Opioid-containing antitussives in Switzerland: a descriptive cross-sectional time-series
analysis of pharmacy sales 2013–2022
DOI: https://doi.org/https://doi.org/10.57187/s.4188
Lucia
Gasparovica,
Dominik
Stämpflia,
Shanzeh
Chaudhryb,
Mina
Tadrousbcd,
Andrea M. Burdena
a Institute of Pharmaceutical
Sciences, Department of Chemistry and Applied Biosciences, ETH Zurich, Zurich,
Switzerland
b Leslie Dan Faculty of Pharmacy,
University of Toronto, Toronto, Canada
c Institute for Clinical Evaluative
Sciences (ICES), Toronto, Canada
d Women’s College Research
Institute and Department of Medicine, Women’s College Hospital, Toronto, Canada
Summary
BACKGROUND: Opioid-containing antitussives are
used to symptomatically treat a dry, irritative cough but bring a risk of misuse
as recreational drugs. Since the update of the Swiss Therapeutic Products Act in
2019, opioid-containing antitussives are regulated more strictly, being available
only on prescription and no longer over the counter.
AIM: This study aimed to describe the
sales trends of opioid-containing antitussives in Switzerland between 2011 and 2022
to assess the impact of the regulation change.
METHODS: We descriptively analysed cross-sectional
data of opioid sales from wholesalers to pharmacies and self-dispensing physicians
as an indicator of community use.
MAIN FINDINGS: An estimated 369 million standard
units of opioid-containing antitussives were sold over the whole observation period,
of which 59% contained dextromethorphan as the active ingredient. Sales decreased
slowly between 2011 and 2019, then dropped substantially in 2020 (−30.4% compared
to previous year) and 2021 (−15.2%), then partially recovered in 2022. The sales
of codeine-containing antitussives did not recover until the end of the study period
(quarter 3 of 2022) and remained 37.3% lower than before the rescheduling (quarter
4 of 2018).
DISCUSSION: It is likely that repeated media
attention on cases of misuse of opioid-containing antitussives led to more cautious
dispensing in Switzerland leading up to the revision of the Therapeutic Products
Act in 2019. The substantial decrease in sales in 2020 and 2021 was likely related
to the COVID-19 pandemic rather than the rescheduling of opioid-containing antitussives.
Longer data collection will be needed to assess the impact of the regulation change
post-pandemic.
Introduction
Acute respiratory tract infections, including acute
coughs, are among the most frequent conditions pharmacists encounter in their daily
practice [1]. Medications containing codeine, codeine derivatives or dextromethorphan,
hereinafter “opioid-containing antitussives”, are used to symptomatically treat
a dry, irritative cough – despite a relative lack of evidence of their efficacy
[2].
In the late 2000s, concerns started emerging
in the USA about the misuse of opioid-containing antitussives as recreational drugs
by teenagers, a trend which subsequently spread to Europe, including Switzerland
[3, 4]. A survey conducted in 2018 among 1180 Swiss adolescents found that one in
six 20-year-olds had used codeine cough syrup recreationally [5], a number which
might even be an underestimate, as found in later hair analyses of the survey participants
[6]. Risks of codeine misuse include respiratory
depression and dependence. Dextromethorphan overdose can lead to seizures and coma
[7, 8].
In 2019, the Swiss Therapeutic Products Act
was updated and included a revision of the Swiss drug dispensing categories. All
medications had previously been classified into categories A–E based on the required
dispensing authority, with category A being the most restrictive (single dispensing
requiring a medical prescription) and category E being the least restrictive (over-the-counter
[OTC] without medical advice). Category C (medications dispensed by
pharmacists) was abolished in the new classification, and all medications in this
category underwent a risk-based reclassification to either category D (over-the-counter
dispensing with specialist advice) or category B (dispensing requiring a medical
prescription). More specifically, drugs reclassified from category C to category
B received a special status, in that these can be dispensed by pharmacists after
a documented consultation. Opioid-containing antitussives, which were previously
in category C, fell into this so-called B+ dispensing category due to their risk
of misuse [9, 10].
Despite regular media attention and this significant
regulation change, an investigation of the utilisation of opioid-containing antitussives
in Switzerland has not been conducted to date. This study aims to close this gap
by describing the sales trends of opioid-containing antitussives in Switzerland
between 2011 and 2022.
Methods
Study design and data sources
We conducted a descriptive interrupted time-series
analysis using repeated cross-sectional data on drug sales in Switzerland. Data
was derived from the IQVIA MIDAS® pharmaceutical sales database for the period
between quarter 1 (Q1) 2013 and Q3 2022. IQVIA MIDAS® is a dataset that tracks sales
volumes and values across 94 countries and over 1.6 million drug products, reflecting
estimates of real-world activity. This includes hospital and retail pharmacy channels
but does not include information about individual facilities. For our analysis,
drug sales within the retail channel, which includes pharmacies and self-dispensing
physicians (i.e. physicians who own their own dispensary), were used as an indicator
of community use. This data included the year, calendar quarter (Q), Anatomical
Therapeutic Chemical (ATC) code of the medication and the total number of standardised
units sold. A standardised drug unit is defined as a single tablet/capsule, patch,
vial or 5 ml of oral liquid. The data exhibited no missing values. To validate the
data, we performed a cross-check of the included products and the products approved
and distributed on the Swiss market and ensured that sales numbers were within a
reasonable range.
We included all sales and variables for the
following ATC codes in the category R05 cough and cold preparations: R05DA04
codeine, R05DA09 dextromethorphan, R05FA02 opium derivatives and expectorants.
Products under the ATC code R05FA02 containing the non-narcotic opioid noscapine
were excluded from our analysis as their regulatory status did not change. The ATC
code R05DA20 opium alkaloids and derivatives was not included as it included
only one product and would thus reveal information outside the scope of the licensing
agreement with IQVIA MIDAS. While dextromethorphan-containing antitussives do not
primarily act at opioid receptors, we included products under R05DA09 dextromethorphan
in our analysis as they pose the same concerns regarding misuse as opioid-containing
antitussives and were thus treated equally to codeine and codeine derivatives and
combination products during the regulation change of 2019 [7, 11]. Combination cold
medicines containing dextromethorphan
are classified under the ATC code N02BE51 Paracetamol, combinations excl. psycholeptics
and were outside the scope of the regulation change and of our analysis. In a post
hoc analysis, the ATC code R05DB13 butamirate was added to the analysis to
serve as a control, as butamirate-containing products were not affected by the regulation
change.
Statistical analysis
We analysed quarterly sales data from Q1 2013
to Q3 2022. The quarterly sales were standardised to the Swiss population (rate
per 100,000). Swiss population statistics for the years 2013 to 2022 were obtained
from the Swiss Federal Statistical Office [12].
We conducted a descriptive time-series analysis
to plot and visualise the development of quarterly sales of antitussives in Switzerland
between Q1 2013 and Q3 2022. To assess time trends, the time series was decomposed
with Seasonal and Trend decomposition using LOESS, resulting in a trend component
which excludes the seasonal fluctuation and random noise from the data.
To assess fluctuations in sales, annual sales
rates were calculated as the sum of the sales rates across all four quarters in
the year for years with complete data (2013–2021). The percent change in sales rate
was determined by comparing it to the previous year.
All analyses were conducted for the cumulative
sales of all ATC codes and stratified by ATC code. To visualise the timing of the
reclassification, we defined a range for each ATC class given that the roll-out
of the reclassification occurred throughout 2019 and the date of upregulation for
each antitussive medication varied (see appendix table S1). Additionally, we included
the start and end of the COVID-19 pandemic in Switzerland as previous studies have
shown the impact of COVID-19 on medication utilisation [13]. The COVID-19 pandemic
start and end dates were determined as 28 February 2020 and 31 March 2022, respectively,
in accordance with the proclamation and end of the “special situation” according
to Article 6 of the Swiss Epidemics Act [14] as communicated
in press releases by the Swiss Federal Council [15, 16].
No protocol has been preregistered for this
descriptive study.
Statistical analyses were performed using R
statistical software (version 4.0.4) [17]: data was plotted using ggplot2 [18] and
the Seasonal and Trend decomposition using LOESS was performed with forecast
[19].
Ethics approval
This study did not use any individual participant
or patient data, and therefore is exempt from research ethics review. This study
followed the Strengthening the Reporting of Observational Studies in Epidemiology
(STROBE) reporting guidelines [20].
Results
Between Q1 2013 and Q3 2022, an estimated 324
million standard units of opioid-containing antitussives were sold. The majority
of sales were for preparations containing dextromethorphan (an estimated 191 million
standard units, 59%), followed by codeine preparations (27%), and opioid and expectorant
combination products (14%) (see table 1).
Table 1Sales volumes and shares of opioid-containing antitussives over the whole study
period, Q1 2013 – Q3 2022. Sales are in standard units. Author analysis based on
IQVIA MIDAS® quarterly
volume sales data for Q1 2013 – Q3 2022, reflecting estimates of real-world activity.
Copyright IQVIA. All rights reserved.
| Substance |
Total estimated sales
Q4 2011 – Q3 2022 in million standard units |
Percent of all antitussive
sales |
| All |
324 |
100% |
| Codeine |
87 |
27% |
| Dextromethorphan |
191 |
59% |
| Opioids and expectorants |
46 |
14% |
The quarterly sales rate trends are plotted
in figure 1 for the overall and individual
ATC classes (appendix table S3 provides the corresponding rates). While there was
variation in the sales rate, the decomposed trend line shows a sharp decrease in
all sales (figure 1) at the end of 2019; however,
the sales rate had nearly returned to baseline by the end of 2021. The sales of
butamirate-containing antitussives showed a very similar trend as opioid-containing
antitussives between 2020 and 2022; however, butamirate-containing antitussive sales
increased further after 2022. The sales rates, decomposed trend and the corresponding
graph can be found in appendix table S4 and appendix figure S2.
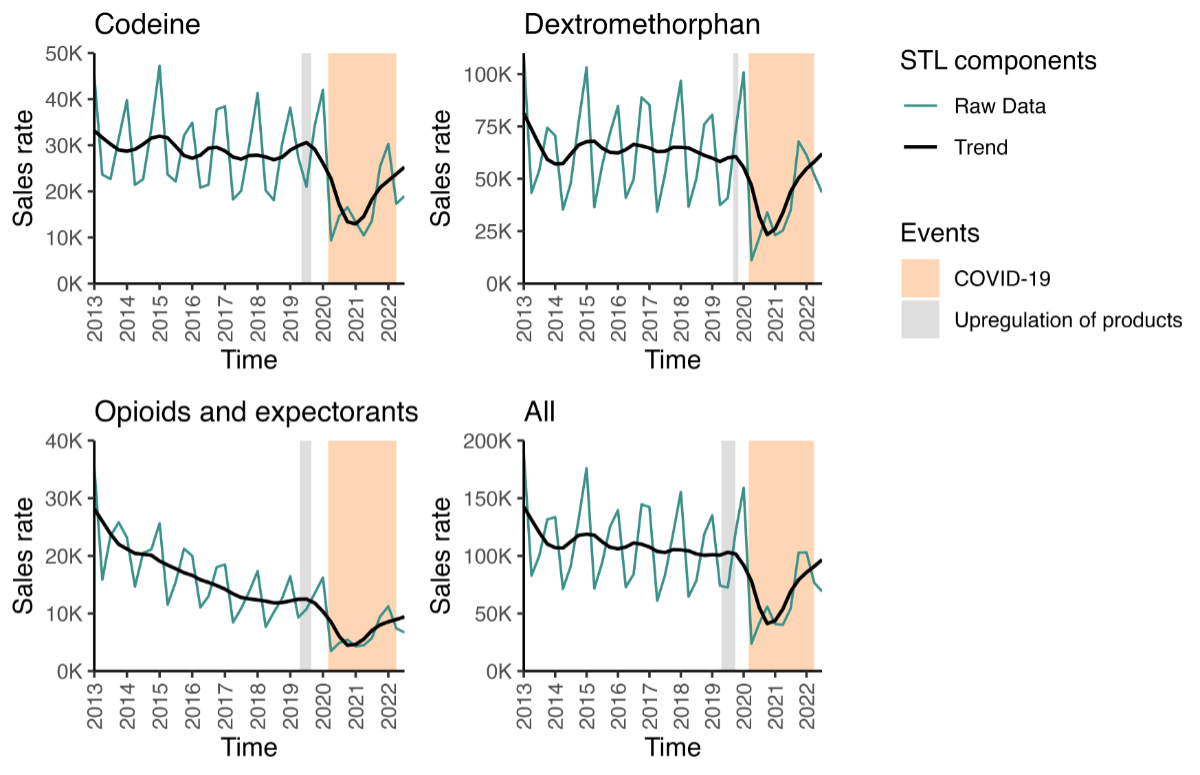
Figure 1Antitussive sales trends per substance class. The sales are units sold per 100,000
population. The green line shows the raw data, while the black line shows the trend
component remaining after time-series decomposition with LOESS. Graphs including
the seasonal and remainder components and the underlying data can be found in appendix
figure S1. The period in which the products in the respective category were upregulated
is marked in gray; the period of the COVID-19 pandemic is marked in orange. Author
analysis based on IQVIA MIDAS® quarterly volume sales data for Q1 2013 – Q3 2022, reflecting estimates
of real-world activity. Copyright IQVIA. All rights reserved.
Table 2
provides the annual sales rate per 100,000 for years with complete data (2013–2021)
and the percent change vs the previous year. Overall, antitussive sales
fluctuated between 2012 and 2019; however, sales decreased substantially in
2020. In 2020, 30.5% less opioid-containing antitussives were sold than in
2019. A similar decline in 2020 was seen for all individual ATC codes with
dextromethorphan sales decreasing the least (−27.8%) and opioid and expectorant
sales decreasing the most (−39.9%) compared to the previous year.
Table 2Estimated cumulative annual sales rates (units sold per 100,000 population; complete
years only) and change in comparison to the immediately preceding year. Crude estimated
sales rates and changes are in appendix table S2. Author analysis based on IQVIA
MIDAS® quarterly volume
sales data for Q1 2013 – Q3 2022, reflecting estimates of real-world activity. Copyright
IQVIA. All rights reserved.
|
All |
Codeine |
Dextromethorphan |
Opioids and expectorants |
| Period |
Estimated sales rate |
Change [%] |
Estimated sales rate |
Change [%] |
Estimated sales rate |
Change [%] |
Estimated sales rate |
Change [%] |
| 2013 |
504,614 |
– |
122,932 |
– |
281,059 |
– |
100,623 |
– |
| 2014 |
427,924 |
−15.2% |
117,531 |
−4.4% |
230,929 |
−17.8% |
79,463 |
−21.0% |
| 2015 |
467,181 |
9.2% |
125,218 |
6.5% |
268,059 |
16.1% |
73,904 |
−7.0% |
| 2016 |
441,222 |
−5.6% |
114,920 |
−8.2% |
264,210 |
−1.4% |
62,092 |
−16.0% |
| 2017 |
405,139 |
−8.2% |
106,904 |
−7.0% |
246,559 |
−6.7% |
51,676 |
−16.8% |
| 2018 |
417,533 |
3.1% |
109,848 |
2.8% |
259,875 |
5.4% |
47,810 |
−7.5% |
| 2019* |
403,176 |
−3.4% |
120,784 |
10.0% |
232,376 |
−10.6% |
50,017 |
4.6% |
| 2020** |
280,402 |
−30.5% |
82,529 |
−31.7% |
167,808 |
−27.8% |
30,065 |
−39.9% |
| 2021** |
238,099 |
−15.1% |
62,832 |
−23.9% |
151,294 |
−9.8% |
23,973 |
−20.3% |
Discussion
While the sales of opioid-containing antitussive
medications were slowly decreasing prior to the reclassification that occurred throughout
2019, the sales rates decreased substantially in Q4 of 2019. Although we observed
a decrease prior to the start of the COVID-19 pandemic in Switzerland, the substantial
decrease in sales in 2020 is likely largely due to the effects of the pandemic rather
than the reclassification.
Having only been completed in late 2019, the
effect of the reclassification of opioid-containing antitussives on the 2019 sales
rates is difficult to discern given the proximity to the start of the COVID-19 pandemic.
However, we do note that the sales rate decreased by 3.4% in 2019 compared to 2018,
and the quarterly data shows that most opioid-containing antitussives have not returned
to 2018 levels. This may indicate that the reclassification has slightly curbed
sales, particularly for codeine-containing antitussives.
The sales of antitussives containing dextromethorphan,
or opioids combined with expectorants appear to be gradually increasing post-pandemic;
however codeine-containing antitussives only saw a partial recovery of the sales
rate. At the end of the study period in Q3 2022, sales were 37.2% lower than in
Q4 of 2018. In 2015, the European Medicines Agency issued a contraindication for
the use of codeine-containing antitussives in children younger than 12 years, as
well as in patients with known ultrarapid metaboliser status in the CYP2D6 enzyme
[21]. This contraindication was adopted in Switzerland in 2017 [22]. Therefore,
it is also possible that combination products containing additional active ingredients
for the symptomatic treatment of acute respiratory infections (e.g. butamirate)
are increasingly favoured over codeine monopreparations with the aim of improving
treatment efficacy, given that the antitussive properties of codeine do not seem
to outperform placebo [23]. However, concerns and additional restrictions placed
on codeine use have little impact on the sales of all opioid-containing antitussives
cumulatively as dextromethorphan remains the most popularly used opioid-containing
antitussive in Switzerland. While dextromethorphan does not act at opioid receptors,
its misuse potential is similar to that of codeine [24] and dextromethorphan monopreparations
have thus undergone the same
regulatory changes as codeine-containing antitussives [11]. The media coverage on
antitussive misuse and the ensuing regulation
change in Switzerland was, however, mainly focused on codeine-containing antitussives
[25–28]. This might suggest that the revision of the Therapeutic Products Act had
a larger impact on codeine-containing than on dextromethorphan-containing antitussives.
On the other hand, calls to the Swiss poisons information centre Tox Info Suisse
for dextromethorphan poisonings have been decreasing since 2017 [29]. As data after
2020 is not available from this
source, it remains unclear how the poisoning trends have been impacted by or evolved
since the COVID-19 pandemic.
However, as identified, the estimation of the
effect of the Therapeutic Products Act revision on the sales of opioid-containing
antitussives is complicated by the start of the COVID-19 pandemic in February 2020,
which affected drug sales through various mechanisms [13]. Firstly, people who contracted
COVID-19 were obliged to isolate at home and were thus unlikely to seek treatment
at a pharmacy. Therefore, as opioid-containing antitussives under the new Therapeutic
Products Act can only be dispensed to the patient personally, non-opioid antitussives
(from dispensing category D) would have been favoured when sold to caregivers. Secondly,
due to the protective measures taken during the pandemic, other respiratory tract
infections such as seasonal influenza had a very low incidence in the 2020/2021
season [30], likely also minimising the necessity for treating cough from respiratory
tract infections other than COVID-19 and decreasing sales of opioid-containing antitussives.
While the pandemic broadly affected supply chains for drugs which led to shortages,
the effect on opioid-containing antitussives specifically was relatively small [31].
Lastly, a study by Gordon and colleagues published in March 2020 found that dextromethorphan
increased the titres of SARS-CoV-2 in vitro [32]. The authors, and subsequently several
news outlets, called for
caution in the use of dextromethorphan-containing antitussives for the symptomatic
treatment of COVID-19 [33–35]. To what extent this affected the sales of dextromethorphan-containing
antitussives is unclear; however, in our study dextromethorphan-containing antitussives
saw a smaller decrease (−27.8%) in 2020 compared to 2019 than codeine-containing
antitussives (−31.7%) and opioid and expectorants (−39.9%). To better distinguish
the effects of COVID-19 from those of the revision, we additionally analysed the
sales trends of butamirate-containing antitussives which were not reclassified and
thus unaffected by the regulation. To date, butamirate-containing antitussives are
still available over-the-counter [36]. We
found that butamirate-containing antitussive sales trends developed very similarly
to the sales trends of opioid-containing antitussives during the COVID-19 pandemic
in that there was a sharp drop during 2020 followed by an increase in 2021. However,
while the sales of opioid-containing antitussives merely returned to pre-upregulation
levels (Q4 2018 vs Q3 2022: −3.8%), the sales of butamirate-containing antitussives
were 40.6% higher at the end of our observation period than in Q4 of 2018. On the
one hand, this suggests that the drop in sales of opioid-containing antitussives
in 2020 was indeed mainly driven by the circumstances of the pandemic; on the other
hand, the following rise in butamirate sales might be a consequence of fewer sales
of codeine-containing antitussives, assuming the overall demand for antitussives
was not impacted long-term by the pandemic or the upregulation.
To
date, this is the first analysis of opioid-containing antitussive sales in Switzerland.
This study has notable strengths. The Swiss IQVIA
MIDAS® data covers pharmacies, self-dispensing physicians and drugstores; the coverage
in Switzerland is estimated at 100% [37]. All sales and changes for pharmaceutical
specialties to pharmacies,
physicians’ practices and drugstores are recorded for the listed drugs. However, our
database only gives information on standardised
units sold and does not inform on the number of patients who acquired opioid-containing
antitussives, the number of prescribers or dispensers, or their characteristics.
Additionally, a comparison to other countries is difficult due to the variety of
regulatory settings. While France experienced a similar upregulation of codeine-containing
products from over-the-counter to prescription-only in 2017 [38], this affected not
only antitussives but also
codeine-containing analgesic products [39].
In France the upregulation led to a drop in overall codeine sales; however, we were
unable to find any published data on the impact on antitussives specifically or
on the dispensing of codeine products during the COVID-19 pandemic to compare it
to our analysis [40]. Nevertheless, our results
provide insight into the general trend observed in opioid-containing antitussive
sales in Switzerland from Q1 2013 to Q3 2022.
Cumulatively, a decreasing trend was observed
for the sales of opioid-containing antitussives between 2013 and 2019. It is likely
that repeated media attention on cases of antitussive misuse has led to more cautious
recommendation of opioid-containing antitussives in pharmacies, especially in a
younger population. It does not appear that the revision of the Swiss Therapeutic
Products Act in 2019 led to a further decrease in the sales of opioid-containing
antitussives. However, as the COVID-19 pandemic occurred shortly following revision,
longer data collection will be needed to comprehensively assess its effects on antitussive
utilisation going forward.
Data availability statement
The IQVIA MIDAS® pharmaceutical sales data
analysed for this study was licensed from IQVIA. Underlying raw data will not
be disclosed, only the data points set out in this paper. The code used in the
analysis can be shared upon request to the corresponding author.
Acknowledgments
Data from this study was provided by the Leslie Dan
Faculty of Pharmacy at the University of Toronto. The statements, findings, conclusions,
views and opinions contained and expressed in this research article are based in
part on data obtained under license from the following IQVIA Solutions Canada Inc.
information service: IQVIA MIDAS®. Copyright IQVIA. All Rights Reserved. The statements,
findings, conclusions, views and opinions contained and expressed herein are not
necessarily those of IQVIA Solutions Canada Inc. or any of its affiliated or subsidiary
entities.
Lucia
Gasparovic, Professorship in
Pharmacoepidemiology
ETH Zurich
Vladimir-Prelog-Weg
1-5/10
CH-8093
Zurich
lucia.gasparovic[at]pharma.ethz.ch
References
1. Eickhoff C, Hämmerlein A, Griese N, Schulz M. Nature and frequency of drug-related
problems in self-medication (over-the-counter drugs) in daily community pharmacy practice
in Germany. Pharmacoepidemiol Drug Saf. 2012 Mar;21(3):254–60. 10.1002/pds.2241
2. Krüger K, Gehrke-Beck S, Holzinger F, Heintze C. S3-Leitlinie Akuter und chronischer
Husten. AWMF online. 2021. Available from: https://register.awmf.org/de/leitlinien/detail/053-013
3. Burns JM, Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil. 2013 Nov;4:75–82.
10.2147/SAR.S36761
4. Bachmann A, Galgano L, Guillaume M. Jugendliche mit Medikamenten- und Mischkonsum:
Situations- und Bedarfsanalyse. Infodrog. 2022. Available from: https://www.infodrog.ch/files/content/ff-de/Bericht_Jugendliche%20Mischkonsum%20Situationsanalsyse%20und%20Empfehlungen.pdf
5. Quednow BB, Steinhoff A, Bechtiger L, Ribeaud D, Eisner M, Shanahan L. High Prevalence
and Early Onsets: Legal and Illegal Substance Use in an Urban Cohort of Young Adults
in Switzerland. Eur Addict Res. 2022;28(3):186–98. 10.1159/000520178
6. Steinhoff A, Shanahan L, Bechtiger L, Zimmermann J, Ribeaud D, Eisner MP, et al. When
Substance Use Is Underreported: Comparing Self-Reports and Hair Toxicology in an Urban
Cohort of Young Adults. J Am Acad Child Adolesc Psychiatry. 2023 Jul;62(7):791–804.
10.1016/j.jaac.2022.11.011
7. Journey JD, Agrawal S, Stern E. Dextromethorphan Toxicity. In: StatPearls. Treasure
Island (FL): StatPearls Publishing; 2023., Available from https://www.ncbi.nlm.nih.gov/books/NBK538502/
8. Weier M, Weier N, O’Mara B. Over-the-Counter Medications and Their Misuse: A Focus
on Codeine. In: Patel VB, Preedy VR, editors. Handbook of Substance Misuse and Addictions.
Cham: Springer International Publishing; 2022. pp. 1–23.
9. Die Bundesversammlung der Schweizerischen Eidgenossenschaft. Bundesgesetz über Arzneimittel
und Medizinprodukte. 2002. Available from: https://www.fedlex.admin.ch/eli/cc/2001/422/de
10. Bundesamt für Gesundheit Erleichterte Abgabe von Arzneimitteln der Liste B. https://www.bag.admin.ch/bag/de/home/medizin-und-forschung/heilmittel/abgabe-von-arzneimitteln.html
11. Swissmedic. Revision des Heilmittelrechts – Liste der von der Abgabekategorie C in
die Abgabekategorie B umgeteilten Arzneimittel. Swissmedic 2019. Available from: https://www.swissmedic.ch/swissmedic/de/home/news/mitteilungen/liste_abgabekategorie_c_abgabekategorie_bumgeteilten_am.html
12. Bundesamt für Statistik. Struktur der ständigen Wohnbevölkerung nach Kanton, 1999-2022.
BAS. 2023. Available from: https://www.bfs.admin.ch/asset/de/26565154
13. Suda KJ, Kim KC, Hernandez I, Gellad WF, Rothenberger S, Campbell A, et al. The global
impact of COVID-19 on drug purchases: A cross-sectional time series analysis. J Am
Pharm Assoc (Wash DC). 2022;62(3):766–774.e6. 10.1016/j.japh.2021.12.014
14. Bundesrat. Bundesgesetz über die Bekämpfung übertragbarer Krankheiten des Menschen.
2012. Available from: https://www.fedlex.admin.ch/eli/cc/2015/297/de
15. Bundesrat. Coronavirus: Bundesrat verbietet grosse Veranstaltungen. 2020. Available
from: https://www.admin.ch/gov/de/start/dokumentation/medienmitteilungen.msg-id-78289.html
16. Bundesrat. Coronavirus: Rückkehr in die normale Lage und Planung der Übergangsphase
bis Frühling 2023. 2022. Available from: https://www.admin.ch/gov/de/start/dokumentation/medienmitteilungen.msg-id-87801.html
17. R Core Team. R: A language and environment for statistical computing. Vienna, Austria:
R Foundation for Statistical Computing; 2022.
18. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2016.
19. Hyndman RJ, Khandakar Y. Automatic time series forecasting: the forecast package for
{R}. J Stat Softw. 2008;27(3):1–22. 10.18637/jss.v027.i03
20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)
statement: guidelines for reporting observational studies. Lancet. 2007 Oct;370(9596):1453–7.
10.1016/S0140-6736(07)61602-X
21. European Medicines Agency. Codeine not to be used in children below 12 years for cough
and cold. 2015. Available from: https://www.ema.europa.eu/en/news/codeine-not-be-used-children-below-12-years-cough-and-cold
22. Swissmedic. Husten- und Erkältungsmittel mit Codein bzw. Dihydrocodein: Anpassungen
der Arzneimittelinformationen. 2017. Available from: https://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/marktueberwachung/health-professional-communication--hpc-/archiv/hpc-husten-und-erkaeltungsmittel-mit-codein-bzw-dihydrocodein.html
23. Morice A, Kardos P. Comprehensive evidence-based review on European antitussives.
BMJ Open Respir Res. 2016 Aug;3(1):e000137. 10.1136/bmjresp-2016-000137
24. Oh S, Agrawal S, Sabir S, Taylor A. Dextromethorphan. In: StatPearls. Treasure Island
(FL): StatPearls Publishing; 2024., Available from https://www.ncbi.nlm.nih.gov/books/NBK538216/
25. Schnyder M. Medikamenten-Missbrauch - Hustensirup mit Codein: Was gilt nun ab Januar?
In: Schweiz. Radio Fernseh. 2018. Available from: https://www.srf.ch/sendungen/kassensturz-espresso/medikamenten-missbrauch-hustensirup-mit-codein-was-gilt-nun-ab-januar
26. Wermelinger R. Medikamenten-Missbrauch - Hustensaft als Partydroge: Kommt die Rezeptpflicht?
In: Schweiz. Radio Fernseh. 2018. Available from: https://www.srf.ch/news/schweiz/medikamenten-missbrauch-hustensaft-als-partydroge-kommt-die-rezeptpflicht
27. Roth S. Missbrauch: Mit Hustensirup zum Rausch. In: St Galler Tagblatt. 2018. Available
from: https://www.tagblatt.ch/ostschweiz/appenzellerland/missbrauch-mit-hustensirup-zum-rausch-ld.606519
28. 20 Minuten. Jugendliche dröhnen sich mit Hustensaft zu. In: 20 Minuten. 2018. Available
from: https://www.20min.ch/story/jugendliche-droehnen-sich-mit-hustensaft-zu-190224395258
29. Tox Info Suisse. Jahresbericht 2020. Availabe from: https://www.toxinfo.ch/customer/files/878/9211581_Tox_JB-2020_DE_Web.pdf
30. Bundesamt für Gesundheit BAG. Infoportal übertragbare Krankheiten: Influenza (Grippe).
Available from: https://idd.bag.admin.ch/diseases/influenza/statistic
31. drugshortage.ch. Lieferengpässe von Medikamenten - Abgeschlossen. Available from:
https://www.drugshortage.ch/index.php/abgeschlossen/
32. Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein
interaction map reveals targets for drug repurposing. Nature. 2020 Jul;583(7816):459–68.
10.1038/s41586-020-2286-9
33. Healy M. Why you should avoid some cough syrups if you think you’ve got the coronavirus.
In: Los Angel. Times. 2020. Available from: https://www.latimes.com/science/story/2020-04-30/why-you-should-avoid-cough-syrup-if-you-think-youve-got-the-coronavirus
34. Zimmer C. Old Drugs May Find a New Purpose: Fighting the Coronavirus. In: N. Y. Times.
2020. Available from: https://www.nytimes.com/2020/04/30/health/coronavirus-antiviral-drugs.html
35. Hüttemann D. Nature-Studie: Neue Kandidaten gegen Coronaviren und Warnung vor Dextromethorphan.
In: Pharm. Ztg. Online. 2020. Available from: https://www.pharmazeutische-zeitung.de/neue-kandidaten-gegen-coronaviren-und-warnung-vor-dextromethorphan-117321/
36. HCI Solutions AG Compendium. R05DB13 Butamirat. Available from: https://compendium.ch/de/register/atc/R05DB13
37. Ju C, Wei L, Man KK, Wang Z, Ma TT, Chan AY, et al. Global, regional, and national
trends in opioid analgesic consumption from 2015 to 2019: a longitudinal study. Lancet
Public Health. 2022 Apr;7(4):e335–46. 10.1016/S2468-2667(22)00013-5
38. Ministère du Travail de la Santé et des Solidarités. Agnès Buzyn décide d’inscrire
la codéine et d’autres dérivés de l’opium à la liste des médicaments disponibles uniquement
sur ordonnance. 2017. Available from: https://sante.gouv.fr/archives/archives-presse/archives-communiques-de-presse/article/agnes-buzyn-decide-d-inscrire-la-codeine-et-d-autres-derives-de-l-opium-a-la
39. Natali I. Economic Opportunity and Opioid Regulation: the Case of Codeine in France.
Toulouse School of Economics (TSE). 2024. Working paper 24-1563. Available from: https://www.tse-fr.eu/publications/economic-opportunity-and-opioid-regulation-case-codeine-france
40. Richards GC, Aronson JK, MacKenna B, Goldacre B, Hobbs FD, Heneghan C. Sales of Over-the-Counter
Products Containing Codeine in 31 Countries, 2013-2019: A Retrospective Observational
Study. Drug Saf. 2022 Mar;45(3):237–47. 10.1007/s40264-021-01143-2
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.4188.
