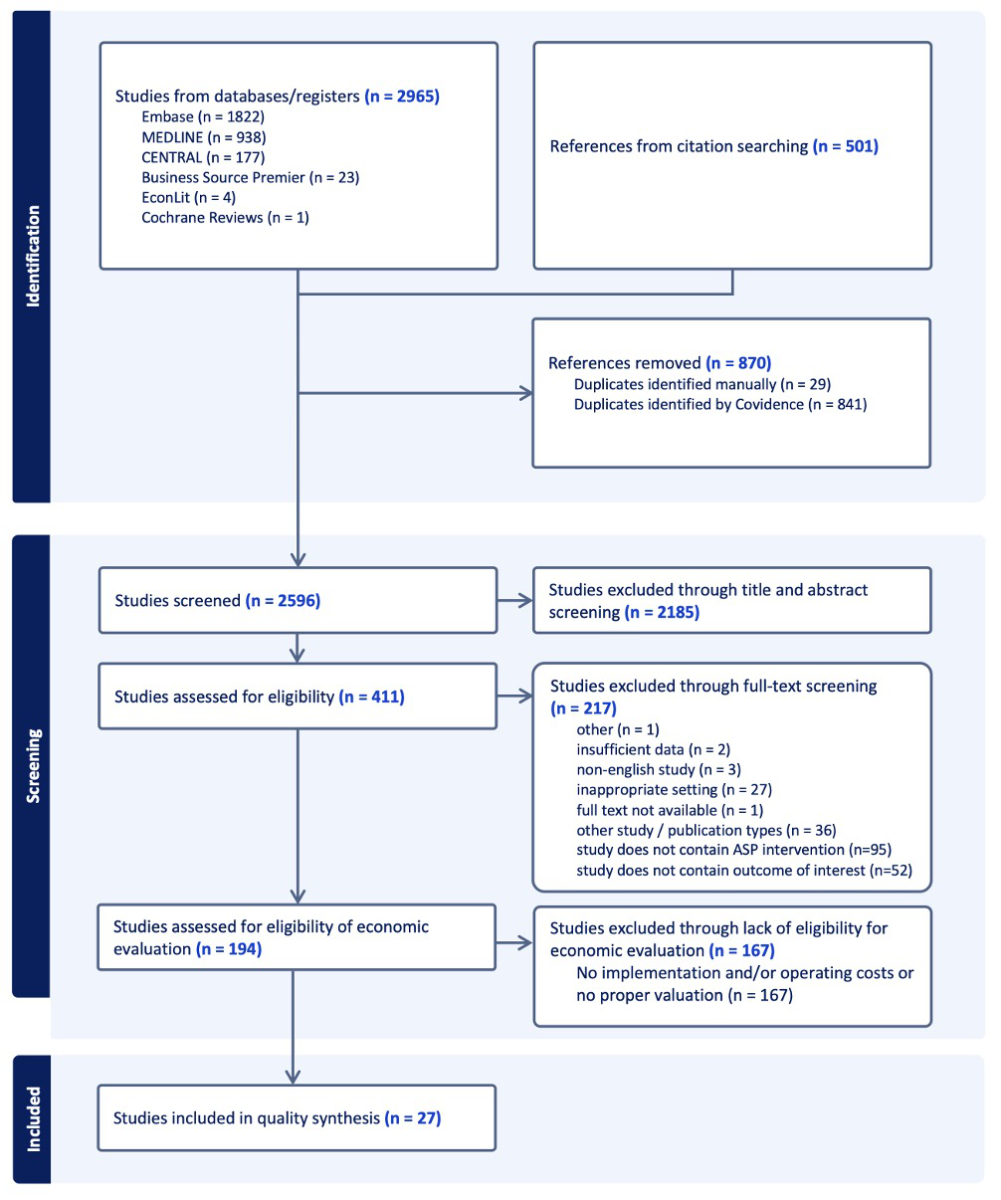
Figure 1PRISMA flow diagram.
DOI: https://doi.org/https://doi.org/10.57187/s.4217
Consolidated Health Economic Evaluation Reporting Standards
Diagnosis-Related Group
As early as the 1990s, the misuse and overuse of antibiotics and the resulting development of antibiotic-resistant bacteria highlighted the need for systematic approaches to managing antibiotic use [1]. As reported by the Organisation for Economic Co-operation and Development (OECD) in 2023, the issue of antibiotic resistance has reached a critical point, with the financial burden estimated to exceed US$ 28.9 billion per year worldwide [2].
Antibiotic Stewardship Programmes represent systematic efforts within healthcare facilities to promote the appropriate use of antimicrobials through targeted measures adapted to the local context [3]. A number of studies, such as those by Lee et al. [4] and Baur et al. [5], have demonstrated that Antibiotic Stewardship Programmes can contribute to a reduction of antibiotic resistance by minimising unnecessary prescriptions. In accordance with the Centers for Disease Control and Prevention (CDC), this objective is complemented by the need to mitigate the numerous other adverse effects on society, the environment and the economy [6].
From a societal perspective, Antibiotic Stewardship Programmes represent a logical approach to reducing antibiotic resistance, as they have the potential to lower antibiotic resistance-related mortality and associated costs [7–10]. In addition to the well-documented health impact, the economic benefits of Antibiotic Stewardship Programmes are increasingly being recognised. A growing field of research indicates that Antibiotic Stewardship Programmes contribute to healthcare cost savings by reducing the length of hospital stays, overall cost savings and antibiotic expenses. These metrics serve as indicators of cost-effectiveness, with the objective of determining whether Antibiotic Stewardship Programmes represent an economically viable solution for hospitals. This leads to the important question of who should bear the financial responsibility for implementing Antibiotic Stewardship Programme measures: policymakers or individual facilities?
Previous literature reviews, such as those by Dik et al. [11] or Nathwani et al. [8], demonstrated significant discrepancies in the methodologies employed to assess costs and frequently observed the lack of a standardised approach to documenting expenses. The complexity of determining all relevant costs led to expenses, such as implementation costs, not being considered. Such discrepancies, along with the uncertainty regarding which interventions are cost-effective, made comparisons challenging and precluded the formulation of clear recommendations for action. Furthermore, there have also been significant developments since these reviews. For instance, the WHO has accorded antibiotic resistance the status of a public health threat since 2015 and has developed a Global Action Plan to combat antimicrobial resistance. As part of this plan, a series of campaigns were initiated, including the GLASS report, the TrACSS website and the World Antimicrobial Resistance Awareness Week [12, 13]. These initiatives emphasise the increase in global awareness about antibiotic resistance and underscore the need to evaluate the economic aspects of Antibiotic Stewardship Programmes as a component of these efforts.
The present study builds on previous findings by Dik et al. [11], which covered the period between 2000 and 2014, and aims to provide a comprehensive and updated overview of the literature published between 2015 and 2024 to examine the impact of these various developments on the economic analysis of Antibiotic Stewardship Programmes. The review also assesses the quality of the included studies using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist, to identify trends in the documentation of economic outcomes and provide recommendations for future research.
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA-P) statement and has been registered with the international prospective register for systematic reviews (PROSPERO) (registration number: CRD42023441237), available at https://www.crd.york.ac.uk/prospero/ [11, 14]. A full study protocol was not prepared beforehand.
Studies published between 2015 and June 2024 and explicitly labelled as economic analyses examining the economic impact of Antibiotic Stewardship Programmes in the acute care setting were included. Only full-length publications in peer-reviewed journals, written in English and designed as randomised controlled trials, observational studies (cohort and case-control studies) or quasi-experimental studies (before-and-after studies) were considered eligible. Other study types and any studies with a sample size lower than five were excluded. Finally, studies investigating paediatric populations or patients in long-term care were not considered. This was done to enhance the comparability of results as the specificities of these populations might not only be reflected in the choice of interventions, but also the cost structure of these settings may differ considerably from adult acute care facilities, potentially distorting the economic analysis.
The search was conducted with the assistance of an information specialist librarian (AB) from the University of Zurich medical library. The electronic databases Medline (via Ovid), Embase (via Elsevier), Cochrane Reviews and Trials (via Cochrane Library), Business Source Premier (via EBSCOhost) and EconLit (via EBSCOhost) were searched using a combination of medical indexing terms and free-text terms. The full search strategies for all databases are reported in the appendix (tables S1–S5). The initial searches were performed in August 2023 and an update search was performed in June 2024. In addition, we performed citation searching using Scopus and Web of Science (forward and backward citation searching). The seed references were the articles included for analysis following full-text screening.
Search results from each database (including update searches and citation searching results) were exported and uploaded individually to Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org). Deduplication was performed using Covidence. Title and abstract screening was conducted independently by three authors (AB, TD, JH). All records were screened by JH. The concurrent screening performed by AB and TD was split, with the initial 100 being done by AB and the remainder by TD. Disagreements were resolved by a fourth review author (SK).
Eligibility was determined by a two-stage full-text screening process, performed independently by two authors (TD and JH), with disagreements again resolved by SK. Initially, exclusion criteria were applied, and then, in a second step, relevant studies for the economic evaluation identified.
For this analysis, we only included studies that were labelled as economic analyses and could be clearly categorised as Cost-Benefit Analyses (CBA), Cost-Effectiveness Analyses (CEA), Cost-Consequence Analyses (CCA), Cost-Utility Analyses (CUA), Cost Analyses (CA) or Cost-Minimisation Analyses (CMA) [11, 15]. A more detailed explanation of the categories can be found in table 1.
In accordance with the recommendations of Drummond et al., the studies were required to include the implementation and/or operating costs associated with the interventions [15].
Table 1Overview of economic analysis methods and their applications. The cost measurement for all categories is expressed in monetary units.
| Type of economic analysis | Outcome measures (effect) | Application |
| Cost-effectiveness analysis (CEA) | Clinical parameters (e.g. life years gained) | Compares relative costs and health outcomes to determine the most efficient option. |
| Cost-benefit analysis (CBA) | Monetary value | Evaluates costs and benefits by converting outcomes into monetary terms to assess economic impact. |
| Cost-consequence analysis (CCA) | Various outcomes presented separately | Examines both the varying costs and effects associated with two methods. |
| Cost-minimisation analysis (CMA) | Assumes effects are equal; compares costs only | Analyses cost differences under the assumption of similar effects across methods. |
| Cost analysis (CA) | Not applicable | Focuses only on the cost aspect, without evaluating the outcomes. |
| Cost-utility analysis (CUA) | Utility measures (e.g. quality-adjusted life years [QALYs]) | Measures outcomes based on their benefits, focusing on the quality and quantity of life. |
Data extraction was conducted by two authors (TD and JH). Major disagreements were resolved by discussion with a third author (SK). Besides bibliographic data (i.e. publication year, journal, authors), we extracted information on study characteristics (i.e. study design, setting, hospital size, number of patients included, time horizon), details about the interventions (categorised as “giving education”, “therapy evaluation, review and/or feedback”, “alteration of therapy guidelines”, “pre-analytic consultations” and “rapid diagnostic tools”), classification as an Antibiotic Stewardship Programme (i.e. structured programme promoting the appropriate use of antibiotics, requiring the involvement of qualified personnel) and financial outcomes (i.e. type of economic analysis). For the latter, the framework used by Dik et al. [11] was applied, measuring impact in monetary units per type of cost accounted for. The following cost categories were extracted: Antibiotic Stewardship Programme implementation costs, Antibiotic Stewardship Programme operational costs (personnel and/or equipment), morbidity and/or mortality costs, and societal costs. Also, data regarding cost changes attributed to Antibiotic Stewardship Programmes, such as length-of-stay costs, overall antimicrobial costs and overall costs, were collected, along with data on implemented price adjustments and/or discounting measures.
All statistics were descriptive, including differences, medians with ranges and percentages. To ensure comparability of outcomes, data were converted to US dollars (USD) per year. While this approach facilitates standardised reporting across studies from different countries, it does not account for variations in local purchasing power or economic conditions, which may influence cross-country comparisons. In studies that did not employ inflation-adjusted values during the intervention period, the inflation adjustment was performed separately. To assess the effectiveness of the interventions, the aim was to achieve a percentage reduction in costs between the pre- and post-intervention periods for the outcomes length of stay, antimicrobial costs and overall costs. The cost reduction was calculated by determining the difference between the costs before and after the intervention. This difference was then divided by the pre-intervention costs and expressed as a percentage by multiplying by 100.
The variability in cost reporting was minimised by dividing the assessed costs and Antibiotic Stewardship Programme measures into subgroups and tabulating categories such as study design, geographical region and hospital size, and making inflation adjustments for values from previous currency years. Nevertheless, due to the considerable heterogeneity among the included studies, particularly in relation to the methods employed for cost reporting and the measures utilised for outcomes, sensitivity analyses could not be performed. Furthermore, the risk of bias resulting from incomplete results was not formally evaluated. However, for studies with incomplete data, missing data were documented as “not available” and no assumptions or imputations were made. Studies that exhibited significant data gaps were excluded during the data extraction process (see tables S1–S5).
The quality of studies was evaluated by two reviewers independently (TD and JH) using the CHEERS checklist, version 2022 [16]. Discrepancies were discussed and resolved by consensus. The CHEERS checklist assesses the fulfilment of 28 quality criteria. For each, a score of 1 point was allocated if information was adequately disclosed and documented. In cases of incomplete or absent information, 0.5 or 0 points, respectively, were assigned. The category “not applicable (N/A)” was used if the criterion did not apply to the included study. The values were converted into a scale ranging from 0 to 1, with higher numbers indicating better quality. To enable a more precise quality assessment of the included studies, we adopted the quality classification standard by Degeling et al. [17], which divides quality into three categories: high quality (>80%), moderate quality (60–80%), and low quality (<60%). A detailed description of the CHEERS checklist items is provided in table S6 in the appendix.
Not applicable.
A total of 2596 publications were detected in our literature search after deduplication. After title and abstract review, 411 papers were included in the full-text review of which 194 studies matched the eligibility criteria. Based on their fulfilment of the essential parameters required for an economic analysis, 27 were selected and included in the systematic review [18–44]. The review process is summarised in figure 1.

Figure 1PRISMA flow diagram.
Overall, the 23 studies focusing on an economic analysis included a total of 10,827 patients undergoing Antibiotic Stewardship Programme interventions and 9405 in the comparison groups. In four studies, the exact number of patients or the distinction between the two groups was unclear [21, 25, 30, 41].
Most of the studies were conducted in North America (10/27, 37%) [25–28, 35, 36, 38–40, 44] or Europe (8/27, 30%) [19–21, 23, 24, 30, 34, 42]. The most common study period was 2015–2017 (9/27, 33%) [21, 23, 30, 33, 36, 39, 40, 42, 43]. Observational studies, including cohort and case-control studies, were the most common study design (8/27, 30%) [23–27, 29, 32, 35], followed by retrospective evaluations (6/27, 22%) [18, 20, 28, 33, 40, 41] or quasi-experimental studies (5/27, 19%) [22, 30, 36, 39, 43]. One study (1/27, 4%) employed a randomised controlled trial (RCT) methodology, specifically a stepped-wedge cluster RCT conducted by van Daalen et al. [42]. Table S7 in the appendix provides a summary of the general characteristics of the 27 included studies.
Therapy evaluation, review and/or feedback (23/27, 85%) [18, 20, 21, 23–34, 36–41, 43, 44] were the most frequently used interventions, followed by alteration of therapy guidelines (9/27, 33%) [18, 19, 21, 22, 26, 31, 35, 39, 42] and education (6/27, 22%) [22, 27, 28, 34, 42, 43]. Pre-analytic consultations (3/27, 11%) [20, 26, 43] and rapid diagnostic tools (3/27, 11%) [28, 36, 38] were less often utilised. Out of the 27 studies, 14 (52%) combined multiple interventions, qualifying them as “service bundles” [18, 20–22, 26–28, 31, 34, 36, 38, 39, 42, 43]. Of these, 12 (86%) contained the intervention “therapy evaluation, review and/or feedback”. In contrast, all studies that considered only one intervention (13/27, 48%) analysed the intervention category “therapy evaluation, review and/or feedback” [19, 23–25, 29, 30, 32, 33, 35, 37, 40, 41, 44].
The majority of the identified studies (19/27, 70%) were categorised as cost analyses [18–20, 24, 27, 28, 30, 31, 33–36, 38–44] in general. Furthermore, 19% (5/27) of the studies conducted cost-benefit analyses [21, 22, 25, 26, 35], with an equivalent number (5/27, 19%) categorised as cost-effectiveness analyses [20, 28, 29, 35, 42]. Cost-minimisation analyses were reported (2/27, 7%) [23, 32]. Only Psaltikidis et al. [37] conducted a cost-utility analysis (1/27, 4%), while Durojaiye et al. [24] employed a cost-consequence analysis. Some studies addressed multiple categories (5/27, 19%) [20, 24, 28, 35, 42].
Costs were measured in various currencies, including USD (18/27, 67%) [18, 19, 25–29, 31–33, 35–41, 43], Euros (EUR) (4/27, 15%) [21, 23, 34, 42], Canadian Dollars (CAD) (1/27, 4%) [44], Turkish Lira (TRY) (1/27, 4%) [20], Swedish Krona (SEK) (1/27, 4%) [30] and British Pounds (GBP) (1/27, 4%) [24]. Only the cost-benefit study of Butt et al. used a benefit-to-cost ratio [22]. The methods used to estimate costs in the 27 studies varied widely, including “per patient”, “per year”, “per bloodstream infection”, “per five years”, “per 1000 cases”, “per month”, “average cost” and no specific method. Only one third (9/27, 33%) considered inflation and performed price adjustments [18, 20, 23, 24, 29–31, 37, 42].
In all studies, the costs and benefits were analysed from the perspective of the hospital (27/27, 100%). While all studies reported operational costs, implementation costs were only reported in 8/27 (30%) of the studies [22–24, 28–30, 36, 42]. Additionally, only 14/27 (52%) papers examined morbidity/mortality costs [18, 20–24, 26, 28, 29, 35, 37, 40, 43, 44] and societal costs were considered in only 3 of the 27 studies (11%) [22, 36, 37]. While the need for inclusion of social costs was highlighted in a few studies, only Psaltikidis et al. also evaluated costs and benefits from a societal perspective (1/27, 4%) [37]. A comprehensive analysis of the efficacy of length of stay, antimicrobial cost savings and overall cost savings is presented in table 2.
Table 2Overview of relative cost savings for different interventions. An en dash (–) indicates that the study did not consider the particular value. “Unquantifiable” means the effectiveness of the measures regarding overall cost savings could not be quantified due to insufficient data. Studies are organised alphabetically by first author.
| Author(s) (Year) | Intervention category | Length of stay reduction | Antimicrobial reduction | Overall cost reduction |
| Abushanab et al. (2024) | Altered therapy guidelines, Therapy evaluation, review and/or feedback | – | −2% | unquantifiable |
| Asilturk et al. (2024) | Altered therapy guidelines | – | −37% | – |
| Bastug et al. (2021) | Therapy evaluation, review and/or feedback, Pre–analytic consultations | −85% | – | −25% |
| Borde et al. (2016) | Therapy evaluation, review and/or feedback, Altered therapy guidelines | – | −67% | unquantifiable |
| Butt et al. (2019) | Giving education, Altered therapy guidelines | −35% | −26% | unquantifiable |
| Dik et al. (2015) | Therapy evaluation, review and/or feedback | – | DRG1: −23.4% / DRG2: −26.6% | unquantifiable |
| Durojaiye et al. (2018) | Therapy evaluation, review and/or feedback | – | – | −85% |
| Howell et al. (2019) | Therapy evaluation, review and/or feedback | – | unquantifiable | −20% |
| Hyland et al. (2022) | Therapy evaluation, review and/or feedback, pre-analytic consultations, altered therapy guidelines | – | unquantifiable | unquantifiable |
| Jaggar et al. (2023) | Therapy evaluation, review and/or feedback, Giving education | – | −32% (academic hospital) −63% (non-academic hospital) | unquantifiable |
| Karimaghaei et al. (2022) | Therapy evaluation, review and/or feedback, Giving education, Rapid diagnostic tools | – | – | −86% |
| Kim et al. (2022) | Therapy evaluation, review and/or feedback | +21% | −39% | −27% |
| Lanbeck et al. (2016) | Therapy evaluation, review and/or feedback | −3% | −23% | −3% |
| Lester et al. (2020) | Altered therapy guidelines, Therapy evaluation, review and/or feedback | – | −69% | −30% |
| Loesch et al. (2021) | Therapy evaluation, review and/or feedback | – | – | −50% |
| Malone et al. (2015) | Therapy evaluation, review and/or feedback | unquantifiable | unquantifiable | unquantifiable |
| Mouwen et al. (2020) | Giving education, Therapy evaluation, review and/or feedback | unquantifiable | unquantifiable | unquantifiable |
| Olson et al. (2023) | Altered therapy guidelines | – | – | unquantifiable |
| Patel et al. (2017) | Rapid diagnostic tools, Therapy evaluation, review and/or feedback | – | – | −5% |
| Psaltikidis et al. (2019) | Therapy evaluation, review and/or feedback | – | – | −47% |
| Ramsey et al. (2020) | Therapy evaluation, review and/or feedback, Rapid diagnostic tools | – | – | unquantifiable |
| Ross et al. (2015) | Therapy evaluation, review and/or feedback, Giving education, Altered therapy guidelines | – | unquantifiable | – |
| Ruh et al. (2015) | Therapy evaluation, review and/or feedback | – | −92% / −81% | – |
| Salman et al. (2021) | Therapy evaluation, review and/or feedback | – | – | unquantifiable |
| van Daalen et al. (2017) | Giving education, Altered therapy guidelines | unquantifiable | – | unquantifiable |
| Wang et al. (2015) | Giving education, Therapy evaluation, review and/or feedback, Pre-analytic consultations | – | −95% | −20% |
| Yadav et al. (2022) | Therapy evaluation, review and/or feedback | – | – | −80% |
DRG: Diagnosis-related group.
Overall cost savings were reported by 24 studies (89%) [18, 20–38, 41–44]. Only 12 (44%) quantified the effectiveness of their interventions in terms of overall cost savings[18–20, 24, 25, 28–32, 36, 37, 43, 44]. Yadav et al. [44] reported a significant reduction in total costs of 80% and, similarly, Durojaiye et al. [24] achieved a relative cost reduction of 85%. Karimaghaei et al. [28] also showed a substantial total cost reduction of 86%. Cost reductions in the remaining nine studies ranged from 3% to 50% [20, 25, 29–32, 36, 37, 43].
The cost of antimicrobials was analysed in 16/27 studies (59%) [18–23, 25–27, 29–31, 33, 34, 39–41, 43], and all showed a reduction, with figures ranging from 23% [30] to 95% [43]. Furthermore, the study by Jaggar et al. [27] identified differences in costs between academic and non-academic hospitals and Dik et al. presented minimal differences in percentage reduction in antibiotic costs based on Diagnosis-Related Groups (DRGs) [23, 27].
Of the seven studies that assessed length of stay [20, 22, 29, 30, 33, 34, 42], six reported that an Antibiotic Stewardship Programme intervention led to a reduction in costs. Kim et al. [29] reported that although the intervention group had higher hospitalisation costs (USD +266.8 or +21%), the overall hospital length of stay was reduced from 10.8 to 9.5 days, yielding an overall cost saving. Three studies demonstrated cost savings between 2.9% and 85% [20, 22, 30] and in three studies [33, 34, 42], it was not possible to quantify cost savings attributable to change in length of stay.
The quality assessment yielded a median score of 64% (range 43–87%). The reporting quality of the included studies varied significantly. As illustrated in table 2, nine studies (33%) scored 60% or lower [22, 25, 27, 28, 36, 38–41], placing them in the low-quality category and 16 studies (59%) fell into the moderate-quality category [19–21, 23, 24, 26, 29–35, 42–44]. Only Psaltikidis et al. [37] and Abushanab et al. [18] (2/27, 7%) achieved the high-quality category, scoring 83% and 87%, respectively.
Furthermore, there were several aspects for which the studies often lacked the required reporting according to the CHEERS checklist criteria. For instance, item 16, “Rationale and description of the model”, was only assessable in four studies (15%), as most of the economic evaluations were not model-based [23, 37, 41, 42]. A plan for health economic analyses was developed but not fully reported in three studies (11%) [18, 42, 43]. Of the 27 studies included, only van Daalen et al. [42] mentioned their approach to involving patients and other stakeholders in the study, while none of the studies addressed the impact of this involvement in the results section. Of note, none of the 27 studies had a complete abstract, with information on discount rate, perspective, currency year and time horizon mostly missing. Only two studies (7%) characterised the distributional effects completely [24, 44] and three (11%) considered the discount rate [18, 31, 37]. Figure 2 shows the proportions of fulfilment categories per item.
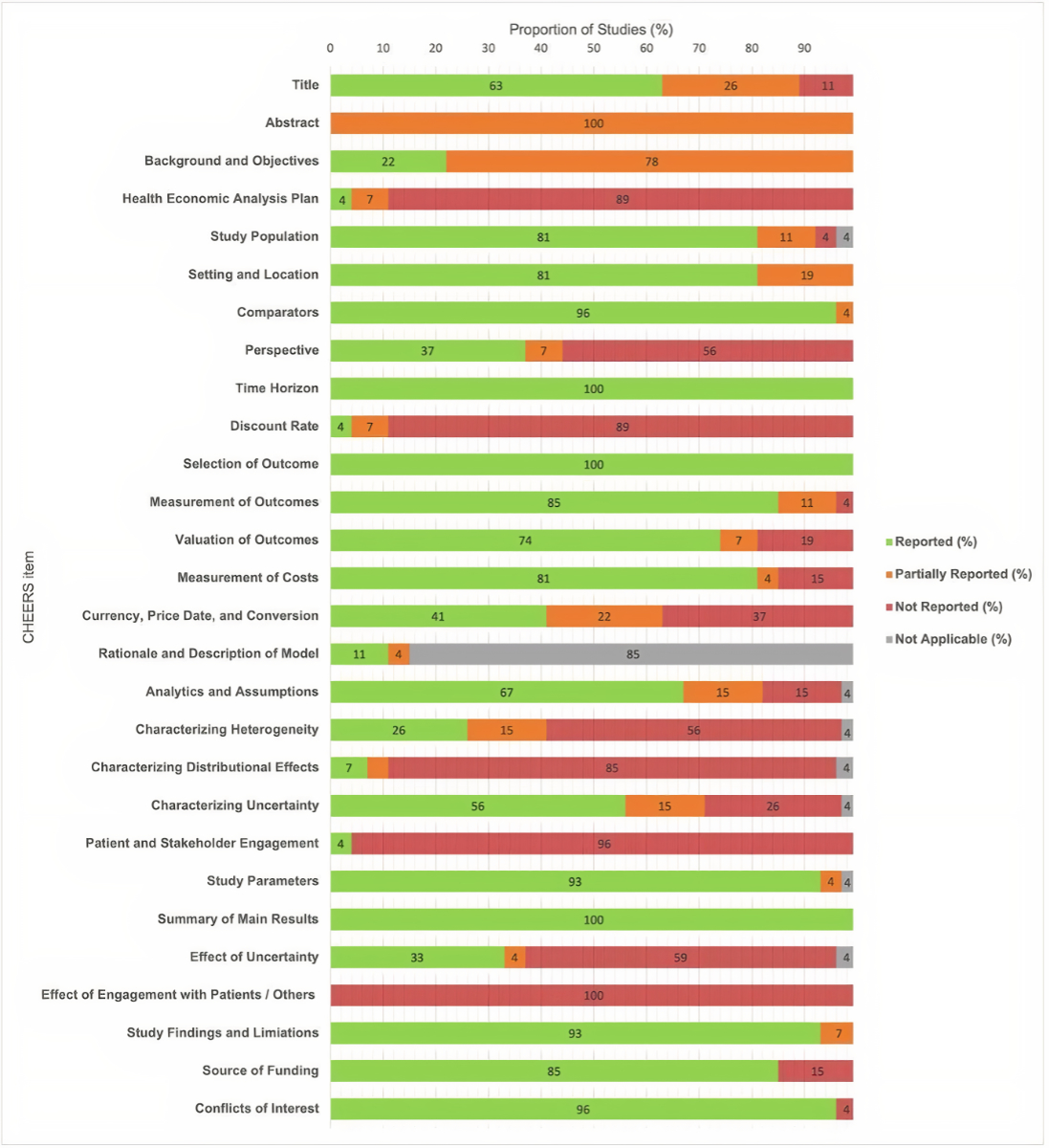
Figure 2Overview of reported items from the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist among the 27 included studies. Numbers are percentages and have been rounded.
In the present systematic literature review, focusing on economic studies examining Antibiotic Stewardship Programme interventions in the acute care setting and their attributed economic impact for a hospital, published between 2015 and 2024, we observe that Antibiotic Stewardship Programmes are consistently associated with reduction of overall costs, length of stay costs and antimicrobial costs. Furthermore, the intervention category “evaluation, review and feedback of therapeutic interventions” was most common and was consistently shown to be effective in reducing costs. Additionally, the implementation of rapid diagnostic tools and educational programmes contributed to these reductions. Similar to Dik et al. [11], we observed significant heterogeneity between studies. However, with the increasing efforts in papers such as those by Psaltikidis et al. [37] and Abushanab et al. [18] to document cost changes in a standardised manner, we noted that the quality of studies evaluating economic aspects of Antibiotic Stewardship Programme interventions has improved over time. This improvement in comparison with the publications before 2015 allowed us to apply the recommended CHEERS checklist for quality assessment in the updated period from 2015 to 2024.
Despite the potential for cost reduction, the quantitative documentation of overall costs, length of stay costs and antimicrobial costs varied considerably. Among the included studies, length of stay costs were less frequently documented than antimicrobial costs and overall savings. Dik et al. [11] emphasised the financial importance of reducing length of stay costs, noting that in addition to the financial benefit for a hospital resulting from the freed-up beds, there can also be a positive effect on patients by allowing them to leave hospital earlier.
Furthermore, it was challenging to ascertain the financial benefits of specific interventions, as so-called “service bundles” were frequently implemented. While the intervention “therapy evaluation, review and/or feedback” was the most prevalent intervention in our review, accounting for 23 of the 27 studies (85%), its actual cost-effectiveness could not be clearly determined, as in most cases it was combined with other interventions such as education or alteration of therapy guidelines. Van Dorst et al. [45] highlight the need to evaluate the economic impact of individual Antibiotic Stewardship Programme interventions. This is essential to facilitate comparisons and determine the most effective intervention in each setting. This may prove to be especially important in settings with limited resources and not only guide health policy stakeholders in the selection of Antibiotic Stewardship Programme interventions but also provide motivationfor their implementation.
As became evident when applying the CHEERS checklist, economic reporting quality is not consistent, preventing comparability of study results. The variations in the quality of the studies summarised in table 2 are principally due to discrepancies in adherence to methodological rigour. For instance, high-quality studies provided comprehensive justifications for the selection of economic models and contained clear information on discount rates and sensitivity analyses. Conversely, lower-quality studies frequently exhibited deficiencies in these aspects and demonstrated a lack of transparency in cost reporting and valuation approaches. In general, the absence and heterogeneity of crucial information on parameters such as study design, number of patients or beds, currency, and inflation or price adjustment posed a significant challenge during data synthesis and indicates that the improvements and recommendations proposed by Dik et al. [11] have not yet been widely implemented in the economic evaluation of Antibiotic Stewardship Programmes. In particular, the inclusion of all relevant cost types, including implementation, operating and societal costs, was considered in a few studies in our review. Our results are consistent with those reported by Dik et al. [11], which also found that only 11% of the included studies considered implementation costs, and none of them considered societal costs.
Recent literature supports this observation. Elshenawy et al. [46] identified that numerous studies lacked comprehensive consideration of all relevant costs, limiting their broader applicability. Similarly, Painter et al. [47] emphasised that the variability in cost calculation methods and the inconsistent reporting of implementation costs continue to hinder the comparability of Antibiotic Stewardship Programme studies. Nevertheless, it is evident that interventions require time, resources and specific equipment, which could represent a significant challenge for small hospitals. Stenehjem et al. [48] identified practical challenges that small hospitals face in implementing Antibiotic Stewardship Programmes and notes that these costs are often underreported, which affects the comparability of study results. In addition to insufficient funding, these challenges include limited access to infectious diseases specialists and difficulties in collecting and analysing data on antibiotic use due to inferior data infrastructure. Telemedical support, optimisation of resource allocation within healthcare networks and training of non-specialised healthcare professionals to assume leadership roles in Antibiotic Stewardship Programmes are potential approaches to meet these challenges. Furthermore, the CDC [49] emphasised the need for robust implementation strategies tailored to the capabilities of small and critical access hospitals.
Therefore, the need to improve the quality of economic evaluations of Antibiotic Stewardship Programmes persists. The implementation of simplified measures, including antibiotic time-outs, clinical algorithms and regional collaborations, has the potential to further facilitate the implementation of Antibiotic Stewardship Programmes in resource-limited settings [48]. These improvements are of paramount importance to ensure the comparability of different interventions, facilitate transferability of results to other settings and motivate both small and large hospitals to invest in Antibiotic Stewardship Programmes on their own.
Our study has several limitations. First and foremost, our review was limited to studies that explicitly categorised their economic evaluations as cost-benefit analyses, cost-effectiveness analyses, cost-consequence analyses, cost analyses or cost-minimisation analyses. Nevertheless, it is possible that other studies addressing costs and their consequences may also have been pertinent, but did not explicitly identify themselves as such economic analyses and were therefore not included in the present review. Second, it is possible that publication bias may have influenced the results: all the selected studies reported cost reductions and it may be that studies in which no effects on cost reduction could be demonstrated were not published. This has the potential to result in an overestimation of the cost-saving potential of Antibiotic Stewardship Programme interventions. Furthermore, the paucity of access to unpublished data makes it difficult to comprehensively assess the true extent of this cost effect. Thirdly, the considerable variability in study design, as well as the geographical representation of mainly high-income countries such as North America and Europe, may limit the generalisability of our results to low-resource settings. This emphasis on high-income settings has the potential to introduce a bias by overestimating the feasibility and cost-effectiveness of Antibiotic Stewardship Programmes in resource-limited contexts, where specific challenges, such as limited infrastructure and higher implementation costs, may be of critical importance. Fourthly, it is important to acknowledge the implications of the exclusion criteria, paediatric populations, studies not in English and long-term care facilities, on the generalisability and external validity of the results.
It is important to note that cost measures might be influenced by external factors, such as disease severity or events such as pandemics. Thus, the evaluation of costs does not allow for measuring the appropriateness of the interventions or clinical results. The cost calculations and timeframe of the intervention that led to the observed cost reductions were highly heterogeneous and not always clearly stated, which could affect the external validity of our results. Finally, the factor “loss to follow-up” in the original studies could be indirectly relevant for our systematic review as a significant loss of participants in the original studies could cause systematic bias and affect the robustness of the results. However, this potential bias is reflected in the quality analysis using the CHEERS checklist, which takes aspects such as inadequate reporting or methodological weaknesses into account.
In summary, our systematic review provides a comprehensive and transparent overview of the current state of knowledge and demonstrates that Antibiotic Stewardship Programmes represent, in addition to the known social benefits, a promising approach to reducing costs in acute care facilities. These findings have the potential to motivate both small and large hospitals to independently invest in the implementation of Antibiotic Stewardship Programmes. Nevertheless, there remain unanswered questions regarding the long-term impact on overall healthcare costs and the methods used to assess this impact. Further studies should aim to address these limitations by employing standardised economic evaluation methodologies, thus facilitating the comparability of research results and ensuring the attainment of more robust and generally valid outcomes. Furthermore, the reporting process should be transparent, and the long-term economic impact of Antibiotic Stewardship Programmes should be analysed comprehensively. This will further reinforce the evidence base for the economic evaluation of Antibiotic Stewardship Programmes and facilitate the formulation of well-informed decisions regarding the implementation of such programmes.
Datasets used and/or analysed in this study are available on request from the corresponding author.
Author contributions: JH, TD and SK were involved in the conception and design of the work. JH, TD, SK and AB were involved in study selection and data extraction. JH contributed as first author and drafted the original version of the manuscript. SK contributed as final author. TD, AB, PK and SPK critically reviewed and approved the final manuscript.
This review received no external funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Monroe S, Polk R. Antimicrobial use and bacterial resistance. Curr Opin Microbiol. 2000 Oct;3(5):496–501. doi: https://doi.org/10.1016/S1369-5274(00)00129-6
2. OECD. Embracing a One Health Framework to Fight Antimicrobial Resistance. 2023.
3. Fishman N. Antimicrobial stewardship. Am J Med. 2006 Jun;119(6 Suppl 1):S53–61. Discussion S62–70. doi: https://doi.org/10.1016/j.amjmed.2006.04.003
4. Lee CR, Cho IH, Jeong BC, Lee SH. Strategies to minimize antibiotic resistance. Int J Environ Res Public Health. 2013 Sep;10(9):4274–305. doi: https://doi.org/10.3390/ijerph10094274
5. Baur D, Gladstone BP, Burkert F, Carrara E, Foschi F, Döbele S, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017 Sep;17(9):990–1001. doi: https://doi.org/10.1016/S1473-3099(17)30325-0
6. CDC. Core Elements of Hospital Antibiotic Stewardship Programs. 2019. Available from: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
7. Boyles TH, Whitelaw A, Bamford C, Moodley M, Bonorchis K, Morris V, et al. Antibiotic stewardship ward rounds and a dedicated prescription chart reduce antibiotic consumption and pharmacy costs without affecting inpatient mortality or re-admission rates. PLoS One. 2013 Dec;8(12):e79747. doi: https://doi.org/10.1371/journal.pone.0079747
8. Nathwani D, Varghese D, Stephens J, Ansari W, Martin S, Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control. 2019 Feb;8(1):35. doi: https://doi.org/10.1186/s13756-019-0471-0
9. Akpan MR, Ahmad R, Shebl NA, Ashiru-Oredope D. A Review of Quality Measures for Assessing the Impact of Antimicrobial Stewardship Programs in Hospitals. Antibiotics (Basel). 2016 Jan;5(1):5. doi: https://doi.org/10.3390/antibiotics5010005
10. Coulter S, Merollini K, Roberts JA, Graves N, Halton K. The need for cost-effectiveness analyses of antimicrobial stewardship programmes: A structured review. Int J Antimicrob Agents. 2015 Aug;46(2):140–9. doi: https://doi.org/10.1016/j.ijantimicag.2015.04.007
11. Dik JW, Vemer P, Friedrich AW, Hendrix R, Lo-Ten-Foe JR, Sinha B, et al. Financial evaluations of antibiotic stewardship programs-a systematic review. Front Microbiol. 2015 Apr;6:317. doi: https://doi.org/10.3389/fmicb.2015.00317
12. Ajulo S, Awosile B. Global antimicrobial resistance and use surveillance system (GLASS 2022): investigating the relationship between antimicrobial resistance and antimicrobial consumption data across the participating countries. PLoS One. 2024 Feb;19(2):e0297921. doi: https://doi.org/10.1371/journal.pone.0297921
13. Mendelson M, Matsoso MP. The World Health Organization Global Action Plan for antimicrobial resistance. S Afr Med J. 2015 Apr;105(5):325. doi: https://doi.org/10.7196/SAMJ.9644
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar;372:n71. doi: https://doi.org/10.1136/bmj.n71
15. Michael F. Drummond, M.J.S., Karl Claxton, Greg L. Stoddart, and George W. Torrance, Methods for the Economic Evaluation of Health Care Programmes. 4. ed. 2015, The United States of America by Oxford University Press. 437.
16. Husereau D, Drummond M, Augustovski F, Briggs AH, Carswell C, Caulley L, et al.; CHEERS 2022 ISPOR Good Research Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BJOG. 2022 Feb;129(3):336–44. doi: https://doi.org/10.1111/1471-0528.17012
17. Degeling K, Vu M, Koffijberg H, Wong HL, Koopman M, Gibbs P, et al. Health Economic Models for Metastatic Colorectal Cancer: A Methodological Review. PharmacoEconomics. 2020 Jul;38(7):683–713. doi: https://doi.org/10.1007/s40273-020-00908-4
18. Abushanab D, Al-Marridi W, Al Hail M, Abdul Rouf PV, ElKassem W, Thomas B, et al. The cost associated with the development of the antimicrobial stewardship program in the adult general medicine setting in Qatar. J Pharm Policy Pract. 2024 Mar;17(1):2326382. doi: https://doi.org/10.1080/20523211.2024.2326382
19. Asiltürk D, Güner R, Kaya Kalem A, Özkoçak Turan I, Hasanoğlu I, Eser F, et al. Antibiotic management programme in a tertiary intensive care unit: effects of a carbapenem-restricted period on clinical and laboratory parameters and costs of infections. J Hosp Infect. 2024 Jun;148:87–94. doi: https://doi.org/10.1016/j.jhin.2024.03.006
20. Bastug A, Oksuz E, Kazancioglu S, Malhan S, Ozbay BO, Bodur H. Efficacy and cost-effectivity analysis of outpatient parenteral antimicrobial therapy unit in infectious disease clinical practices: turkey perspective. Int J Clin Pract. 2021 Jun;75(6):e14147. doi: https://doi.org/10.1111/ijcp.14147
21. Borde JP, Nussbaum S, Hauser S, Hehn P, Hübner J, Sitaru G, et al. Implementing an intensified antibiotic stewardship programme targeting daptomycin use in orthopaedic surgery: a cost-benefit analysis from the hospital perspective. Infection. 2016 Jun;44(3):301–7. doi: https://doi.org/10.1007/s15010-015-0854-y
22. Butt SZ, Ahmad M, Saeed H, Saleem Z, Javaid Z. Post-surgical antibiotic prophylaxis: impact of pharmacist’s educational intervention on appropriate use of antibiotics. J Infect Public Health. 2019;12(6):854–60. doi: https://doi.org/10.1016/j.jiph.2019.05.015
23. Dik JW, Hendrix R, Friedrich AW, Luttjeboer J, Panday PN, Wilting KR, et al. Cost-minimization model of a multidisciplinary antibiotic stewardship team based on a successful implementation on a urology ward of an academic hospital. PLoS One. 2015 May;10(5):e0126106. doi: https://doi.org/10.1371/journal.pone.0126106
24. Durojaiye OC, Bell H, Andrews D, Ntziora F, Cartwright K. Clinical efficacy, cost analysis and patient acceptability of outpatient parenteral antibiotic therapy (OPAT): a decade of Sheffield (UK) OPAT service. Int J Antimicrob Agents. 2018 Jan;51(1):26–32. doi: https://doi.org/10.1016/j.ijantimicag.2017.03.016
25. Howell CK, Jacob J, Mok S. Remote Antimicrobial Stewardship: A Solution for Meeting The Joint Commission Stewardship Standard? Hosp Pharm. 2019 Feb;54(1):51–6. doi: https://doi.org/10.1177/0018578718769240
26. Hyland SJ, Kusumi RK, Lopez LF, Kramer BJ, Fada RA, Mohan VS, et al. Antimicrobial Stewardship in Total Joint Arthroplasty: Outcomes of a Collaborative Program Implementation. J Am Acad Orthop Surg. 2022 Oct;30(20):e1327–36. doi: https://doi.org/10.5435/JAAOS-D-21-00722
27. Jaggar J, Cleveland KO, Twilla JD, Patterson S, Hobbs AL. Leveling Up: Evaluation of IV v. PO Linezolid Utilization and Cost after an Antimicrobial Stewardship Program Revision of IV to PO Conversion Criteria within a Healthcare System. Pharmacy (Basel). 2023 Apr;11(2):70. doi: https://doi.org/10.3390/pharmacy11020070
28. Karimaghaei S, Rao A, Chijioke J, Finch N, Nigo M. Characteristics, safety and cost-effectiveness analysis of self-administered outpatient parenteral antibiotic therapy via a disposable elastomeric continuous infusion pump at two county hospitals in Houston, Texas, United States. J Clin Pharm Ther. 2022 Feb;47(2):211–7. doi: https://doi.org/10.1111/jcpt.13550
29. Kim Y, et al.; Clinical Response and Hospital Costs of Therapeutic Drug Monitoring for Vancomycin in Elderly Patients. J Pers Med. 2022;12(2):163. doi: https://doi.org/10.3390/jpm12020163
30. Lanbeck P, Ragnarson Tennvall G, Resman F. A cost analysis of introducing an infectious disease specialist-guided antimicrobial stewardship in an area with relatively low prevalence of antimicrobial resistance. BMC Health Serv Res. 2016 Jul;16(1):311. doi: https://doi.org/10.1186/s12913-016-1565-5
31. Lester R, Haigh K, Wood A, MacPherson EE, Maheswaran H, Bogue P, et al. Sustained Reduction in Third-generation Cephalosporin Usage in Adult Inpatients Following Introduction of an Antimicrobial Stewardship Program in a Large, Urban Hospital in Malawi. Clin Infect Dis. 2020 Dec;71(9):e478–86. doi: https://doi.org/10.1093/cid/ciaa162
32. Loesch GH, Cruz JA, Gasparetto J, Oliveira DD, Telles JP, Tuon FF. Cost minimization analysis of outpatient parenteral/oral antibiotic therapy at a trauma hospital: public health system. Infect Control Hosp Epidemiol. 2021 Dec;42(12):1445–50. doi: https://doi.org/10.1017/ice.2021.22
33. Malone M, West D, Xuan W, Lau NS, Maley M, Dickson HG. Outcomes and cost minimisation associated with outpatient parenteral antimicrobial therapy (OPAT) for foot infections in people with diabetes. Diabetes Metab Res Rev. 2015 Sep;31(6):638–45. doi: https://doi.org/10.1002/dmrr.2651
34. Mouwen AM, Dijkstra JA, Jong E, Buijtels PC, Pasker-de Jong PC, Nagtegaal JE. Early switching of antibiotic therapy from intravenous to oral using a combination of education, pocket-sized cards and switch advice: A practical intervention resulting in reduced length of hospital stay. Int J Antimicrob Agents. 2020 Jan;55(1):105769. doi: https://doi.org/10.1016/j.ijantimicag.2019.07.020
35. Olson B, Ship N, Butera ML, Warm K, Oen R, Howard J. Clostridioides difficile infection in a skilled nursing facility (SNF): cost savings of an automated, standardized probiotic antimicrobial stewardship programme (ASP) policy. JAC Antimicrob Resist. 2023 Sep;5(5):dlad102. doi: https://doi.org/10.1093/jacamr/dlad102
36. Patel TS, Kaakeh R, Nagel JL, Newton DW, Stevenson JG. Cost Analysis of Implementing Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Plus Real-Time Antimicrobial Stewardship Intervention for Bloodstream Infections. J Clin Microbiol. 2016 Dec;55(1):60–7. doi: https://doi.org/10.1128/JCM.01452-16
37. Psaltikidis EM, Silva EN, Moretti ML, Trabasso P, Stucchi RS, Aoki FH, et al. Cost-utility analysis of outpatient parenteral antimicrobial therapy (OPAT) in the Brazilian national health system. Expert Rev Pharmacoecon Outcomes Res. 2019 Jun;19(3):341–52. doi: https://doi.org/10.1080/14737167.2019.1541404
38. Ramsey A, Mustafa SS, Holly AM, Staicu ML. Direct Challenges to Penicillin-Based Antibiotics in the Inpatient Setting. J Allergy Clin Immunol Pract. 2020;8(7):2294–301. doi: https://doi.org/10.1016/j.jaip.2020.02.033
39. Ross JL, Rankin S, Marshik P, Mercier RC, Brett M, Walraven CJ. Antimicrobial Stewardship Intervention and Feedback to Infectious Disease Specialists: A Case Study in High-Dose Daptomycin. Antibiotics (Basel). 2015 Jul;4(3):309–20. doi: https://doi.org/10.3390/antibiotics4030309
40. Ruh CA, Parameswaran GI, Wojciechowski AL, Mergenhagen KA. Outcomes and Pharmacoeconomic Analysis of a Home Intravenous Antibiotic Infusion Program in Veterans. Clin Ther. 2015 Nov;37(11):2527–35. doi: https://doi.org/10.1016/j.clinthera.2015.09.009
41. Salman B, Al-Hashar A, Al-Khirbash A, Al-Zakwani I; Clinical and Cost Implications of Clinical Pharmacist Interventions on Antimicrobial Use at Sultan Qaboos University Hospital in Oman. Clinical and Cost Implications of Clinical Pharmacist Interventions on Antimicrobial Use at Sultan Qaboos University Hospital in Oman. Int J Infect Dis. 2021 Aug;109:137–41. doi: https://doi.org/10.1016/j.ijid.2021.07.002
42. van Daalen FV, Opmeer BC, Prins JM, Geerlings SE, Hulscher ME. The economic evaluation of an antibiotic checklist as antimicrobial stewardship intervention. J Antimicrob Chemother. 2017 Nov;72(11):3213–21. doi: https://doi.org/10.1093/jac/dkx259
43. Wang J, Dong M, Lu Y, Zhao X, Li X, Wen A. Impact of pharmacist interventions on rational prophylactic antibiotic use and cost saving in elective cesarean section. Int J Clin Pharmacol Ther. 2015 Aug;53(8):605–15. doi: https://doi.org/10.5414/CP202334
44. Yadav K, Kumar S, Chhabra S, Rosenberg H, Eagles D, Suh KN, et al. Outpatient parenteral antibiotic therapy (OPAT) and inpatient treatment strategies for emergency department patients with cellulitis: a cost analysis. CJEM. 2022 Aug;24(5):520–8. doi: https://doi.org/10.1007/s43678-022-00320-1
45. van Dorst PW, van der Pol S, Salami O, Dittrich S, Olliaro P, Postma M, et al. Evaluations of training and education interventions for improved infectious disease management in low-income and middle-income countries: a systematic literature review. BMJ Open. 2022 Feb;12(2):e053832. doi: https://doi.org/10.1136/bmjopen-2021-053832
46. Elshenawy RA, Umaru N, Alharbi AB, Aslanpour Z. Antimicrobial stewardship implementation before and during the COVID-19 pandemic in the acute care settings: a systematic review. BMC Public Health. 2023 Feb;23(1):309. doi: https://doi.org/10.1186/s12889-023-15072-5
47. Painter C, Faradiba D, Chavarina KK, Sari EN, Teerawattananon Y, Aluzaite K, et al. A systematic literature review of economic evaluation studies of interventions impacting antimicrobial resistance. Antimicrob Resist Infect Control. 2023 Jul;12(1):69. doi: https://doi.org/10.1186/s13756-023-01265-5
48. Stenehjem E, Hyun DY, Septimus E, Yu KC, Meyer M, Raj D, et al. Antibiotic Stewardship in Small Hospitals: Barriers and Potential Solutions. Clin Infect Dis. 2017 Aug;65(4):691–6. doi: https://doi.org/10.1093/cid/cix407
49. CDC. Implementation of Antibiotic Stewardship Core Elements at Small and Critical Access Hospitals. 2023. Available from: https://www.cdc.gov/antibiotic-use/core-elements/small-critical.html
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.4217.