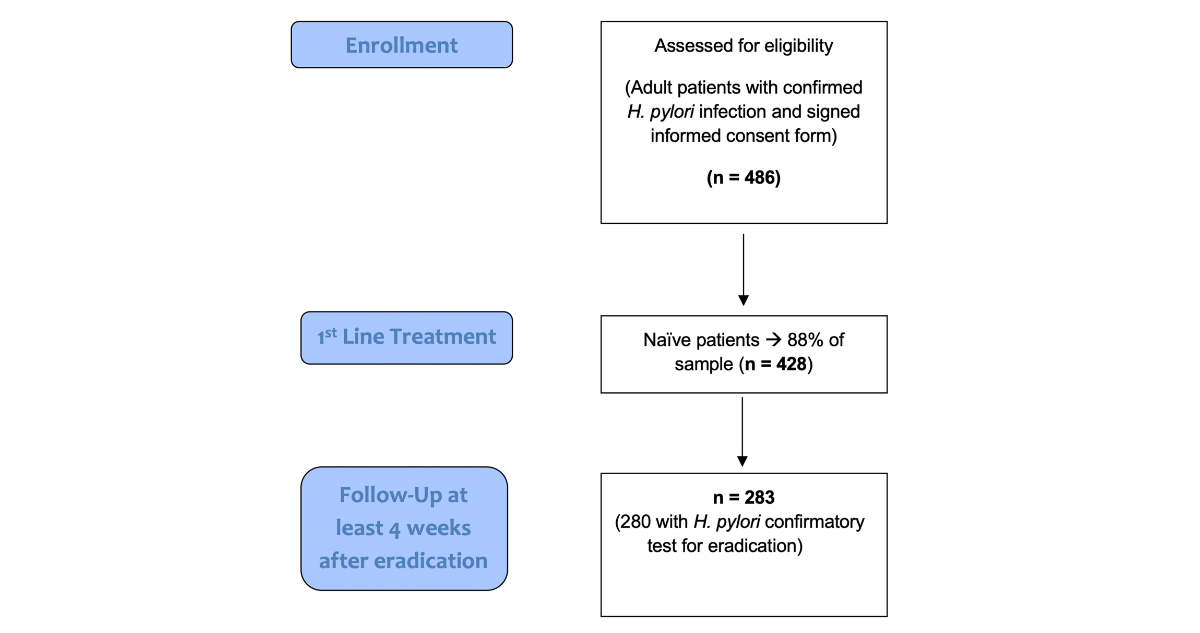
Figure 1Flow chart with selection algorithm.
DOI: https://doi.org/https://doi.org/10.57187/s.4191
Helicobacter pylori is a Gram-negative, microaerophilic bacterium with a flagellated, helical shape that resides primarily in the human stomach [1, 2]. Despite worldwide endeavours to improve diagnosis and achieve eradication, it still infects about 43% of the global population, according to estimates from a recent meta-analysis [3].
Helicobacter pylori infection has been linked aetiologically with peptic ulcer disease and gastric cancer, namely gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma [4, 5]. The World Health Organization officially classified Helicobacter pylori as a definite (or Class I) carcinogen in 1994. This classification was reviewed in 2012 and 2019, with the conclusion that the evidence continues to support its unchanged status as a Class I carcinogen [5]. The importance of Warren and Marshall’s discovery of Helicobacter pylori is highlighted by their receipt of the Nobel Prize in Physiology or Medicine in 2005 [6].
Beyond the well-substantiated causality linking gastroduodenal ulcers and cancer, strong evidence associates Helicobacter pylori infection with idiopathic (immune) thrombocytopenic purpura, as well as iron and vitamin B12 deficiency [4, 5]. Additionally, many other extragastric manifestations have been reported with variable grades of evidence [4, 5].
The above-mentioned factors underscore the importance of successful treatment for Helicobacter pylori infection. Furthermore, antibiotic resistance has increased considerably worldwide, and in Europe, the prevalence of clarithromycin resistance has reportedly doubled in the last twenty years [7]. Migration may be contributing to this increase – a systematic review and meta-analysis [8] reported that refugees and asylum seekers may affect the increase in antibiotic resistance in Europe. In Switzerland, a 2017 study reported that this migrant population (primarily individuals from Afghanistan, Syria, and Eritrea) was colonised up to ten times more frequently with resistant bacterial strains compared to the Swiss population [9].
According to numerous recommendations, including the recent Maastricht VI-Florence consensus, the optimal eradication rate of Helicobacter pylori is above 90%, an arbitrary but clinically reasonable threshold [7, 10–12]. However, clinicians face several challenges with Helicobacter pylori eradication. Firstly, several treatment regimens encompassing different drugs are used globally, and the accessibility of treatments and drugs varies by geographical region and country; therefore, no regimen is universally accepted as optimal. Additionally, although Helicobacter pylori prevalence is decreasing worldwide, antimicrobial resistance is increasing. Clinical trial evidence may not always represent real-world clinical practice, as inclusion criteria are restrictive, and patient care time and follow-up are limited. Long-term studies are warranted to collect data over time, identify trends, and evaluate treatment strategies and health outcomes. These real-time data, gathered at local, regional, and global levels, can help generate hypotheses for further research and gather and interpret epidemiological data [13].
In this context, the European Registry on Helicobacter pylori Management (Hp-EuReg) brings together information on the real clinical practice of European gastroenterologists, encompassing over 70,000 patients recruited to date from 38 countries, with variable bacterial resistance prevalence patterns and therapy accessibility [14]. While this analysis was not pre-specified in the original protocol, it aligns with the registry’s overarching objective to periodically analyse national-level data, identify trends, and provide timely insights into clinical outcomes.
In Switzerland, Helicobacter pylori infection has a low prevalence of around 25%. Nevertheless, existing studies show a high degree of discrepancy, with reported prevalence ranging from 9 to 73%. This phenomenon may be explained by increasing migration [1]. Furthermore, little scientific evidence is available regarding the empirical treatment for Helicobacter pylori eradication.
Therefore, this interim analysis evaluated the effectiveness and safety of first-line empirical treatment regimens for Helicobacter pylori eradication in Switzerland using data from Hp-EuReg.
The European Registry on Helicobacter pylori Management (Hp-EuReg) is an ongoing international, European, multicentre, prospective, non-interventional registry that has collected information on Helicobacter pylori infection management since May 2013 [13].
The Hp-EuReg protocol was approved by the Ethics Committee of La Princesa University Hospital (Madrid, Spain), which acted as a reference Institutional Review Board (20 December 2012). It was conducted according to the guidelines of the Declaration of Helsinki, was classified by the Spanish Drug and Health Product Agency, and was prospectively registered at Clinical Trials.gov under the code NCT02328131. It was promoted by the European Helicobacter and Microbiota Study Group (www.helicobacter.org). Comprehensive information regarding this study has been previously published in the protocol [14, 15].
The current study is an interim sub-analysis focusing on a Swiss cohort of adult patients with Helicobacter pylori infections, collected from the Hp-EuReg. The study was approved by the Swiss ethics committee (BASEC 2021-01334).
Recruiting centres were selected by the national coordinator (Michael Doulberis) for Switzerland based on their clinical activity and adherence to eligibility criteria, which included the involvement of gastroenterologists managing adult patients with confirmed Helicobacter pylori infection and obtaining signed informed consent. Selection also required access to diagnostic and eradication confirmation tests. In Switzerland, a total of nine gastroenterology centres participated, with clinicians systematically recording Helicobacter pylori-related routine clinical practice data in the electronic case report form (e-CRF).
Criteria for country selection, national coordinators, and gastroenterologists acting as recruiting investigators are reported in the protocol [14]. The main criterion for the eligible investigators was that they were gastroenterologists managing patients with Helicobacter pylori infection. All adult patients diagnosed with Helicobacter pylori infection and receiving empirical eradication therapy were included. The main outcome – confirmed eradication – required a valid confirmatory test (e.g. a urea breath test or stool antigen test) at least four weeks after treatment. Cases without follow-up confirmatory test results were excluded from the effectiveness analysis, resulting in a reduced cohort for statistical evaluation.
Data were recorded in an e-CRF using a web-based application, REDCap (Research Electronic Data Capture), a platform managed and hosted by the Asociación Española de Gastroenterología (AEG; www.aegastro.es), a non-profit scientific and medical society that focuses primarily on gastroenterological research. Patient demographic information, previous eradication attempts, treatment algorithms used, and outcomes (cure rates, compliance, adverse events, and follow-up) were recorded. All patient data were anonymised. The main outcome was confirmed eradication at least four weeks after treatment.
After the data extraction, the database was reviewed for inconsistencies and underwent subsequent data cleaning. The data quality review process involved evaluating whether the study selection criteria had been met and whether the data had been collected correctly, ensuring that the study was conducted according to the highest scientific and ethical standards. Data discordances were resolved by querying the investigators and through group emailing. Before statistical analysis, the Hp-EuReg Scientific Director ensured coherence, data quality, and scientific integrity.
For the purpose of the current study, all patient records registered up to December 2023 and treated with first-line empirical therapy were included in the analysis.
Reporting of this study was conducted according to STROBE guidelines [16].
Continuous variables are summarised as the median and interquartile range (IQR), as the data did not follow a normal distribution, whereas qualitative variables are presented as absolute and relative frequencies, displayed as percentages (%). Fisherʼs exact test and the χ2 test were used where applicable. Statistical analysis was performed using IBM SPSS Statistics for PC (Version 25.0, Armonk, NY, USA) and Microsoft Office Excel 365 (Redmond, WA, USA). The selected level of statistical significance was set at p <0.05 (two-tailed).
Effectiveness was assessed using a modified intention-to-treat (mITT) analysis, including all patients with a valid follow-up test at least four weeks after treatment, regardless of compliance. Patients without a confirmatory test result were excluded from this analysis.
Compliance was defined as taking at least 90% of the prescribed medications. This was assessed through patient self-reporting and/or clinician evaluation.
Adverse events were documented as any unintended medical occurrences during treatment and were classified according to severity as mild, moderate, or severe. Serious adverse events were defined as those resulting in hospitalisation, permanent disability, or death.
The primary outcome was treatment effectiveness, measured as the proportion of patients with confirmed Helicobacter pylori eradication among those prescribed first-line therapy. Secondary outcomes included the incidence and severity of adverse events as well as compliance rates.
Regarding laboratory methods, Helicobacter pylori infection was diagnosed prior to treatment using validated techniques, such as histology (with or without immunohistochemistry), urea breath tests, stool antigen tests, or rapid urease tests. The choice of diagnostic method was determined by local clinical practices. Eradication was confirmed at least four weeks after treatment using either a urea breath test or a stool antigen test; all laboratories adhered to standardised protocols to ensure accuracy and validity.
Treatment length was categorised into three groups corresponding to the most frequent treatment durations: 7, 10, or 14 days. Proton pump inhibitor (PPI) doses were stratified based on acid inhibition potency, as defined previously [17, 18]: low dose (4.5–27 mg omeprazole equivalents bis in die [bid], i.e. 20 mg omeprazole equivalents bid), standard dose (32–40 mg omeprazole equivalents bid, i.e. 40 mg omeprazole equivalents bid), and high dose (ranging from 54–128 mg omeprazole equivalents bid, i.e. 60 mg omeprazole equivalents bid).
A total of 486 adult patients diagnosed with Helicobacter pylori infection were initially identified. Of these, 428 (88%) were treatment-naïve (figure 1). Second-line prescriptions were given to 44 patients (9.1%), third-line treatments were administered in 11 cases (2.3%), and fewer patients received fourth-line therapy. Male patients represented almost half of the population (n = 210, 49%), and the median age was 51 years. Most patients were Caucasian (n = 375, 88%), and the most common indication for Helicobacter pylori status investigation was non-investigated dyspepsia (n = 228, 54%). The most common diagnostic modality for pre-treatment Helicobacter pylori status was histology (with/without accompanying immunohistochemistry; n = 349, 81.5%). Penicillin allergy was reported in 14 patients (3.3%). A total of 98 patients (23%) received concomitant medication. Acetylsalicylic acid was administered in 13 patients (3%). Further demographic information is presented in table 1.

Figure 1Flow chart with selection algorithm.
Table 1Baseline characteristics of treatment-naïve patients in Switzerland (n = 428). The results were considered statistically significant at a level of p <0.05.
| Variables | Patients | p-value | |
| Gender | Male | 210 (49.1%) | |
| Female | 218 (50.9%) | ||
| Median age (IQR): 51 years (23) | |||
| Ethnic background | Caucasian | 375 (87.6%) | <0.001 |
| Black | 13 (3.0%) | ||
| Asian | 12 (2.8%) | ||
| Other | 22 (5.1%) | ||
| Unknown/not available | 6 (1.4%) | ||
| Penicillin allergy | 14 (3.3%) | ||
| Diagnostic method (pre-treatment) | <0.001 | ||
| Rapid urease test | 290 (67.8%) | ||
| Histology | 349 (81.5%) | ||
| Stool antigen | 3 (0.7%) | ||
| Culture | 0 (0%) | ||
| Urea breath test | 14 (3.3%) | ||
| Serology | 1 (0.2%) | ||
| Indication | <0.001 | ||
| Non-investigated dyspepsia | 228 (53.3%) | ||
| Dyspepsia with normal endoscopy | 61 (14.3%) | ||
| Gastric ulcer | 25 (5.8%) | ||
| Unexplained iron deficiency anaemia | 19 (4.4%) | ||
| Duodenal ulcer | 13 (3.0%) | ||
| Rest | 82 (19.1%) | ||
| Concomitant medication | Proton pump inhibitors (PPI) | 38 (8.9%) | |
| Acetylsalicylic acid | 13 (3%) | ||
| Non-steroidal anti-inflammatory drugs (NSAIDs) | 11 (2.6%) | ||
| Statins | 19 (4.4%) | ||
IQR: interquartile range.
Two first-line regimens accounted for over 90% of cases (amoxicillin-clarithromycin triple therapy, 49% and single-capsule bismuth 42%). Further notable schemes included quadruple therapy with amoxicillin-clarithromycin-metronidazole (n = 11, 2.6%) and sequential therapy using the same drug combination (n = 8, 1.9%). A triple regimen with clarithromycin and levofloxacin was prescribed in seven cases (1.6%).
Regarding the duration of eradication schemes, over half of the cases underwent 10-day treatment (230, 54%), and 157 patients (37%) underwent 14-day treatment. Regarding proton pump inhibitor potency, almost 70% of the prescriptions included a low-dose proton pump inhibitor. High-dosage proton pump inhibitors were prescribed to 123 patients (29%) (table 2).
Table 2First-line prescriptions in Switzerland (n = 428). The results were considered statistically significant at a level of p <0.05.
| Variable | Patients | p-value | |
| Regimen | Triple clarithromycin + amoxicillin | 206 (48.5%) | <0.001 |
| Bismuth quadruple* | 179 (42.1%) | ||
| Quadruple clarithromycin + amoxicillin + metronidazole | 11 (2.6%) | ||
| Sequential clarithromycin + amoxicillin + metronidazole | 8 (1.9%) | ||
| Triple clarithromycin + levofloxacin | 7 (1.6%) | ||
| Quadruple-metronidazole + tetracycline + bismuth salts | 6 (1.4%) | ||
| Triple clarithromycin + metronidazole | 2 (0.5%) | ||
| Quadruple metronidazole + tetracycline | 2 (0.5%) | ||
| Triple metronidazole + tetracycline | 1 (0.2%) | ||
| Triple amoxicillin + metronidazole | 1 (0.2%) | ||
| Sequential clarithromycin + metronidazole + levofloxacin | 1 (0.2%) | ||
| Dual clarithromycin + amoxicillin | 1 (0.2%) | ||
| Duration | 10 days | 230 (54.1%) | <0.001 |
| 14 days | 157 (36.9%) | ||
| 7 days | 38 (8.9%) | ||
| PPI potency | Low | 291 (69.1%) | <0.001 |
| High | 123 (29.2%) | ||
| Standard | 7 (1.7%) | ||
* Bismuth quadruple therapy as a single capsule containing tetracycline, metronidazole, and a bismuth salt. Proton pump inhibitor (PPI) potency was categorised as follows: low dose (4.5–27 mg omeprazole equivalents bis in die [bid], i.e. 20 mg omeprazole equivalents bid), standard dose (32–40 mg omeprazole equivalents bid, i.e. 40 mg omeprazole equivalents bid), and high-dose (54–128 mg omeprazole equivalents bid, i.e. 60 mg omeprazole equivalents bid).
A total of 283 patients with available follow-up data were evaluated for effectiveness, adverse events, and compliance. The overall first-line treatment effectiveness (per modified intention-to-treat) was 92% (table 3), achieving 91% in the low-dose proton pump inhibitor group (i.e. 20 mg omeprazole equivalent twice daily) and 96% in the high-dose group (i.e. 80 mg omeprazole equivalent twice daily). The lowest effectiveness (82%, 28/34 cases) was reported for 7-day amoxicillin-clarithromycin triple therapy, and the highest (97%, 100/103 cases) was observed with 10-day single-capsule bismuth quadruple therapy containing metronidazole, tetracycline, and bismuth. The overall effectiveness of the amoxicillin-clarithromycin triple therapy scheme was 87% (126/145).
Table 3Effectiveness by modified-intention-to-treat of first-line prescription regimens in Switzerland (n = 283). No statistically significant differences were observed between the different schemes (p >0.05).
| Scheme* | Patients | 95% CI |
| Triple clarithromycin + levofloxacin | 3/3 (100%) | 29–100 |
| Triple clarithromycin + amoxicillin | 126/145 (86.9%) | 81–93 |
| Sequential clarithromycin + amoxicillin + metronidazole | 8/8 (100%) | 63–100 |
| Quadruple metronidazole + tetracycline + bismuth | 6/6 (100%) | 54–100 |
| Quadruple clarithromycin + amoxicillin + metronidazole | 11/11 (100%) | 72–100 |
| Single-capsule bismuth** | 100/103 (97.1%) | 92–99 |
| Overall cure rate | 261/283 (92.2%) | 89–96 |
* Schemes with only one patient/case have been omitted.
** Bismuth quadruple therapy as a single capsule containing tetracycline, metronidazole, and a bismuth salt.
Regarding the influence of proton pump inhibitor potency and effectiveness, the effectiveness of high-dose proton pump inhibitors ranged from 83% for clarithromycin-amoxicillin triple therapy (10/12) to 100% for quadruple therapy (metronidazole-tetracycline-bismuth, 6/6) and clarithromycin-amoxicillin-metronidazole (11/11). In the low-dose proton pump inhibitor group, the main regimen was the amoxicillin-clarithromycin scheme, with an effectiveness reaching 87% (114/137). Further relevant information is presented in table 4.
Table 4First-line therapy effectiveness by modified intention-to-treat analysis according to therapy duration and proton pump inhibitor (PPI) dose (n = 283).
| Parameter | Scheme* | Patients | 95% CI** | |
| Duration | 7 days | Triple levofloxacin + amoxicillin | 28/34 (82.4%) | 65–93 |
| 10 days | Triple levofloxacin + amoxicillin | 23/25 (92%) | 74–99) | |
| Sequential levofloxacin + amoxicillin + metronidazole | 8/8 (100%) | 63–100 | ||
| Quadruple metronidazole + tetracycline + bismuth salts | 2/2 (100%) | 16–100 | ||
| Quadruple levofloxacin + amoxicillin + metronidazole | 9/9 (100%) | 66–100 | ||
| Single-capsule bismuth*** | 99/102 (97.1%) | 92–99 | ||
| 14 days | Triple levofloxacin + levofloxacin | 3/3 (100%) | 29–100 | |
| Triple levofloxacin + amoxicillin | 75/86 (87.2%) | 80–95 | ||
| Quadruple metronidazole + tetracycline + bismuth salts | 4/4 (100%) | 40–100 | ||
| Quadruple levofloxacin + amoxicillin + metronidazole | 2/2 (100%) | 16–100 | ||
| PPI inhibition | Low | Triple levofloxacin + levofloxacin | 3/3 (100%) | 29–100 |
| Triple levofloxacin + amoxicillin | 114/131 (87%) | 81–93 | ||
| Sequential levofloxacin + amoxicillin + metronidazole | 8/8 (100%) | 63–100 | ||
| Single-capsule bismuth* | 48/49 (98%) | 89–100 | ||
| Standard | Single-capsule bismuth* | 2/2 (100%) | 16–100 | |
| High | Triple levofloxacin + amoxicillin | 10/12 (83.3%) | 52–98 | |
| Quadruple metronidazole + tetracycline + bismuth salts | 6/6 (100%) | 54–100 | ||
| Quadruple levofloxacin + amoxicillin + metronidazole | 11/11 (100%) | 72–100 | ||
| Single capsule bismuth* | 49/50 (98%) | 89–100 | ||
| Overall cure rate | 261/283 (92.2%) | 89–96 | ||
* Schemes with only one patient/case have been omitted.
** No statistically significant differences were observed between different treatment lengths and the PPI dosages (p >0.05). PPI potency was categorised as follows: low dose (4.5–27 mg omeprazole equivalents bis in die [bid], i.e. 20 mg omeprazole equivalents bid), standard dose (32–40 mg omeprazole equivalents bid, i.e. 40 mg omeprazole equivalents bid), and high dose (54–128 mg omeprazole equivalents bid, i.e. 60 mg omeprazole equivalents bid).
*** Bismuth quadruple therapy as a single capsule containing tetracycline, metronidazole, and a bismuth salt.
Regarding safety and compliance, the eradication treatments were well tolerated (table 5), and no serious adverse events were reported. The overall incidence of at least one adverse event was 8.5% (24/283). The following adverse events were reported in some cases: dysgeusia (n = 1), diarrhoea (n = 6), nausea (n = 6), and dyspepsia (n = 3). Regarding compliance, 99% (280/283) of patients adhered to treatment.
Table 5Safety of first-line empirical treatment (n = 283).
| Adverse event | Cases |
| Nausea | 6 (1.4%) |
| Vomits | 0 |
| Dyspepsia | 3 (0.7%) |
| Heartburn | 1 (0.2%) |
| Abdominal pain | 4 (0.8%) |
| Asthenia | 2 (0.4%) |
| Dysgeusia/metallic taste | 1 (0.2%) |
| Diarrhoea | 6 (1.4%) |
| Anorexia | 0 (0%) |
| Others | 13 (3%) |
| Serious adverse event* | 0 |
| Treatment cessation due to adverse events | 5 (1.1%) |
| Overall incidence of adverse events | 24 (8.5%) |
* Serious adverse events were defined as those leading to patient hospitalisation, disability, or death (or birth defects in pregnant patients).
This is the first (interim) study using Swiss Hp-EuReg-derived data, and it drew meaningful conclusions. The first-line empirical treatment yielded a satisfactory overall effectiveness of 92%, exceeding the optimal arbitrary but clinically acceptable 90% threshold. Comparable effectiveness was reported in the analysis encompassing all Hp-EuReg-participating European countries [15], as well as in local, national-level analyses from neighbouring countries.
The most frequently prescribed regimens were single-capsule bismuth quadruple therapy (Pylera®), given to almost half of the patients (42%), and amoxicillin-clarithromycin triple therapy (49%).
An additional noteworthy finding was the high proportion of cases (nearly 70%) prescribed low-dose proton pump inhibitors alongside eradication therapy. Theoretically, such a decision by the attending gastroenterologists may lead to suboptimal eradication rates, which is why the Maastricht VI recommendations endorse high-dose regimens. In particular, the guidelines underscore that using high‐dose proton pump inhibitors twice daily enhances the efficacy of triple therapy. However, they also state that it is unclear whether twice daily high‐dose proton pump inhibitors improve the efficacy of quadruple therapies. This was also discussed in a previous Italian study from the Hp-EuReg [12, 19]. Nevertheless, a satisfactory overall effectiveness was achieved in our setting.
Regarding the issue of clarithromycin resistance prevalence in Europe, no antibiogram data were available in the studied cohort, and thus the current study focused on assessing empirical therapy only. However, in a 2023 study conducted in Switzerland, Braendli et al. investigated the effectiveness of clarithromycin-based versus non-clarithromycin-based eradication treatments among 608 patients. They reported that the so-called “French” scheme (clarithromycin, amoxicillin, and a proton pump inhibitor) was the most common (71%), and no difference was observed between clarithromycin-based treatments and non-clarithromycin-based treatments (both yielded a success rate of 71%) [20]. Moreover, a recent systematic review and meta-analysis shed light on worldwide clarithromycin resistance, revealing that Switzerland was among the countries with the highest estimated clarithromycin resistance for Helicobacter pylori infection (67%) [21, 22].
Regarding treatment tolerance, the overall incidence of at least one adverse event was 8.5% (24/283), and none of the recruited patients experienced serious events. In comparison, a recent publication from Hp-EuReg, encompassing real-world data from 22,000 European patients, reported an adverse event incidence of 23% [23]. The adverse event incidence in the current Swiss study was notably lower. The interim nature of the analysis and the potential underreporting of mild adverse events may partially explain this discrepancy. While bismuth-containing regimens are generally considered less well-tolerated, the high use of these regimens in our cohort (42%) did not translate into a higher incidence of adverse events. This could reflect better-than-anticipated patient tolerance in routine clinical settings or variations in reporting practices. Additionally, the reliance on physician- and patient-reported data highlights the potential for underestimation of adverse events, particularly for subjective symptoms such as nausea or dyspepsia. Further analyses with larger datasets may help clarify this observation.
Despite these interesting findings, our study also has several limitations. First and foremost, a relatively small number of patients was included in this interim analysis, providing a limited sample size for the effectiveness analyses by treatment duration and proton pump inhibitor dosage. A concerning finding was that of 428 treatment-naïve patients registered, only 280 (65% of the total cohort) had confirmatory Helicobacter pylori eradication test results, reducing the sample size for the effectiveness and safety analysis. Patients with missing information either had no routine eradication control or were lost to follow-up. A possible explanation for this bias in the Swiss arm of the Hp-EuReg is that eradication confirmation is typically performed via stool antigen testing during a visit to the general practitioner. It is possible that these patients were correctly assessed after treatment but were lost to follow-up by the attending gastroenterologist who initially prescribed the therapy and thus were not entered into AEG-REDCap.
In the future, a much larger number of included centres and therefore patients is anticipated. Furthermore, this was not a randomised controlled trial (RCT), so any comparison of effectiveness among different regimens should be conducted with caution due to the potential presence of unidentified biases. Second, no antibiograms were performed among the eligible patients, which would have clarified the status of Helicobacter pylori antibiotic resistance in Switzerland. Nevertheless, the Maastricht VI-Florence recommendations advise that in countries without known clarithromycin resistance rates, systematic antimicrobial resistance testing should be performed to guide treatment decisions whenever feasible. However, due to the practical constraints associated with routine testing in Switzerland, including the necessity of endoscopic biopsy for culture, empirical treatment strategies are commonly employed. Given prior studies indicating clarithromycin resistance rates exceeding 15% in Western Europe, bismuth quadruple therapy remains the preferred first-line option, aligning with international guidelines. This is supported by our data, with bismuth-based treatment showing a markedly higher eradication rate (97%) compared to standard triple therapy (83–87%). All quadruple rescue regimens, including those with levofloxacin or tetracycline, achieved 100% cure rates. As this is an interim analysis, we acknowledge the limitation of the absence of antibiograms in this dataset and aim to incorporate a larger cohort with routine antimicrobial resistance testing in a future study to provide a more comprehensive assessment of local resistance patterns.
The 10-day single-capsule bismuth quadruple therapy (containing metronidazole, tetracycline, and bismuth) represents a reasonable empirical option, yielding >90% effectiveness when local clarithromycin resistance is unknown. Proton pump inhibitor dosage did not appear to substantially influence eradication success. Treatments were generally well tolerated, and patients from Switzerland adhered to therapy. However, the relatively large proportion of patients who did not undergo confirmatory eradication testing is a limitation that warrants caution when interpreting the results. Further local studies from our registry are needed to confirm these interim findings.
All data relevant to the study are included in the article or uploaded as supplementary information. However, previously published data from the Hp-EuReg study, de-identified raw data related to the current study, and further information on the methods used to explore the data may be shared without time constraints. Individual participant data will not be shared.
We want to thank the Spanish Association of Gastroenterology (AEG) for providing the e-CRF service free of charge. We also thank the patients who consented to this study and their families for their support and commitment.
Author contributions: Michael Doulberis coordinated the study; analysed, synthesised, and interpreted the data; wrote the first draft; and approved the submitted manuscript. He also obtained funding for Switzerland. Daniele Riva, Ioannis Linas, Patrick Mosler, Tom Völler, Imen Jallouli, Jürg Knuchel, Claudia Gregoriano, Pablo Gressot, Thrasyvoulos Gkretzios, Christos Kiosses, Thomas Balanis, Radu Tutuian, Hasan Kulaksiz, and Thomas Kuntzen collected data, critically reviewed the manuscript drafts, and approved the final submitted manuscript. Anna Cano-Català and Pablo Parra performed the monitoring and quality check of the data and approved the final manuscript. Olga P. Nyssen, Hp-EuReg Scientific Director, planned and coordinated the study; extracted the data; supervised the monitoring and quality control; assisted with the analysis, interpretation, and synthesis of data; critically reviewed the manuscript drafts; and approved the final submitted manuscript. Anna Cano-Català, Pablo Parra, Leticia Moreira, Olga P. Nyssen, Francis Mégraud, Colm O’Morain, and Javier P. Gisbert, all members of the Hp-EuReg Scientific Committee, critically reviewed the manuscript drafts and approved the final submitted manuscript. Javier P. Gisbert, principal investigator of the registry, directed the project, obtained funding, designed the protocol, planned and coordinated the study, critically reviewed the manuscript drafts, and approved the final submitted manuscript.
This project was promoted and funded by the European Helicobacter and Microbiota Study Group (EHMSG) and received support from the Spanish Association of Gastroenterology (AEG) and the Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd).
The Hp-EuReg was co-funded by the European Union programme HORIZON (grant agreement number 101095359) and supported by UK Research and Innovation (grant agreement number 10058099). However, the views and opinions expressed are those of the author(s) only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency (HaDEA). Neither the European Union nor the granting authority can be held responsible for them.
The Hp-EuReg was co-funded by the European Union programme EU4Health (grant agreement number 101101252).
This study was funded by Diasorin, Biocodex, and Juvisé Pharmaceuticals; however, clinical data were not accessible to the companies, and they were not involved in any stage of the Hp-EuReg study (design, data collection, statistical analysis, or manuscript writing). We thank Diasorin, Biocodex, and Juvisé for their support.
This study was also supported by funding from the Research Council of Cantonal Hospital Aarau (Project number: 1410.000.175).
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. MD reports travel fees from Takeda, Dr Falk, and AbbVie, as well as consulting fees from Takeda. IL reports travel fees from AbbVie, Schwabe Pharma AG, and Tillotts Pharma; consulting fees from Schwabe Pharma, AbbVie, and Implantica AG; and expert testimony fees from Schwabe Pharma AG, Takeda Pharma AG, and Implantica AG. A patent is also planned with the latter company. He participated on a Data Safety Monitoring Board or Advisory Board for Schwabe Pharma AG. TV reports support for attending meetings and/or travel from Gilead, Johnson & Johnson, Schwabe, and Takeda. RT reports travel fees from Schwabe Pharma AG, as well as consulting fees from Laborie and Schwabe Pharma AG. OPN has served as a speaker or received research funding from Allergan, Mayoly Spindler, Richen, Biocodex, and Juvisé. JPG has served as a speaker, consultant, and advisory board member for, or has received research funding from, Mayoly, Allergan, Diasorin, Biocodex, Juvisé, and Richen. Dr Nyssen has also received research funding from Mayoly, Allergan, Diasorin, Biocodex, Juvisé, and Richen.
1. Doulberis M, Pierre NT, Manzini G, Papaefthymiou A, Kountouras J, Klukowska-Rötzler J, et al. Helicobacter pylori-Related Metabolic Parameters and Premalignant Gastric Mucosa Histological Lesions in Swiss Bariatric Patients. Microorganisms. 2021 Jun;9(7):1361. doi: https://doi.org/10.3390/microorganisms9071361
2. Rupp S, Papaefthymiou A, Chatzimichael E, Polyzos SA, Spreitzer S, Doulberis M, et al. Diagnostic approach to Helicobacter pylori-related gastric oncogenesis. Ann Gastroenterol. 2022;35(4):333–44. doi: https://doi.org/10.20524/aog.2022.0725
3. Li Y, Choi H, Leung K, Jiang F, Graham DY, Leung WK. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023 Jun;8(6):553–64. doi: https://doi.org/10.1016/S2468-1253(23)00070-5
4. Doulberis M, Kotronis G, Thomann R, Polyzos SA, Boziki M, Gialamprinou D, et al. Review: Impact of Helicobacter pylori on Alzheimer’s disease: What do we know so far? Helicobacter. 2018 Feb;23(1):e12454. doi: https://doi.org/10.1111/hel.12454
5. Doulberis M, Kountouras J, Rogler G. Reconsidering the “protective” hypothesis of Helicobacter pylori infection in eosinophilic esophagitis. Ann N Y Acad Sci. 2020 Dec;1481(1):59–71. doi: https://doi.org/10.1111/nyas.14449
6. Hellström PM. Spotlight on gastroenterology - the Nobel Prize Laureates in Physiology or Medicine 2005: John Robin Warren and Barry James Marshall. Scand J Gastroenterol. 2005 Dec;40(12):1383–5. doi: https://doi.org/10.1080/00365520500390402
7. Jonaitis P, Kupcinskas J, Nyssen OP, Puig I, Gisbert JP, Jonaitis L. Evaluation of the Effectiveness of Helicobacter pylori Eradication Regimens in Lithuania during the Years 2013-2020: Data from the European Registry on Helicobacter pylori Management (Hp-EuReg). Medicina (Kaunas). 2021 Jun;57(7):642. doi: https://doi.org/10.3390/medicina57070642
8. Nellums LB, Thompson H, Holmes A, Castro-Sánchez E, Otter JA, Norredam M, et al. Antimicrobial resistance among migrants in Europe: a systematic review and meta-analysis. Lancet Infect Dis. 2018 Jul;18(7):796–811. doi: https://doi.org/10.1016/S1473-3099(18)30219-6
9. Piso RJ, Käch R, Pop R, Zillig D, Schibli U, Bassetti S, et al. A Cross-Sectional Study of Colonization Rates with Methicillin-Resistant Staphylococcus aureus (MRSA) and Extended-Spectrum Beta-Lactamase (ESBL) and Carbapenemase-Producing Enterobacteriaceae in Four Swiss Refugee Centres. PLoS One. 2017 Jan;12(1):e0170251. doi: https://doi.org/10.1371/journal.pone.0170251
10. Boltin D, Beniashvili Z, Lahat A, Hirsch J, Nyssen OP, Mégraud F, et al. European Registry on Helicobacter pylori management (Hp-EuReg): first-line Therapy in Israel. Isr Med Assoc J. 2021 Jan;23(1):38–42.
11. Caldas M, Pérez-Aisa Á, Castro-Fernández M, Bujanda L, Lucendo AJ, Rodrigo L, et al.; Hp-EuReg Investigators. European Registry on Helicobacter pylori Management: Effectiveness of First and Second-Line Treatment in Spain. Antibiotics (Basel). 2020 Dec;10(1):13. doi: https://doi.org/10.3390/antibiotics10010013
12. Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022 Aug 8:gutjnl-2022-327745. doi: https://doi.org/10.1136/gutjnl-2022-327745
13. Nyssen OP, Moreira L, García-Morales N, Cano-Català A, Puig I, Mégraud F, et al. European Registry on Helicobacter pylori Management (Hp-EuReg): most relevant results for clinical practice. Front Gastroenterol (Lausanne). 2022;1:965982. doi: https://doi.org/10.3389/fgstr.2022.965982
14. McNicholl AG, O’Morain CA, Megraud F, Gisbert JP; As Scientific Committee of the Hp-Eureg on Behalf of the National Coordinators. Protocol of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg). Helicobacter. 2019 Oct;24(5):e12630. doi: https://doi.org/10.1111/hel.12630
15. Nyssen OP, Bordin D, Tepes B, Pérez-Aisa Á, Vaira D, Caldas M, et al.; Hp-EuReg Investigators. European Registry on Helicobacter pylori management (Hp-EuReg): patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut. 2021 Jan;70(1):40–54. doi: https://doi.org/10.1136/gutjnl-2020-321372
16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct;370(9596):1453–7. doi: https://doi.org/10.1016/S0140-6736(07)61602-X
17. Graham DY, Lu H, Dore MP. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter. 2019 Feb;24(1):e12554. doi: https://doi.org/10.1111/hel.12554
18. Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009 Jan;65(1):19–31. doi: https://doi.org/10.1007/s00228-008-0576-5
19. Gatta L, Nyssen OP, Fiorini G, Saracino IM, Pavoni M, Romano M, et al. Effectiveness of first and second-line empirical treatment in Italy: results of the European registry on Helicobacter pylori management. United European Gastroenterol J. 2023 Feb;11(1):103–13. doi: https://doi.org/10.1002/ueg2.12348
20. Braendli T, Schindler V, Braun DL, Murray FR, Hente JM, Pohl D. Clarithromycin-based Helicobacter pylori eradication therapy is not associated with higher treatment failure compared with non-clarithromycin-based regimens in a tertiary referral hospital in Switzerland. Swiss Med Wkly. 2023 Jan;153(1):40024. doi: https://doi.org/10.57187/smw.2023.40024
21. Sholeh M, Khoshnood S, Azimi T, Mohamadi J, Kaviar VH, Hashemian M, et al. The prevalence of clarithromycin-resistant Helicobacter pylori isolates: a systematic review and meta-analysis. PeerJ. 2023 Mar;11:e15121. doi: https://doi.org/10.7717/peerj.15121
22. Megraud F, Bruyndonckx R, Coenen S, Wittkop L, Huang TD, Hoebeke M, et al.; European Helicobacter pylori Antimicrobial Susceptibility Testing Working Group. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut. 2021 Oct;70(10):1815–22. doi: https://doi.org/10.1136/gutjnl-2021-324032
23. Nyssen OP, Perez-Aisa A, Tepes B, Castro-Fernandez M, Kupcinskas J, Jonaitis L, et al.; Hp-EuReg Investigators. Adverse Event Profile During the Treatment of Helicobacter pylori: A Real-World Experience of 22,000 Patients From the European Registry on H. pylori Management (Hp-EuReg). Am J Gastroenterol. 2021 Jun;116(6):1220–9. doi: https://doi.org/10.14309/ajg.0000000000001246