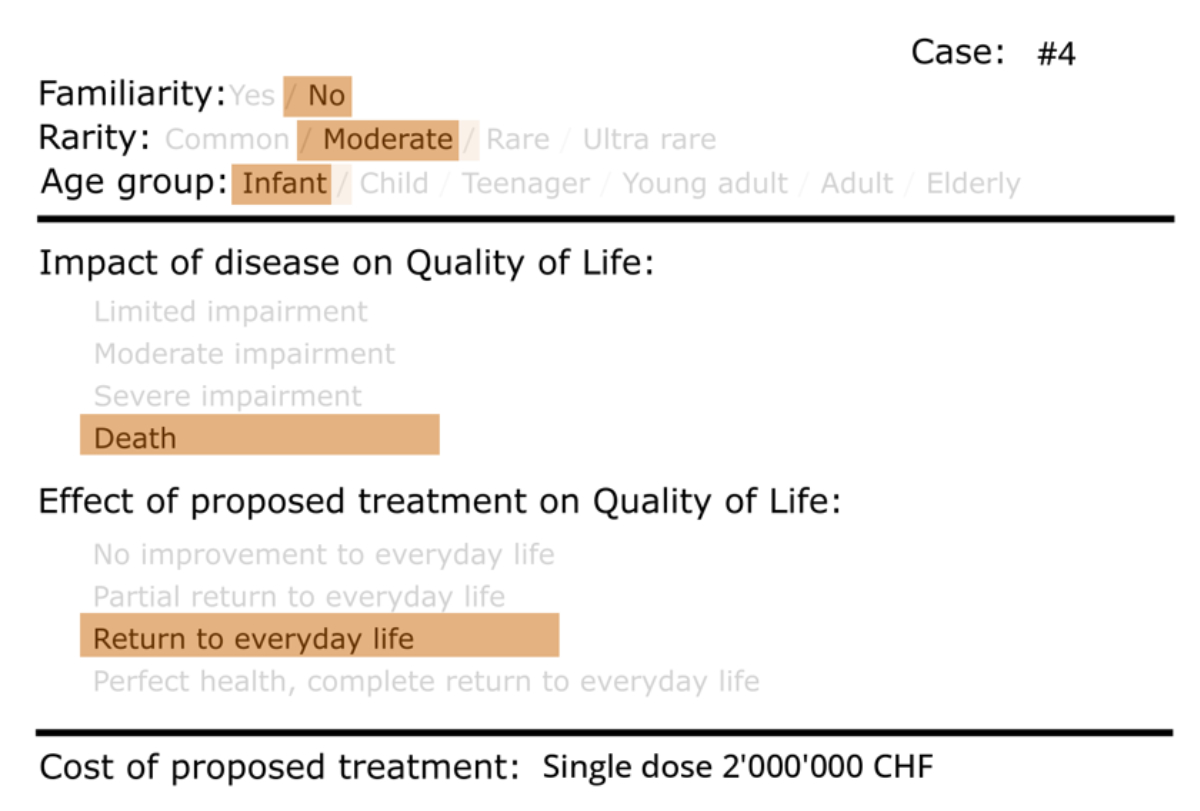
Figure 1Scenario card sample. CHF: Swiss francs.
DOI: https://doi.org/https://doi.org/10.57187/s.4243
Rare diseases, while affecting only 5–8% of the European population [1], are linked with disproportionately high costs for diagnosis, treatment and management [2]. Their individually low prevalence and substantial economic burden raise ethical, economic and social questions that demand careful consideration within healthcare systems.
The low incidence within the general population complicates the allocation of limited resources, as the principle of distributive justice seeks to ensure equitable access to healthcare services and treatments for all individuals [3]. The allocation of resources for rare diseases is determined by the interplay between medical necessity, ethics and economic sustainability [3]. The ethical dimension of this challenge lies in the responsibility to offer treatment options, whenever available, to individuals regardless of the rarity of their disease [4]. Thus, healthcare systems face the challenge of balancing the moral obligation to provide care with fiscal constraints that shape resource allocation decisions.
Economically, the high costs associated with the treatment of rare diseases can strain healthcare budgets [5], and some may argue that this could lead to diversion of resources from more prevalent conditions that may affect a larger portion of the population. This prompts policymakers to confront complex questions of prioritisation [6].
The social aspect of rare disease resource allocation also involves considerations beyond immediate medical treatment. It encompasses the broader impact on patients’ quality of life, their families and society at large [7]. The burden of rare diseases extends beyond medical costs, with emotional, psychological and social ramifications [8]. These relevant aspects add an additional layer of complexity to the resource allocation equation.
Within this context, understanding how the public comprehends these intricate dynamics and their implications for resource allocation becomes pivotal in shaping healthcare policies within a democratic society [9]. Public understanding and opinion regarding which costs should be allocated for specific treatments hold significant sway over healthcare decision-making processes [10]. While medical experts and policymakers contribute invaluable technical insights, the involvement of the public is essential due to several critical reasons. Public opinion generally reflects the collective values and ethical considerations of a society. Healthcare resource allocation decisions often require making choices that align with societal norms and preferences [11, 12]. As rare diseases present ethical and moral dilemmas, involving the public allows for a broader dialogue that encompasses diverse viewpoints, fostering a sense of ownership and legitimacy in the decision-making process [13].
That said, it is important to distinguish between descriptive ethics, which concerns what people believe to be right, and classic prescriptive or normative ethics, which addresses what is actually right [14, 15]. Understanding public opinion does not necessarily dictate the correct course of action, as it is not per se a source of moral norms, but remains nonetheless a crucial element to consider. The understanding and management of rare diseases, often challenging to comprehend fully due to their rarity and complexity, benefit from diverse perspectives. In return, understanding public perception of resource allocation for rare diseases may help policymakers and communicators to identify information voids and misunderstanding about rare diseases, the needs of patients and the societal costs, in terms of physical, psychological and financial costs.
Switzerland’s distinctive healthcare landscape, characterised by mandatory health insurance coverage, offers an intriguing case study for probing the dynamics of resource allocation for rare diseases. Switzerland lacks a predefined budget cap for funding high-cost treatments. Despite this, the Swiss Supreme Court’s ruling that insurers do not need to cover treatments exceeding exorbitant costs has raised ethical and financial questions about the necessity to regulate such expenditures, how and in which contexts [16].
Outside of Switzerland, international studies have offered valuable insights into the factors that shape public preferences and resource allocation decisions in the context of rare diseases, revealing that as the costs associated with rare disease treatments escalate, individuals tend to prioritise funding for diseases with a higher prevalence [17]. One study from Sweden – a country with a free national healthcare system, funded by the government with taxes [18] – indicated that there is no inclination towards prioritising patients with rare diseases over those suffering with common diseases [19]. Instead, preferences for prioritising patients with common diseases over those with rare diseases were more frequently displayed, with the propensity for such preferences intensifying when the cost of treating the rare disease was higher [19]. In another study, a substantial portion of respondents demonstrated strong support for treating patients with rare diseases even if the treatments were more expensive [20]. However, once faced with decision-making, in scenarios where the rare disease was four times more expensive to treat than a common disease, the proportion of respondents favouring treatment for the rare disease declined, with a notable increase in respondents favouring the treatment of common diseases [20].
This paper presents an exploratory study designed to elucidate the intricate interplay of factors that influence the willingness of Swiss citizens to allocate resources for the treatment of rare diseases, exploring variables that include disease rarity, treatment costs, patient age, disease severity, treatment benefits and the impact on quality of life. By analysing these elements, the study aims to provide an initial understanding of the trade-offs that guide resource allocation decisions in rare disease treatment.
This study was conducted within the framework of the University Research Priority programme “Innovative Therapies in Rare Diseases of the University of Zurich” (ITINERARE). Recognising the limitations of traditional survey-based research due to framing effects [21], the study drew inspiration from Bourke et al.’s approach [17]. Our exploratory study employed visual discrete-choice surveys. By presenting scenario cards depicting various medical situations, participants were prompted to evaluate their willingness to allocate financial resources to treat patients in varying contexts.
A representative sample of Swiss citizens, spanning the three language regions of Switzerland (German, French and Italian), was recruited for participation; recruitment was coordinated by Demoscope, a reputable polling and research company based in Switzerland. We aimed to obtain a sample size of about n = 450 participants for the study. Participants were selected from Demoscope’s online panel, which comprises web-active Swiss residents. Swiss residents able to provide informed consent and aged 18–80 were eligible. The sample was stratified by language region, age and sex, and invitations were sent accordingly to achieve representativeness across Switzerland’s language regions. Respondents were invited via email.
The survey was built using PubliCo, an online data collection tool with features allowing participatory science, developed by the Institute of Biomedical Ethics and History of Medicine, University of Zurich [13]. Participants provided demographic information including nationality, canton of residency, age, sex, education level, living arrangements, health status, personal experiences with rare diseases and profession.
The core of the study involved presenting participants with a series of vignettes representing different scenarios. Each participant was presented with 10 randomly selected scenarios from a pool of 157 scenarios, each depicting a unique situation, including various combinations of factors influencing resource allocation decisions for rare disease treatments: disease rarity, patient age, familiarity of the disease within the family (defined as the occurrence of the same disease within multiple members of the same family), impact of disease on quality of life and survival, effect of proposed treatment and treatment costs. The categories of the factors are summarised in table 1. The randomised selection of scenario cards ensured an equal presentation of each case to minimise bias.
Table 1Categories for each factor depicted in the scenario cards.
| Factor | Levels |
| Rarity | Common: >1:100 |
| Moderate: >1:50,000 | |
| Rare: <1:50,000 | |
| Ultra-rare: <1:1,000,000 | |
| Age group | Infant: 0–3 y/o |
| Child: 4–12 y/o | |
| Teenager: 13–19 y/o | |
| Young adult: 20–35 y/o | |
| Adult: 36–65 y/o | |
| Elderly: 66+ y/o | |
| Familiarity | Yes |
| No | |
| Impact of disease | Premature death |
| Severe impairment to everyday life | |
| Moderate impairment to everyday life | |
| Slight impairment to everyday life | |
| Effect of proposed treatment | No improvement to everyday life |
| Partial return to everyday life | |
| Return to everyday life | |
| Perfect health, complete return to everyday life | |
| Cost of the treatment | Annual cost: 450 CHF; 500 CHF; 1000 CHF; 1500 CHF; 2000 CHF; 2500 CHF; 3000 CHF; 7000 CHF; 15,000 CHF; 20,000 CHF; 25,000 CHF; 30,000 CHF; 52,500 CHF; 85,000 CHF; 350,000 CHF; 400,000 CHF; 900,000 CHF; 1,000,000 CHF. |
| Single dose: 450,000 CHF; 1,000,000 CHF; 2,000,000 CHF; 2,500,000 CHF. | |
| Single intervention: 5000 CHF; 18,000 CHF; 20,000 CHF; 50,000 CHF; 135,000 CHF; 200,000 CHF. |
Participants evaluated each scenario and determined whether they agreed with allocating financial resources for the treatment of the depicted case. Each card represented one real-world disease for a hypothetical patient, based on a combination of factors (figure 1). We created a total of 157 scenarios; an Excel file with the full list of scenarios is available for download at https://doi.org/10.57187/s.4243. We created the images of the cards programmatically in Python, using the library PIL. Various real-life scenarios associated with real diseases were considered when drafting the cards, including spinal muscular atrophy, cystic fibrosis, biotinidase deficiency, Pompe disease, Gaucher disease type 1, Hunter disease, severe combined immunodeficiency (SCID), Fabry disease, chronic granulomatous disease (CGD), complete progranulin deficiency Neuronal Ceroid Lipofuscinosis (NCL11), partial progranulin deficiency Frontotemporal Dementia (FTD), ataxia telangiectasia (AT), severe congenital neutropenia (SCN), common variable immunodeficiency (CVID), Gitelman syndrome, autosomal-dominant polycystic kidney disease, atypical haemolytic uraemic syndrome, primary hyperoxaluria, Alport syndrome, cystinosis, diabetes mellitus type 1, hypertension, coronary artery disease, asthma, osteoarthritis, migraine, depression, anxiety disorders, inflammatory bowel disease, chronic obstructive pulmonary disease (COPD), osteoporosis, psoriasis, cataract, hearing loss, obesity and respiratory distress syndrome (RDS). Of note, specific disease names were not shown to participants. Rather, each disease was mapped to one or more scenario cards only for analysis purposes.

Figure 1Scenario card sample. CHF: Swiss francs.
The study was conducted online on the PubliCo platform and was available in Switzerland’s national languages – German, French and Italian – and in English. All materials were translated and adapted for linguistic clarity.
Data cleaning and parsing were performed in Python; the Jupyter notebook with the code is available as supplementary material. Data visualisations were performed in Python and GraphPad Prism. Descriptive statistics were used to summarise participant characteristics and overall response patterns: for each of the 157 unique vignettes, the proportion of participants recommending treatment funding (probability of positive decision to treat) was calculated. Probability of positive decision to treat was then analysed across different attribute levels – such as disease rarity, patient age, treatment cost and outcome. No formal statistical analysis was performed for this exploratory phase. Differences across conditions were interpreted descriptively to identify emerging patterns of preference, which require formal significance testing and validation in future research. All raw data are openly accessible and can be downloaded from our Open Science Framework (OSF) repository [22].
The survey was distributed to a representative sample of Swiss residents with the assistance of the polling company Demoscope. We collected 417 responses. After filtering out responses from individuals who did not consent to the use of their data and those who completed the survey in under three minutes, we obtained 375 responses. Most respondents were German-speaking, followed by French and Italian speakers, with a small number of English speakers (figure 2A). The majority of respondents were Swiss nationals (figure 2B), the sex ratio was balanced (figure 2C) and the age distribution reflected the Swiss demographic profile (figure 2D).
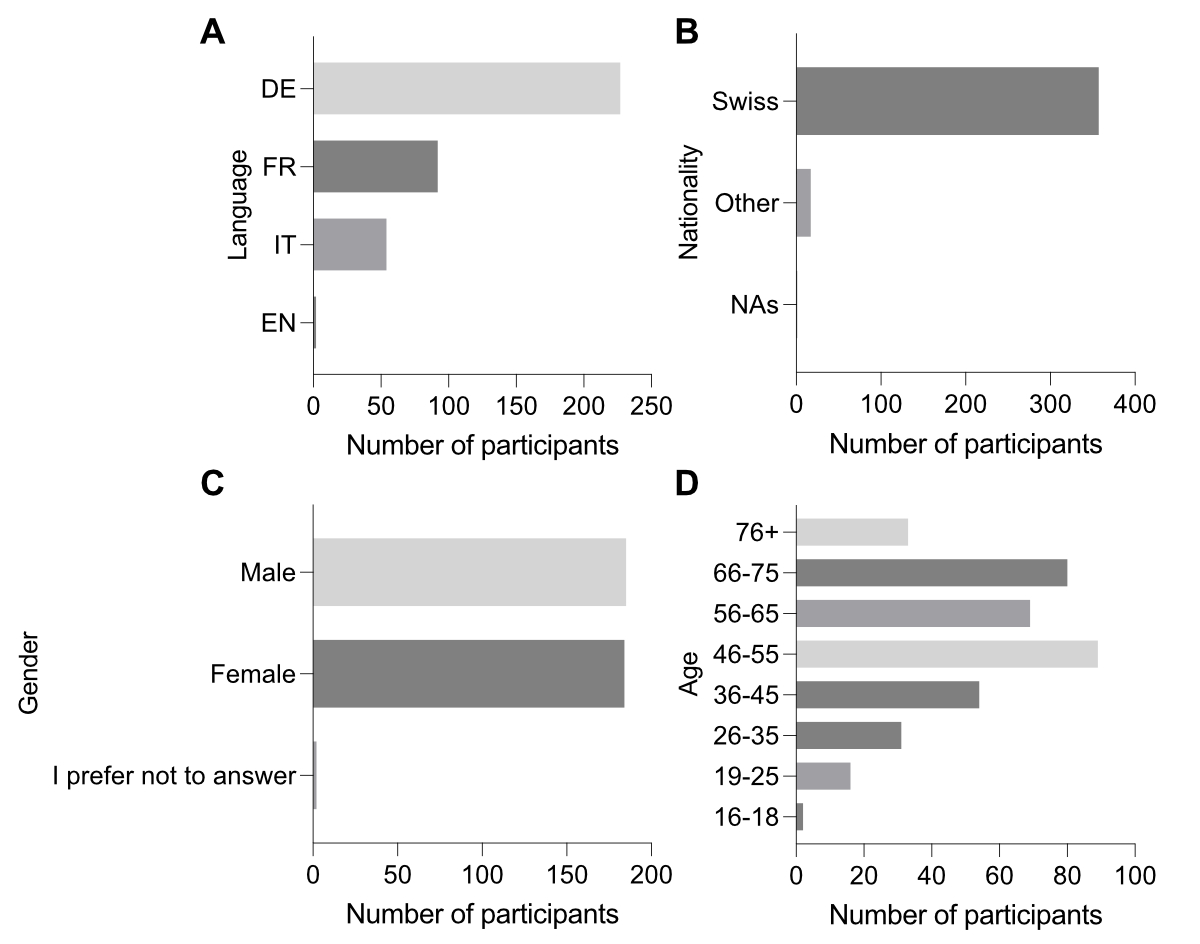
Figure 2Survey demographics. Distribution of respondents by language (A), nationality (B), sex (C) and age (D). Missing values (NAs) reflect instances where respondents chose not to answer.
We also asked our respondents if they are affected by a rare disease. The ratio of affected to non-affected respondents aligns with the statistic that 6–8% of the Swiss population is affected by a rare disease (figure S1A in the appendix) [23]. Additionally, some respondents reported having family members affected by a rare disease (figure S1C). We recorded the self-reported health status of our respondents, with most indicating that they are in good or very good health (figure S1B). Finally, we asked whether they are healthcare professionals and, as expected, most respondents answered no (figure S1D).
Most of our survey participants reside in the canton of Ticino, reflecting the high concentration of Italian-speaking people in that region (figure S2). We also recorded participants’ education levels within the Swiss education system, with the majority indicating that they had received tertiary or higher education (figure S3). Additionally, most respondents reported having children (figure S4), and the majority live with a spouse or unmarried partner (figure S5).
Each respondent evaluated scenario cards and, for each, they indicated whether they would recommend treatment; we calculated the probability of a positive decision to treat based on the proportion of affirmative responses across participants. We analysed whether the respondents’ demographics influenced the probability of a positive decision to treat (results are presented in appendix table S1). The probability of a positive decision to treat was highly dependent on the characteristics of the individual scenario cards presented to the respondents. Some scenarios received a probability of a positive decision to treat of 1, indicating that respondents always advised treatment, while other scenarios had a probability of a positive decision to treat below 0.5, meaning that fewer than 50% of respondents decided to treat the patient (figure S6).
Each scenario card was associated with a real underlying disease, but the disease was not disclosed to the respondents, as only the characteristics of the disease were presented and categorised in each card to reduce bias and focus the respondents’ judgement on the characteristics of the case. Note that each disease could be represented by multiple cards (see supplementary materials available at https://osf.io/p4h8u/), as some diseases under scrutiny can affect different age groups or manifest with varying symptoms.
We calculated the probability of a positive decision to treat for each disease: our results show that Gitelman syndrome, asthma and Alport syndrome were associated with the highest probability of a positive decision to treat (0.97, 0.95 and 0.95, respectively), whereas ataxia telangiectasia, Fabry disease and coronary artery disease had the lowest probability of a positive decision to treat (0.51, 0.65 and 0.70, respectively) (table 2). It is important to note that this does not indicate a general reluctance among participants to treat ataxia telangiectasia compared to Gitelman syndrome, for example, as the diseases were not disclosed to the respondents. Instead, the reduced willingness is likely associated with specific factors related to the individual diseases presented in the scenario cards.
Table 2Probability of a positive decision to treat per disease. The likelihood of a positive treatment decision varied by disease, ranging from 0.97 for Gitelman syndrome to 0.51 for ataxia telangiectasia. Of note, diseases were not explicitly mentioned in the scenario cards analysed by respondents; the probability of a positive treatment decision per disease was therefore influenced by other factors included in the scenario cards. One or more scenario cards were presented for each disease. The value in brackets is the number of assessments for each disease.
| Disease | Probability of a positive decision to treat (number of assessments) |
| Gitelman syndrome | 0.97 (153) |
| Asthma | 0.95 (475) |
| Alport syndrome | 0.95 (37) |
| Diabetes mellitus (type 1) | 0.94 (293) |
| Biotinidase deficiency | 0.94 (63) |
| Autosomal-dominant polycystic kidney disease | 0.92 (39) |
| Hypertension | 0.91 (79) |
| Severe combined immunodeficiency (SCID) | 0.91 (65) |
| Cystic fibrosis | 0.89 (73) |
| Cystinosis | 0.89 (35) |
| Atypical haemolytic uraemic syndrome | 0.84 (126) |
| Complete progranulin deficiency (NCL11) | 0.84 (438) |
| Common variable immunodeficiency (CVID) | 0.83 (70) |
| Severe congenital neutropenia (SCN) | 0.82 (68) |
| Spinal muscular atrophy (SMA) | 0.81 (224) |
| Hunter syndrome | 0.80 (40) |
| Chronic granulomatous disease (CGD) | 0.79 (413) |
| Gaucher disease type 1 | 0.76 (93) |
| Progranulin deficiency frontotemporal dementia | 0.76 (221) |
| Pompe disease | 0.75 (182) |
| Primary hyperoxaluria | 0.72 (32) |
| Coronary artery disease | 0.70 (139) |
| Fabry disease | 0.65 (150) |
| Ataxia telangiectasia | 0.51 (75) |
For example, in the case of ataxia telangiectasia, the currently available treatments do not significantly improve the quality of life (QoL) of affected children, and are mostly supportive, aiming to manage symptoms rather than cure the disease. In contrast, treatments for Gitelman syndrome (magnesium and potassium supplements) can drastically improve the living conditions of those affected. This effectiveness is likely a major factor contributing to the different probabilities of a positive decision to treat for these diseases.
We also examined how disease rarity and the age group of the affected patient were associated with the probability of a positive decision to treat. Consistent with existing literature, common diseases showed a slightly higher probability of a positive decision to treat (0.91) compared to moderate, rare and ultra-rare diseases (0.79, 0.81 and 0.83, respectively) (table 3). For age groups, elderly patients had a much lower probability of a positive decision to treat (0.65) compared to all other age groups (infant: 0.84; child: 0.85; teenager: 0.86; young adult: 0.85; adult: 0.82) (table 3). As noted in the analysis of the relationship between diseases and the probability of a positive decision to treat, it is important to mention that the higher or lower probability of a positive decision to treat can be influenced by other factors listed on the scenario cards associated with certain categories of rarity or age group, and not necessarily by rarity or age group.
Table 3Probability of a positive decision to treat based on disease rarity and age group. Common diseases had a higher probability of receiving a positive treatment decision (0.91) compared to moderate (0.79), rare (0.81) and ultra-rare diseases (0.83) (A). Elderly patients were chosen for treatment less frequently (0.65) compared to infants (0.84), children (0.85), teenagers (0.86), young adults (0.85) and adults (0.82) (B). Of note, the higher probability of a positive treatment decision can be influenced by and be dependent on other factors included in the scenario cards. The value in brackets is the number of assessments for each disease rarity category / age group.
| Probability of a positive decision to treat (number of assessments) | ||
| (A) Disease rarity | Common | 0.91 (986) |
| Moderate | 0.79 (1125) | |
| Rare | 0.81 (1002) | |
| Ultra-rare | 0.83 (470) | |
| (B) Age group | Infant | 0.84 (523) |
| Child | 0.85 (744) | |
| Teenager | 0.86 (690) | |
| Young adult | 0.85 (608) | |
| Adult | 0.92 (804) | |
| Elderly | 0.65 (214) | |
Further, we observed a higher probability of a positive decision to treat for patients with diseases that severely impaired their everyday life (0.93), when compared with diseases causing a limited or a moderate impairment to everyday life, or with a disease causing premature death (0.78, 0.80 and 0.81, respectively) (table 4). Patients with diseases for which available treatments do not substantially improve everyday life had the lowest probability of a positive decision to treat (0.51) compared to those with treatments that result in a partial return to everyday life, a return to everyday life or a complete return to everyday life/perfect health (0.82, 0.87 and 0.82, respectively) (table 4). As before, a higher or lower probability of a positive decision to treat can be influenced by other factors listed on the scenario cards associated with certain categories; these categories follow our classification of the different impacts of the disease on quality of life or the different effects of the proposed treatment on quality of life, and are not necessarily influenced by the impact of the disease or the effectiveness of the treatment.
Table 4Probability of a positive decision to treat based on the impact of the disease on quality of life (QoL) and the effect of the proposed treatment on QoL. Diseases causing a limited impairment to everyday life in affected patients were chosen for treatment less frequently (0.78) compared to diseases causing a moderate (0.80) or severe (0.93) impairment to everyday life, or premature death (0.93) (A). Patients undergoing treatments causing no meaningful improvement to everyday life were chosen for treatment less frequently (0.51) compared to patients undergoing treatments causing partial return to everyday life (0.82), a return to everyday life (0.87) or a complete return to everyday life, achieving perfect health (0.82) (B). Of note, the probability of a positive treatment decision can be influenced by and be dependent on other factors included in the scenario cards. The value in brackets is the number of assessments for each variable.
| Probability of a positive decision to treat (number of assessments) | ||
| (A) Impact of the disease on quality of life | Limited impairment to everyday life | 0.78 (297) |
| Moderate impairment to everyday life | 0.80 (465) | |
| Severe impairment to everyday life | 0.93 (819) | |
| Premature death | 0.81 (2002) | |
| (B) Effect of proposed treatment on quality of life | No improvement to everyday life | 0.51 (75) |
| Partial return to everyday life | 0.82 (1282) | |
| Return to everyday life | 0.87 (1382) | |
| Perfect health / Complete return to everyday life | 0.82 (844) | |
Furthermore, lower treatment costs are generally associated with a very high probability of a positive decision to treat. High-cost treatments (ranging from 135,000 CHF for a single intervention up to 1 million CHF annually) show varying probabilities of a positive decision to treat, ranging from 0.65 to 0.91 (table 5). As with previous disclaimers, the influence of treatment costs on the probability of a positive decision to treat cannot be isolated from the effects of other factors. This is particularly apparent in this case, as the oscillations in the probability of a positive decision to treat for high-cost treatments are likely influenced by other factors in the scenario cards. An illustrative example is for treatments costing 25,000 CHF annually, which have a probability of a positive decision to treat of 0.65, lower than much more expensive treatments with annual costs of 1 million CHF (probability of a positive decision to treat of 0.84). The probability of a positive decision to treat of 0.65 is due to the low positive impact of treatments for ataxia telangiectasia. These treatments happen to cost about 25,000 CHF per year. Thus, the low probability of a positive decision to treat for 25,000 CHF treatments is due to their lack of effectiveness, rather than the cost of the treatment itself. Finally, familial diseases with phenotypic presentation in multiple family members show a slightly higher probability of a positive decision to treat when compared with non-familial diseases (0.85 vs 0.82, respectively) (appendix table S2).
Table 5Probability of a positive decision to treat based on the cost of treatment. Patients with diseases whose treatments were less expensive generally had a higher probability of receiving a positive treatment decision. However, high costs did not appear to significantly limit the participants’ decisions to treat patients. Of note, a higher or lower probability of a positive treatment decision may be influenced by and be dependent on other factors included in the scenario cards. Costs are listed from least to most expensive, based on the assumption that annual expenses will persist for ten years. The value in brackets is the number of assessments for each treatment cost category.
| Cost of treatment | Probability of a positive decision to treat (number of assessments) |
| Annual cost 250 CHF | 0.95 (475) |
| Annual cost 450 CHF | 0.94 (63) |
| Annual cost 500 CHF | 0.95 (37) |
| Annual cost 1500 CHF | 0.97 (153) |
| Annual cost 2000 CHF | 0.93 (372) |
| Single intervention 135,000 CHF | 0.70 (139) |
| Single intervention 200,000 CHF | 0.89 (73) |
| Annual cost 25,000 CHF | 0.65 (114) |
| Single dose 450,000 CHF | 0.91 (65) |
| Annual cost 52,500 CHF | 0.83 (70) |
| Annual cost 85,000 CHF | 0.82 (68) |
| Single dose 1,000,000 CHF | 0.89 (35) |
| Single dose 2,000,000 CHF | 0.81 (224) |
| Annual cost 200,000 CHF | 0.65 (150) |
| Single dose 2,500,000 CHF | 0.80 (1072) |
| Annual cost 350,000 CHF | 0.77 (133) |
| Annual cost 400,000 CHF | 0.75 (182) |
| Annual cost 900,000 CHF | 0.72 (32) |
| Annual cost 1,000,000 CHF | 0.84 (126) |
Our exploratory study highlighted the Swiss public’s preferences regarding the treatment of rare diseases and associated costs, and identified factors influencing the decision to treat or not treat a disease. Our results offer valuable insights into the decision-making landscape of respondents. We observed a slight preference for treating common diseases over rare ones. This is aligned with previous literature from the UK, which has shown that as the costs of treating rare diseases rise, people tend to favour allocating funds to more prevalent diseases [17]. In Sweden, it was found that treating common diseases was preferred when treatments for rare diseases were more costly [19]. And in Norway, support for treating rare diseases was shown to be decreased in comparison with common diseases when the cost of treating rare diseases was much higher [20].
In our study, elderly patients were less likely to receive treatment compared to younger patients. Participants showed a lower willingness to treat diseases causing limited impairment to everyday life compared to those causing severe impairments, except in cases of diseases causing premature death. This suggests a preference for addressing significant suffering over death. Additionally, treatments showing no substantial improvement to everyday life were associated with the lowest probability of a positive decision to treat, indicating that treatment effectiveness is a particularly relevant factor for respondents. While cheaper treatments had a high probability of a positive decision to treat, expensive treatments costing over 135,000 CHF (single intervention) showed no clear trend in the probability of a positive decision to treat, suggesting that treatment effectiveness played a more crucial role than treatment cost; treatments with annual costs up to 1 million CHF were associated with varying probabilities of a positive decision to treat, emphasising the minor role of cost in respondents’ decision-making.
It is important to note that, despite our efforts to target a representative sample of the Swiss population, the sample size in this study is relatively small (n=375, after cleaning). Given the exploratory nature of our study and the survey structure, we could not perform a multiple logistic regression analysis so we opted for a simple and descriptive probability count based on individual categories for each factor. With this approach, we cannot rule out that the observed effect of one categorical variable might depend on another co-occurring variable. For example, if a treatment costing 25,000 CHF is associated with low effectiveness, it may receive a low probability of a positive decision to treat, leading to the incorrect conclusion that the cost alone is the deterrent.
Overall, the data suggest that treatment cost is a minor concern for our study respondents, who are primarily focused on achieving effective and substantial improvements in patient conditions, regardless of disease rarity.
In light of these findings, we recommend a reevaluation of communication strategies about rare diseases in Switzerland. Current strategies often emphasise the rarity of these diseases as an inherent and important value. However, we should strive for an evidence-based approach focused on outcomes – which requires testing; effective communication strategies in Switzerland, we argue, would likely be more effective if they emphasised the importance of developing appropriate treatments for rare diseases, rather than focusing on their rarity.
All raw data are openly accessible and can be downloaded from our Open Science Framework repository [22].
Funding: University of Zurich, University Research Priority Programme (URPP) ITINERARE
RJ received Research Grants from University Research Priority Programme (URPP) ITINERARE, CRPP Immugene, Wyss Center Zurich and SNSF, not related to present publication. She is a Board member of Somagenetix AG and has stock or stock options from Somagenetix AG with no relation to this manuscript and no payments to her institution. – MB received a research grant from Milupa Metabolics, not related to the present publication, payment or honoraria for lectures at Nordic Metabolic Meeting March 2024 from Immedica and has participated in a Data Safety Monitoring Board for a trial involving a gene therapy for mucopolysaccharidosis IIIa by Lysogene, 2019–2023.
1. The Lancet Neurology. Rare neurological diseases: a united approach is needed. Lancet Neurol. 2011 Feb;10(2):109.
2. Garrison S, Kennedy A, Manetto N, Pariser AR, Rutter JL, Yang G. The Economic Burden Of Rare Diseases: Quantifying The Sizeable Collective Burden And Offering Solutions. Health Aff Forefr. doi:
3. Hughes D. Rationing of drugs for rare diseases. PharmacoEconomics. 2006;24(4):315–6.
4. Kacetl J, Marešová P, Maskuriy R, Selamat A. Ethical Questions Linked to Rare Diseases and Orphan Drugs - A Systematic Review. Risk Manag Healthc Policy. 2020 Oct;13:2125–48.
5. Pearson C, Schapiro L, Pearson SD. The next generation of rare disease drug policy: ensuring both innovation and affordability. J Comp Eff Res. 2022 Oct;11(14):999–1010.
6. Juth N; Sake of Justice. For the Sake of Justice: Should We Prioritize Rare Diseases? Health Care Anal. 2017 Mar;25(1):1–20.
7. Delaye J, Cacciatore P, Kole A. Valuing the “Burden” and Impact of Rare Diseases: A Scoping Review. Front Pharmacol. 2022 Jun;13:914338.
8. Cardinali P, Migliorini L, Rania N. The Caregiving Experiences of Fathers and Mothers of Children With Rare Diseases in Italy: Challenges and Social Support Perceptions. Front Psychol. 2019 Aug;10:1780.
9. Nakada H, Watanabe S, Takashima K, Suzuki S, Kawamura Y, Takai Y, et al. General public’s understanding of rare diseases and their opinions on medical resource allocation in Japan: a cross-sectional study. Orphanet J Rare Dis. 2023 Jun;18(1):143.
10. Eurordis (Rare diseases Europe). Rare diseases: understanding this public health priority. 2005. Available from: https://www.eurordis.org/publications/rare-diseases-understanding-this-public-health-priority/
11. Bentley JP, Larson LN, Brenton MA. Values, participatory democracy, and healthcare resource allocation: an application to a campus community. J Am Coll Health. 1995 Mar;43(5):205–11. doi: https://doi.org/10.1080/07448481.1995.9940478
12. Schoch-Spana M, Brunson EK, Gwon H, Regenberg A, Toner ES, Daugherty-Biddison EL. Influence of Community and Culture in the Ethical Allocation of Scarce Medical Resources in a Pandemic Situation: Deliberative Democracy Study. J Particip Med. 2020 Mar;12(1):e18272.
13. Spitale G, Merten S, Jafflin K, Schwind B, Kaiser-Grolimund A, Biller-Andorno N. A Novel Risk and Crisis Communication Platform to Bridge the Gap Between Policy Makers and the Public in the Context of the COVID-19 Crisis (PubliCo): Protocol for a Mixed Methods Study. JMIR Res Protoc. 2021 Nov;10(11):e33653. doi: https://doi.org/10.2196/33653
14. Prinz JJ. The emotional construction of morals. New York, NY, US: Oxford University Press; 2007. p. xi, 334. ISBN:978-0-19-928301-9
15. Harman G. Moral Relativism Defended. Philos Rev. 1975;84(1):3–22.
16. National Advisory Commission on Biomedical Ethics (NCE). Drug prices. Considerations on the equitable management of expensive new medicines. Opinion no. 35/2020. 2020. Available from: https://www.nek-cne.admin.ch/inhalte/Themen/Stellungnahmen/en/NEK-stellungnahme-medikamentenpreise-EN-rz.pdf
17. Bourke SM, Plumpton CO, Hughes DA. Societal Preferences for Funding Orphan Drugs in the United Kingdom: An Application of Person Trade-Off and Discrete Choice Experiment Methods. Value Health. 2018 May;21(5):538–46.
18. Si. Healthcare in Sweden. sweden.se. 2024. Available from: https://sweden.se/life/society/healthcare-in-sweden
19. Wiss J, Levin LA, Andersson D, Tinghög G. Prioritizing Rare Diseases: Psychological Effects Influencing Medical Decision Making. Med Decis Making. 2017 Jul;37(5):567–76.
20. Desser A S, Gyrd-Hansen D, Olsen J A, Grepperud S, Kristiansen I S. Societal views on orphan drugs: cross sectional survey of Norwegians aged 40 to 67. BMJ. 2010;341:c4715. doi:
21. Goldin J, Reck DH. Framing Effects in Survey Research: Consistency-Adjusted Estimators. SSRN Electron J. 2014; doi:
22. Spitale G, Germani F. ITINERARE - A Swiss Perspective on Rare Disease Resource Allocation. OSF. 2025. doi:
23. Center for Rare Diseases Zurich. University Hospital Zurich (USZ). Available from: https://www.usz.ch/en/department/center-for-rare-diseases-zurich/
The appendix tables and figures are included in the pdf version of the article, and the supplementary materials are available for download as separate files at https://doi.org/10.57187/s.4243.