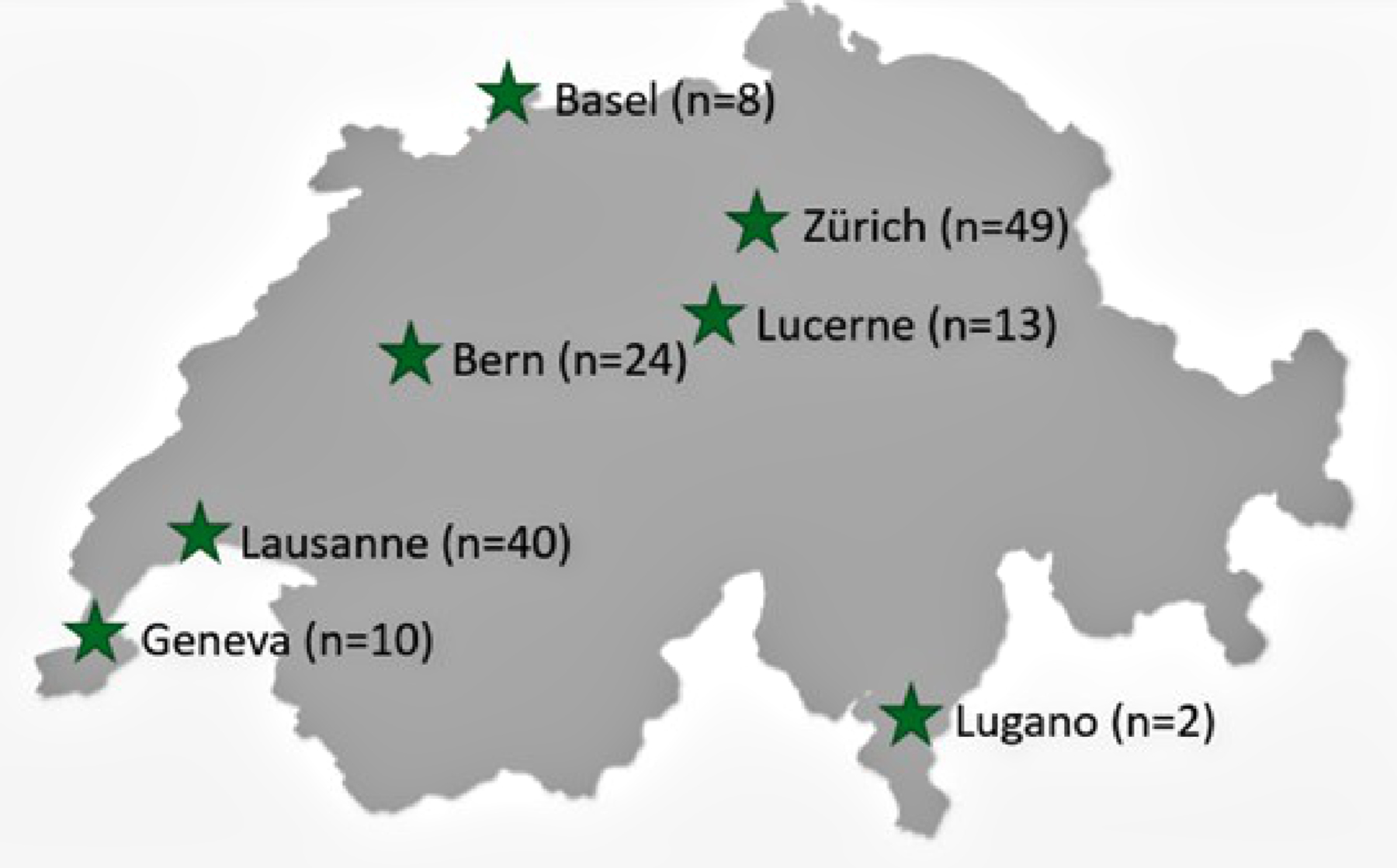
Figure 1The number of patients included during the registry’s first year.
DOI: https://doi.org/https://doi.org/10.57187/s.4193
Bilevel positive airway pressure
Continuous positive airway pressure
Swiss Paediatric Home Respiratory Support
Home respiratory support was first implemented during the poliomyelitis epidemic in the 1950s and has since been constantly evolving. While modern devices with continuous positive pressure were developed in the 1980s for adult patients [1], it was not until the 2000s that these devices began to be used regularly for paediatric patients. Therefore, the use of home respiratory support for children is relatively recent but has increased dramatically over the last two decades in developed countries [2–6]. This increase can be explained by several factors: improvements in sleep-disordered breathing (SDB) diagnosis, the broadening spectrum of indications for home respiratory support in children, the prolonged survival of patients with neuro-muscular diseases, the expansion of home healthcare services and technical innovations in medical devices. Today, home respiratory support is recognised as an effective treatment for sleep-disordered breathing and chronic respiratory failure in children [7].
Home respiratory support includes two main modes, bilevel positive airway pressure (BPAP) and continuous positive airway pressure (CPAP), delivered to the patient either non-invasively via a facial interface or invasively via a tracheostomy. In addition, a high-flow nasal cannula (HFNC), primarily used in neonatal or acute care, has been proposed for long-term home respiratory support in specific paediatric cases [8]; however, there is no clear recommendation yet for this indication. While home respiratory support is now regularly used for children, its technology and medical use are continually developing, and dedicated care networks are constantly evolving. No national paediatric recommendations are currently available, and the European Respiratory Society published the first international statement in 2021 [9]. This statement established a framework for caring for these patients and highlighted the lack of scientific evidence and the need for studies focused on the paediatric population. In Switzerland, the current prevalence of home respiratory support in children is unknown, given that no national data have been released since the survey published in 2001 [10].
In the field of rare diseases and conditions, studies are asking for prospective registries, as they are efficient tools to assess the prevalence and longitudinal evolution and to improve organisation and standards of care at an individual, local and national level by improving knowledge about the patients and their needs [11, 12]. Therefore, in 2019, the Swiss Society of Paediatric Pulmonology appointed paediatric experts to create a special group of interest in home respiratory support to improve collaborations and knowledge. In 2022, this special group created a prospective national registry: Swiss Paediatric Home Respiratory Support (SwissPedHRS).
This article aims to present the methodology of the SwissPedHRS registry and describe the current paediatric population with home respiratory support in Switzerland.
SwissPedHRS is an observational prospective national registry of children and adolescents with home respiratory support in Switzerland. The primary objectives of the registry are (a) to improve knowledge and understanding of the paediatric population with home respiratory support, (b) to support the establishment of Swiss standards of care to homogenise practices over the country, (c) to increase the visibility of this population in the Swiss healthcare system and determine its specific needs, and (d) to help identify patients who could be offered the opportunity to participate in national or international research projects.
More specifically, in the registry’s first year, the objectives were to determine the prevalence of home respiratory support in children in Switzerland and describe the population characteristics.
The inclusion criteria for the SwissPedHRS registry are:
The Swiss Society of Paediatric Pulmonology’s special interest group dedicated to paediatric home respiratory support asked all its members to identify centres caring for children with home respiratory support in Switzerland. The following seven centres were identified and designated as inclusion centres for the registry: Basel, Bern, Geneva, Lausanne, Lugano, Lucerne and Zurich. As the community of paediatric pulmonologists in Switzerland is relatively small and communication within this group excellent, we could ascertain that no child with home respiratory support was followed up in a paediatric pulmonology and/or sleep medicine unit outside the above-mentioned centres.
Physicians identified eligible patients and recruited them during routine medical consultations. The physicians informed eligible patients and their caregivers and collected informed consent from the parents and, if appropriate, the patients during their consultations. The study information and consent forms were available in four languages: French, German, Italian and English.
The patients were followed according to the local standard of care. Data were retrieved from local hospital information systems. A ‘baseline dataset’ was collected at inclusion, and an ‘annual update dataset’ will be collected yearly until the patients turn 18 or up to one year after weaning of home respiratory support. The collected data are detailed in table 1.
Table 1Description of the data collected in the Swiss Paediatric Home Respiratory Support (SwissPedHRS) registry.
| Inclusion dataset | Follow-up dataset | ||
| Informed consent | Date | x | |
| Eligibility | Eligibility criteria | x | |
| Demographics | Month and year of birth, age at consent, sex | x | |
| Medical history | Underlying pathology | x | |
| Initiation | Age at initiation | x | |
| Indication | |||
| Context of initiation | |||
| Decisive test | |||
| Place of initiation | |||
| Home respiratory support status | Is the patient still using home respiratory support? | x | |
| Monitoring | Clinical: weight, height, body mass index | x | x |
| Home respiratory support follow-up over the last 12 months: number of medical controls, home care follow-up, analysis of data from respiratory device software, self-monitoring material | |||
| Home respiratory support compliance | |||
| ENT (ear, nose and throat) follow-up or intervention over the last 12 months | |||
| Maxillofacial follow-up or intervention over the last 12 months | |||
| Orthodontic follow-up or intervention over the last 12 months | |||
| Spine intervention over the last 12 months | |||
| Equipment | Home respiratory support type, device, interface | x | x |
| Additional oxygen | |||
| Use of instrumental help for airway clearance | |||
| Sleep tests | Number of sleep tests conducted over the last 12 months, type of test, main results. | x | x |
| Exacerbations | Number of respiratory exacerbations over the last 12 months, duration, place of management, need for antibiotics, need for additional instrumental airway clearance, need for home respiratory support adaptation | x | x |
| Complications | Pneumothorax, skin problems, bronchoaspiration, digestive complications, discomfort, other | x | x |
Data were collected at each centre by a dedicated person identified by the local project leader regarding its own local organisation (i.e. research team member, doctor, physiotherapist or nurse). These persons were identified and given personal access to the SwissPedHRS RedCap database to allow them to manually enter data directly into the national database.
Data were centralised by the Paediatric Clinical Research Team of Lausanne, the lead centre, and manually checked for completeness, plausibility and consistency. Ambiguities were investigated in collaboration with local investigators during data entry or after the manual quality check. The Research Board, including the national project leader and each centre’s local leader, coordinated the data’s use, analysis and publication.
Local ethics committees approved this project (Kantonale Ethikkommission Bern, Ethikkommission Nordwest und Zentralschweiz, Commission Cantonal d’éthique de la recherche de Genève, Comitato etico cantonale Ticino and Kantonale Ethikkommission Zurich), with final approval by the Lausanne ethical committee in March 2022 (Commission cantonale d’éthique de la recherche sur l’être humain du canton de Vaud [CER-VD], Project ID 2021-01105).
After one year of inclusions (April 2022 to March 2023), data were extracted to describe the cohort of children currently using long-term home respiratory support. At this point, some children with home respiratory support had refused to participate in the registry (refusals) or had been identified as eligible but not yet included by March 2023 (missing). The estimated prevalence of children with home respiratory support in Switzerland was determined as follows: (children included in the registry + refusals + missing) / national population aged <18 years in Switzerland as of 31 December 2022, according to the Swiss Federal Agency of Statistics.
The following data were selected from the SwissPedHRS registry to describe the paediatric population on home respiratory support in Switzerland: sex; age at inclusion; underlying pathology; age, place, and context of home respiratory support initiation; decisive test leading to home respiratory support initiation; breathing disorder mechanism; and type of home respiratory support. The data were analysed using GraphPad Prism (version 10.2.2). Continuous data are reported as medians with ranges, and categorical data are reported as numbers with percentages.
During the registry’s first year, 146 patients were included, with a median (range) age of 10.6 years (0.3–17.9), of whom 86 (59%) were boys and 60 (41%) were girls (figure 1). Eight patients refused to participate in the registry, and 35 had not yet been included at the time of data extraction, increasing the total population of children with home respiratory support in Switzerland to 189. According to the national population data (8,815,385 inhabitants, including 1,583,228 aged <18 years), the prevalence of children with home respiratory support in Switzerland was calculated at 11.9 per 100,000 children (95% confidence interval: 10.2–13.5), equivalent to 2.1 per 100,000 inhabitants.

Figure 1The number of patients included during the registry’s first year.
The patients presented a large spectrum of underlying medical conditions, the most common being related to neuro-muscular and central nervous system diseases (figure 2). Breathing disorder mechanisms leading to home respiratory support were upper airway obstruction (n = 52, 36%), pump failure (n = 44, 30%), central breathing control anomaly (n = 17, 12%), lower airway obstruction (n = 12, 8%) or mixed (n = 21, 14%).
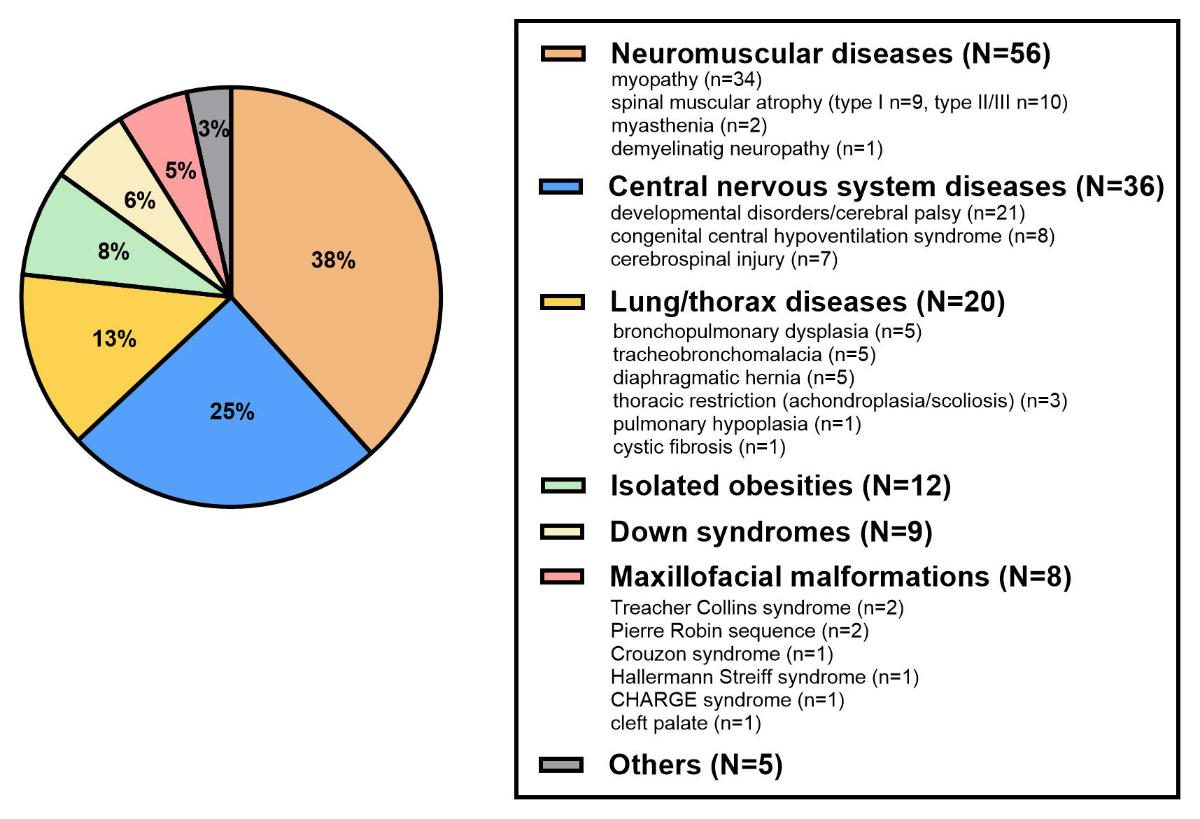
Figure 2The underlying medical conditions (no missing data).
The median (range) age at home respiratory support initiation was 6.5 years (0.0–16.5; figure 3). There was a peak of incidence in the first year of life (n = 35), including congenital central hypoventilation syndrome (CCHS; n = 5), congenital myopathy (n = 5), severe neurological impairment (n = 5), bronchopulmonary dysplasia (n = 4), diaphragmatic hernia (n = 4), craniofacial malformations (n = 3), VACTERL association (Vertebral, Anorectal, Cardiovascular, Tracheal, Esophageal, Renal, Limb) (n = 3), spinal muscular atrophy (SMA; n = 2), thoracic restrictions (n = 2), Down syndrome (n = 1) and tracheomalacia (n = 1).
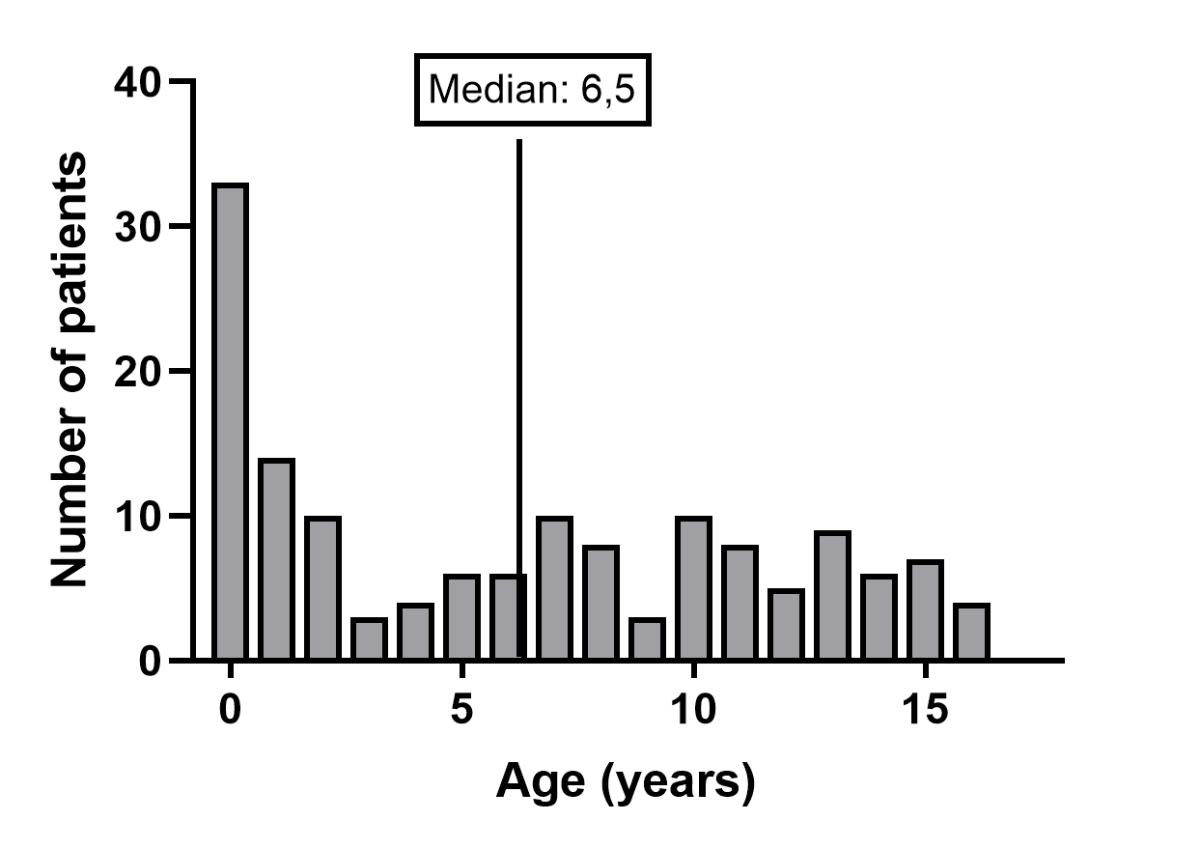
Figure 3Age at home respiratory support initiation (no missing data).
Various investigations were reported as the decisive test for home respiratory support indication (figure 4). The main diagnostic test results are presented in table 2. The decisive test was polysomnography or polygraphy (with or without capnography) in 60% of the patients, increasing to 82% in the elective context.
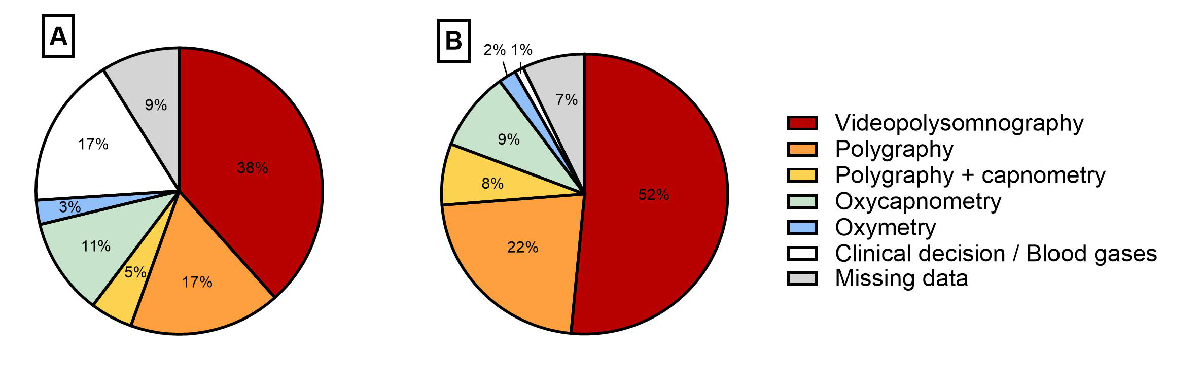
Figure 4Decisive tests for home respiratory support initiation for (A) all and (B) elective initiations.
Table 2The main results of decisive tests for home respiratory support initiation.
| Mean SpO2 (%) | Time with SpO2 <90% (%) | Mean TcPCO2 (mm Hg) | Time with TcPCO2 >50 mm Hg (%) | Apnoea hypopnoea index (/h) | |
| n = 103 | n = 101 | n = 72 | n = 62 | n = 86 | |
| Median | 94.9% | 3.8% | 45.75 | 2.2% | 19.35 |
| Min | 81.0% | 0% | 33.75 | 0% | 0 |
| Max | 100% | 94% | 75.76 | 100% | 184.40 |
SpO2: peripheral oxygen saturation; TcPCO2: transcutaneous carbon dioxide pressure.
Home respiratory support was predominantly non-invasive (n = 125, 86%), and BPAP was the most common mode in non-invasive home respiratory support (n = 79, 63%; figure 5). The population with non-invasive BPAP comprised patients with neuromuscular diseases (n = 48), central nervous system diseases (n = 16), lung/thorax diseases (n = 8), maxillofacial malformations (n = 4), Down syndromes (n = 2) and isolated obesity (n = 1). The population with non-invasive CPAP or high flow nasal cannula comprised patients with central nervous system diseases (n = 15, mainly neurodevelopmental disorders), isolated obesities (n = 11), Down syndrome (n = 7), lung/thorax diseases (n = 4), maxillofacial malformations (n = 4), neuromuscular diseases (n = 1) and other diagnoses (n = 4).
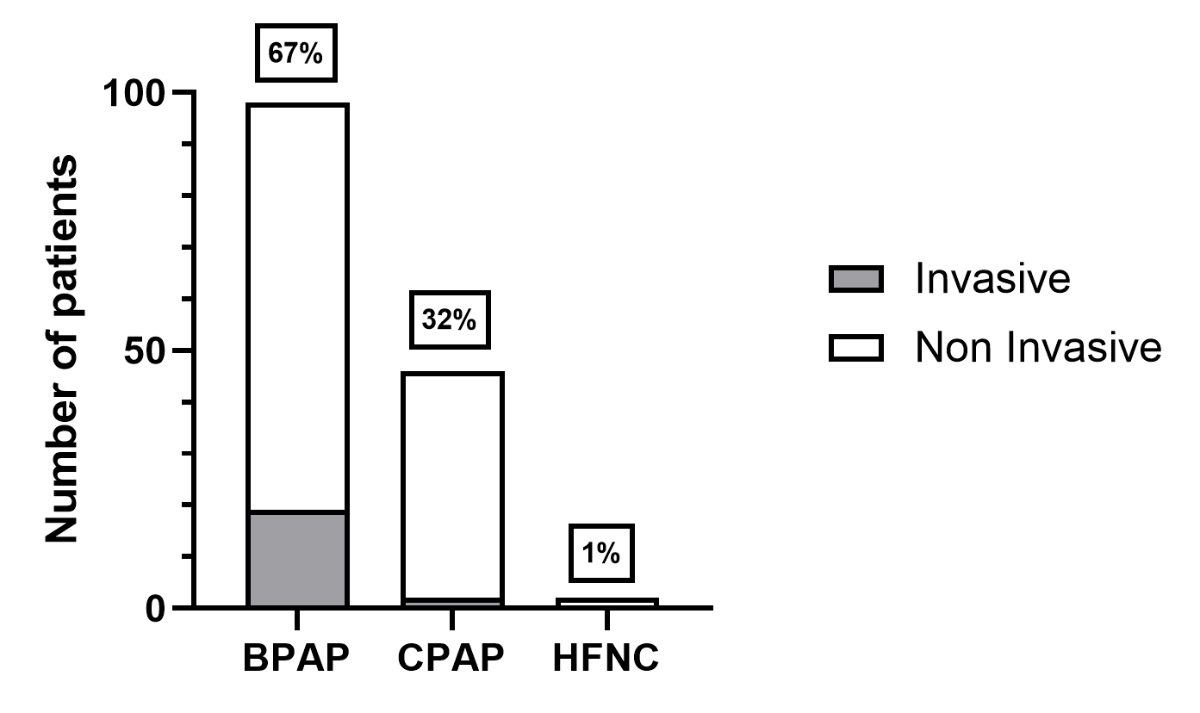
Figure 5The type of home respiratory support (no missing data). BPAP: bilevel positive airway pressure; CPAP: continuous positive airway pressure; HFNC: high-flow nasal cannula.
Only 21 patients (14%) required invasive BPAP (n = 19) or CPAP (n = 2) via tracheostomy (figure 5). The patients requiring BPAP via tracheostomy had neuromuscular diseases (n = 7), lung/thorax diseases (n = 7) and central nervous system diseases (n = 4). The two patients requiring CPAP via tracheostomy had bronchopulmonary dysplasia and tracheomalacia in the context of oesophageal atresia.
Details about underlying medical conditions by home respiratory support type and mode are presented in table 3.
Table 3Underlying medical conditions by type and mode of home respiratory support.
| Invasive (n = 21) | Non-Invasive (n = 125) | All (n = 146) | |||||
| BPAP (n = 19) | CPAP (n = 2) | BPAP (n = 79) | CPAP (n = 44) | High flow nasal cannula (n = 2) | |||
| Neuromuscular diseases | 7 | 0 | 48 | 1 | 0 | 56 | |
| Myopathy | 5 | 0 | 28 | 1 | 0 | 34 | |
| Spinal muscular atrophy | 0 | 0 | 19 | 0 | 0 | 19 | |
| Myasthenia | 2 | 0 | 0 | 0 | 0 | 2 | |
| Demyelinating neuropathy | 0 | 0 | 1 | 0 | 0 | 1 | |
| Central nervous system diseases | 4 | 0 | 16 | 15 | 2 | 36 | |
| Developmental disorders/cerebral palsy | 0 | 0 | 6 | 12 | 2 | 21 | |
| Congenital central hypoventilation syndrome | 2 | 0 | 6 | 0 | 0 | 8 | |
| Cerebrospinal injury | 2 | 0 | 4 | 1 | 0 | 7 | |
| Lung/thorax diseases | 7 | 2 | 8 | 4 | 0 | 20 | |
| Bronchopulmonary dysplasia | 3 | 1 | 1 | 0 | 0 | 5 | |
| Tracheobronchomalacia | 0 | 1 | 2 | 1 | 0 | 4 | |
| Diaphragmatic hernia | 3 | 0 | 2 | 1 | 0 | 6 | |
| Thoracic restriction (achondroplasia/scoliosis) | 1 | 0 | 1 | 1 | 0 | 3 | |
| Pulmonary hypoplasia | 0 | 0 | 1 | 0 | 0 | 1 | |
| Cystic fibrosis | 0 | 0 | 1 | 0 | 0 | 1 | |
| Isolated obesities | 0 | 0 | 1 | 11 | 0 | 12 | |
| Down syndrome | 0 | 0 | 2 | 7 | 0 | 9 | |
| Maxillofacial malformations | 0 | 0 | 4 | 4 | 0 | 8 | |
| Treacher Collins syndrome | 0 | 0 | 1 | 1 | 0 | 2 | |
| Pierre Robin sequence | 0 | 0 | 1 | 1 | 0 | 2 | |
| Crouzon syndrome | 0 | 0 | 1 | 0 | 0 | 1 | |
| Hallermann-Streiff syndrome | 0 | 0 | 1 | 0 | 0 | 1 | |
| CHARGE syndrome | 0 | 0 | 0 | 1 | 0 | 1 | |
| Cleft palate | 0 | 0 | 0 | 1 | 0 | 1 | |
| Others | 1 | 0 | 0 | 4 | 0 | 5 | |
BPAP: bilevel positive airway pressure; CPAP: continuous positive airway pressure.
Home respiratory support was initiated electively in 99 (68%) patients, with the proportion increasing with age and greater for CPAP/high flow nasal cannula than BPAP (figure 6). Non-elective initiations (n = 47, 32%) were related to the failure of respiratory support weaning after an acute respiratory exacerbation (n = 23), neonatal hospitalisation (n = 19) or surgery (n = 5). Home respiratory support was initiated in an outpatient clinic for 28 (19%) patients, exclusively in elective situations, and during hospitalisation in an intensive or intermediate care unit for 95 (66%) patients and in a standard care unit for 21 (15%) patients (figure 7).
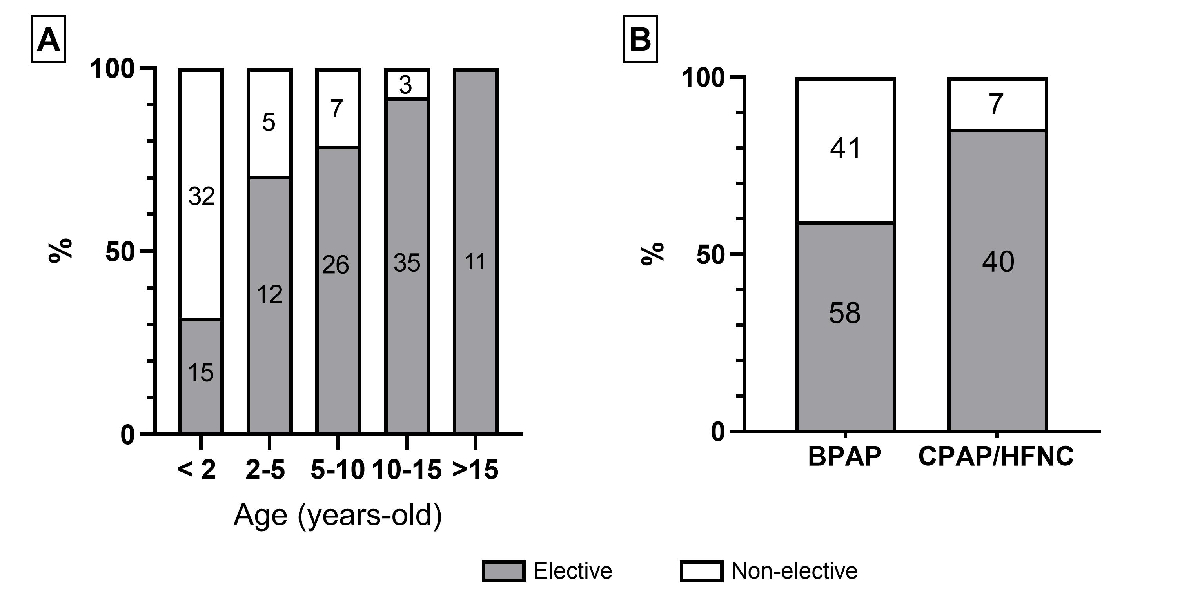
Figure 6Elective versus non-elective context for home respiratory support (HRS) initiation by (A) age at HRS initiation and (B) HRS type (no missing data). The numbers in column boxes are the absolute numbers of patients for each category.
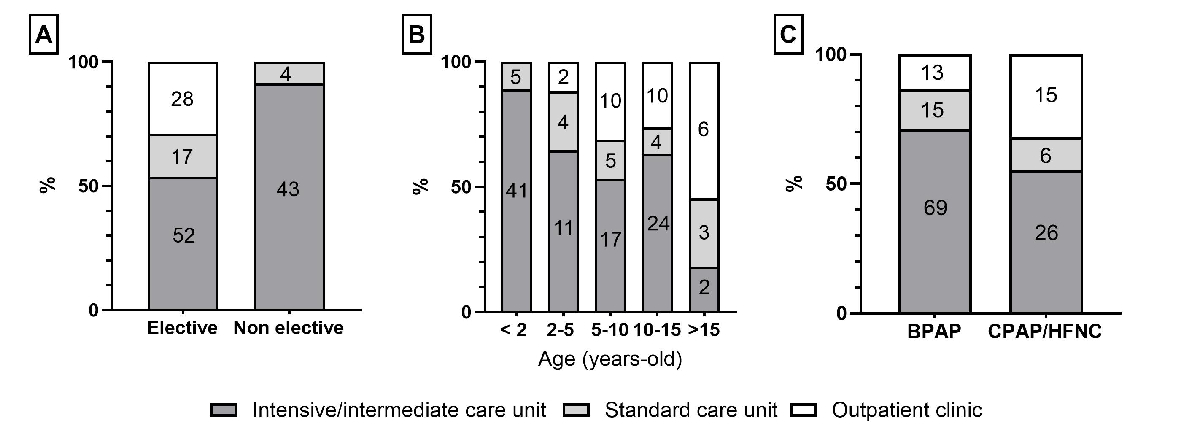
Figure 7The place of home respiratory support (HRS) initiation by (A) context of HRS initiation, (B) age at HRS initiation and (C) HRS type. The numbers in column boxes are the absolute numbers of patients for each category. Missing data: n = 2. BPAP: bilevel positive airway pressure; CPAP: continuous positive airway pressure; HFNC: high-flow nasal cannula.
Swiss Paediatric Home Respiratory Support (SwissPedHRS) is the first national prospective registry dedicated to children with long-term home respiratory support. Its design provides national data on the population of children with home respiratory support and will enable prospective monitoring as it evolves.
In Switzerland, the last data on children with home respiratory support was published in 2001 by Kamm et al., who interviewed all paediatric centres caring for children with home respiratory support [10]. If restricted to the SwissPedHRS inclusion criteria, this survey reported 29 patients, mostly affected by CCHS (n = 11, 38%) and neuromuscular diseases (n = 12, 41%). All home respiratory support was via bilevel positive airway pressure (BPAP), including 10 (34%) on invasive ventilation via tracheostomy. Compared to this ‘historical cohort’ of Kamm et al., our study on the SwissPedHRS cohort shows three main changes:
In addition to the longitudinal comparison with the Kamm et al. survey, we searched for recently published equivalent cohorts from other countries. Very few of the identified cohorts were national. We mostly used French and UK cohorts for comparison, as they were published recently (2021 and 2023, respectively) and included comparable populations (strictly paediatric, equivalent care systems and income levels) [4, 5]. Both cohorts reported data obtained in 2019 via questionnaires completed by physicians involved in paediatric home respiratory support. The French cohort did not include patients with invasive ventilation, whereas the UK cohort did, and neither considered patients using high-flow nasal cannulas. To be exhaustive about paediatric national cohorts, we can include an Austrian cohort published in 2016 in a German language journal [13]. Unlike our cohort, these three cohorts used cross-sectional surveys without, to our knowledge, prospective follow-up.
The prevalence of children with home respiratory support in Switzerland is comparable to other countries. With no information about their completeness, the national surveys in France and United Kingdom reported 1447 and 2383 patients, respectively, corresponding to an estimated national prevalence of 2.1 and 3.6/100,000 inhabitants, respectively [4, 5]. Our results confirm a significant increase in prevalence over recent decades in Switzerland, which increased from 0.4/100,000 inhabitants in 2001 (estimated based on the Kamm et al. survey [10]) to 2.1/100,000 in 2023. This important increase in prevalence has also been observed in other countries. In France and the United Kingdom, the prevalence of children with home respiratory support increased 14- and 16-fold over 20 years, respectively [4, 5]. This increase can be explained by several factors, particularlythe increasing availability of paediatric equipment and the broadening of medical conditions screened for sleep-disordered breathing. Indeed, the current population includes patients with a broad spectrum of pathologies, whereas the Kamm et al. survey in 2001 mainly reported children with neuromuscular diseases and central congenital hypoventilation syndrome [10]. This difference might be due, at least in part, to the increase of obstructive sleep apnoea syndrome (OSAS) screening in children following the dedicated European Respiratory Society recommendations, especially in cases of obesity, whether isolated or syndromic [14]. It can also be partly explained by advances in comfort care practices, leading to more frequent use of home respiratory support in palliative situations and for patients with severe disabilities [15, 16]. Finally, social factors might also have contributed to the increased use of home respiratory support, such as the pressure to reduce hospital stay lengths and the development of homecare services, leading to the home management of patients with multiple morbidities.
To characterise sleep-disordered breathing before elective home respiratory support initiation, most patients in our registry (86%) underwent polygraphy or polysomnography, in line with recommendations for sleep-disordered breathing investigations [9, 14]. Specific groups of patients, such as those with neuromuscular diseases, seem to be more likely screened using oxycapnometry, often used in this population to regularly monitor nocturnal gas exchange to detect the onset of hypoventilation as early as possible. In-depth studies of the tests performed, stratified by disease group, will be conducted in the future once the SwissPedHRS registry includes sufficient patients per disease to allow such analyses. We found a high variation in test results in our cohort, which could be explained by patients’ heterogeneity, underlying medical conditions and breathing disorder mechanisms. However, it also illustrates that sleep test interpretation is not only based on numerical results but also on respiratory curves and/or gas exchange trends. Clinical evaluation is also important and should be combined with sleep test results when deciding to initiate home respiratory support.
The home respiratory support type and mode have evolved in Switzerland since 2001. The proportion of patients on invasive ventilation decreased from 34% in 2001 to 14% in 2023. This decrease was also reported in the UK cohort [4]. It reveals not only changes in the type of patients using home respiratory support but also in our practices with, for example, more patients with CCHS treated with non-invasive ventilation than before. This evolution has been largely supported by the increasing availability of specific devices and interfaces for infants and young children.
Regarding home respiratory support mode, all patients used a BPAP in 2001, whereas in our current cohort from the SwissPedHRS registry, only two-thirds used a BPAP. This change may be due to the evolution of home respiratory support indications over time, marked by the increased use of CPAP for upper airway obstruction over recent decades (no patients in 2001 to 43 [29%] in 2023), partly explained by the increasing prevalence of childhood obesity. Interestingly, the French cohort reported in 2019 had an even lower proportion of BPAP (55%) [5], while non-national cohorts reported highly variable proportions from 25% in a multicentric retrospective Canadian cohort in 2018 [2] to 87% in a monocentric retrospective English cohort in 2015 [17]. While there is no clear explanation for this discrepancy between national cohorts, it may be explained by differences in sleep-disordered breathing screening, health insurance policies, device availability or delays to specific care (i.e. curative surgery for obstructive sleep apnoea syndrome). Regarding non-national cohorts, especially monocentric cohorts, differences could be explained by differences in the type of patients (e.g. a specific rare condition could be more common in a given centre specialised in this condition), different care networks and different backgrounds of home respiratory support teams (i.e. intensive care, pulmonology or sleep medicine).
While a high-flow nasal cannula may not currently be an established mode for home respiratory support, we chose to include patients with a high-flow nasal cannula in the SwissPedHRS registry as it can be proposed as an alternative to CPAP, notably in cases of CPAP failure due to behavioural issues [18]. As this device was not designed to be used at home, a high-flow nasal cannula is unsuitable for this purpose (e.g. no data on the exact pressure delivered nor information about residual apnoeas and few alarms). However, it can be better tolerated than CPAP by some patients, and studies suggest an equal efficacy in treating obstructive sleep apnoea syndrome, such as in children with obesity [19]. In Switzerland, high-flow nasal cannula use at home is still marginal in 2023, with only two patients in our cohort. We cannot compare our cohort to previously reported cohorts as none included this device as a long-term home respiratory support. Through the SwissPedHRS registry, we can prospectively study the evolution of high-flow nasal cannula use at home.
In our cohort, all ages are represented at home respiratory support initiation, but with an important peak in the first year of life. This pattern was also described in a recent meta-analysis on children with long-term mechanical ventilation [6]. It can be explained by the important proportion of congenital conditions that lead to home respiratory support. Interestingly, the SwissPedHRS registry includes only two patients with spinal muscular amyotrophy type 1 among those with early initiation. With the recent marketing of disease-modifying therapies such as nusinersen, risdiplam, and onasemnogene, which dramatically improve the life expectancy of patients with spinal muscular amyotrophy type 1, we anticipate that this population will increasingly be ventilated in the first months of life [20].
In our cohort, home respiratory support initiation occurred in an elective setting for 68% of the patients, significantly fewer than the 92% and 83% reported in the national French cohort in 2019 and the non-national Canadian (Alberta) cohort in 2018, respectively [2, 5]. As recommended in the ERS statement [9], home respiratory support should be initiated in an elective setting whenever possible, given that it leads to a better psychological experience, which provides better chances for good adherence and allows better exploration of sleep-disordered breathing, helping to optimise the choice of home respiratory support device and mode. Therefore, the relatively low rate of elective initiation in the SwissPedHRS registry should lead us to reflect on our practice in Switzerland, promoting early initiation in children with progressive disease and foreseeable respiratory failure. Furthermore, an elective setting allows home respiratory support initiation in outpatient clinics instead of hospitals. In our cohort, 28% of the elective initiations were done in outpatient clinics, whereas this rate reached 82% in the Canadian (Alberta) cohort [2]. There are currently no clear recommendations on the criteria that should guide the choice of setting for home respiratory support initiation (home, outpatient clinic, or hospital), even in the recent ERS statement [9]. Each team has its own criteria based on factors such as age, underlying disease, type and severity of sleep-disordered breathing, home respiratory support type and mode, risk of poor tolerance or complications (i.e. broncho-aspiration), family resources and access to homecare services. In our cohort, home respiratory support in older patients and with CPAP was more likely to be initiated in outpatient clinics, but with important variability between centres due to different teams’ habits and local resources.
The main limitation of these first data from the SwissPedHRS registry is their incompleteness. At the time of extraction, 35 additional patients with home respiratory support had been identified in Switzerland but not yet included in the registry. When the registry was designed, we planned the first data extraction after one year of inclusions. We decided to keep to this schedule despite the delay in inclusions, which led to incomplete inclusions. In addition, for practical reasons, we assumed in the registry design that all children and adolescents with home respiratory support were currently followed up in paediatric centres, and we decided not to ask adult pulmonologists to participate in inclusions in the registry. However, this decision may have compounded the incompleteness, as we cannot discount that some adolescents with simple pathologies such as obstructive sleep apnoea syndrome are followed up by adult teams and were not included in the registry. In the future, strategies will be developed to identify and include potential adolescents with home respiratory support who are followed up by adult teams to improve the completeness of our data.
Finally, the SwissPedHRS registry has enabled us to pool the experiences of all Swiss centres caring for children with home respiratory support, which is crucial to improving and standardising their care in Switzerland. Thanks to its prospective design, the SwissPedHRS registry is and will remain for years a crucial tool for improving our knowledge and, thus, our care of this population. Our further objectives are to monitor the population demography and to study specific aspects such as compliance and side effects of home respiratory support, risk factors and management of respiratory exacerbations and home respiratory support burden of care in paediatrics.
Pseudo-anonymised data can be made available to other teams for research projects. Researchers interested in collaborative work can contact the SwissPedHRS team via the corresponding author to discuss their projects. Per the registry rules, the Research Board of SwissPedHRS will make the final decision on collaboration.
The authors thank all the patients and their families for participating in the registry.
This work was supported by the Lausanne hub of the Swiss Research Network of Clinical Paediatric Hubs (SwissPedNet). SwissPedHRS funding sources: Swiss Lung Association and Swiss Society of Paediatric Pulmonology. The Swiss Paediatric Pulmonology Society provided funding in December 2020 to support the registry’s creation and ethical committee’s fees. In January 2022, the Swiss Lung Association provided funding to cover the costs of the first two years of the registry.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Hind M, Polkey MI, Simonds AK. AJRCCM: 100-Year Anniversary. Homeward Bound: A Centenary of Home Mechanical Ventilation. Am J Respir Crit Care Med. 2017 May;195(9):1140–9. doi: https://doi.org/10.1164/rccm.201702-0285CI
2. Castro-Codesal ML, Dehaan K, Bedi PK, Bendiak GN, Schmalz L, Katz SL, et al. Longitudinal changes in clinical characteristics and outcomes for children using long-term non-invasive ventilation. PLoS One. 2018 Jan;13(1):e0192111. doi: https://doi.org/10.1371/journal.pone.0192111
3. Amin R, Sayal P, Syed F, Chaves A, Moraes TJ, MacLusky I. Pediatric long-term home mechanical ventilation: twenty years of follow-up from one Canadian center. Pediatr Pulmonol. 2014 Aug;49(8):816–24. doi: https://doi.org/10.1002/ppul.22868
4. Barker N, Sinha A, Jesson C, Doctor T, Narayan O, Elphick HE. Changes in UK paediatric long-term ventilation practice over 10 years. Arch Dis Child. 2023 Mar;108(3):218–24. doi: https://doi.org/10.1136/archdischild-2021-323562
5. Fauroux B, Khirani S, Amaddeo A, Massenavette B, Bierme P, Taytard J, et al. Paediatric long term continuous positive airway pressure and noninvasive ventilation in France: A cross-sectional study. Respir Med. 2021 May;181:106388. doi: https://doi.org/10.1016/j.rmed.2021.106388
6. Toussaint M, van Hove O, Leduc D, Ansay L, Deconinck N, Fauroux B, et al. Invasive versus non-invasive paediatric home mechanical ventilation: review of the international evolution over the past 24 years. Thorax. 16 feb 2024;thorax-2023-220888. doi: https://doi.org/10.1136/thorax-2023-220888
7. Castro-Codesal ML, Dehaan K, Featherstone R, Bedi PK, Martinez Carrasco C, Katz SL, et al. Long-term non-invasive ventilation therapies in children: A scoping review. Sleep Med Rev. 2018 Feb;37:148–58. doi: https://doi.org/10.1016/j.smrv.2017.02.005
8. Ignatiuk D, Schaer B, McGinley B. High flow nasal cannula treatment for obstructive sleep apnea in infants and young children. Pediatr Pulmonol. 2020 Oct;55(10):2791–8. doi: https://doi.org/10.1002/ppul.25009
9. Fauroux B, Abel F, Amaddeo A, Bignamini E, Chan E, Corel L, et al. ERS Statement on pediatric long term noninvasive respiratory support. Eur Respir J. 2021 Dec;16:2101404.
10. Kamm M, Burger R, Rimensberger P, Knoblauch A, Hammer J. Survey of children supported by long-term mechanical ventilation in Switzerland. Swiss Med Wkly. 2001 May;131(19-20):261–6. doi: https://doi.org/10.4414/smw.2001.09733
11. Ardura-Garcia C, Goutaki M, Carr SB, Crowley S, Halbeisen FS, Nielsen KG, et al. Registries and collaborative studies for primary ciliary dyskinesia in Europe. ERJ Open Res. 2020 May;6(2):00005–02020. doi: https://doi.org/10.1183/23120541.00005-2020
12. Boulanger V, Schlemmer M, Rossov S, Seebald A, Gavin P. Establishing Patient Registries for Rare Diseases: rationale and Challenges. Pharmaceut Med. 2020 Jun;34(3):185–90. doi: https://doi.org/10.1007/s40290-020-00332-1
13. Weiss S, Van Egmond-Fröhlich A, Hofer N, Pfleger A, Rath R, Schwarz R, et al. Long-Term Respiratory Support for Children and Adolescents in Austria: A National Survey. Klin Padiatr. 2016 Jan;228(1):42–6.
14. Kaditis AG, Alonso Alvarez ML, Boudewyns A, Alexopoulos EI, Ersu R, Joosten K, et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. Eur Respir J. 2016 Jan;47(1):69–94. doi: https://doi.org/10.1183/13993003.00385-2015
15. Grychtol R, Chan EY. Use of non-invasive ventilation in cerebral palsy. Arch Dis Child. 2018 Dec;103(12):1170–7. doi: https://doi.org/10.1136/archdischild-2017-313959
16. Rahman M, Jeffreys J, Massie J. A narrative review of the experience and decision-making for children on home mechanical ventilation. J Paediatr Child Health. 2021 Jun;57(6):791–6. doi: https://doi.org/10.1111/jpc.15506
17. Chatwin M, Tan HL, Bush A, Rosenthal M, Simonds AK. Long term non-invasive ventilation in children: impact on survival and transition to adult care. PLoS One. 2015 May;10(5):e0125839. doi: https://doi.org/10.1371/journal.pone.0125839
18. Amaddeo A, Khirani S, Frapin A, Teng T, Griffon L, Fauroux B. High-flow nasal cannula for children not compliant with continuous positive airway pressure. Sleep Med. 2019 Nov;63:24–8. doi: https://doi.org/10.1016/j.sleep.2019.05.012
19. Fishman H, Al-Shamli N, Sunkonkit K, Maguire B, Selvadurai S, Baker A, et al. Heated humidified high flow nasal cannula therapy in children with obstructive sleep apnea: A randomized cross-over trial. Sleep Med. 2023 Jul;107:81–8. doi: https://doi.org/10.1016/j.sleep.2023.04.017
20. Fitzgerald DA, Doumit M, Abel F. Changing respiratory expectations with the new disease trajectory of nusinersen treated spinal muscular atrophy [SMA] type 1. Paediatr Respir Rev. 2018 Sep;28:11–7.