Swiss Stroke Society position paper on atrial fibrillation monitoring and management
after ischaemic stroke: a shift
from understanding the index stroke to preventing the next one
DOI: https://doi.org/https://doi.org/10.57187/s.4170
Thomas Meinela,
Markus Arnoldb,
Laurent Rotenc,
Philipp Krisaide,
Marie-Luise Monof,
Catherine Gebhardc,
Leo Bonatigh,
Timo Kahlesi,
Urs Fischerj,
Marcel Arnolda,
Mira Katanbj
a Stroke Research Centre Bern, Department of Neurology, Inselspital,
Bern University Hospital, and University of Bern, Bern, Switzerland
b Department for Neurology, University Hospital Zurich, Zurich, Switzerland
c Department
of Cardiology, Inselspital, Bern University Hospital, and University of Bern, Bern,
Switzerland
d Cardiology Division, Department of Medicine, University Hospital
Basel, Basel, Switzerland
e Cardiovascular Research Institute Basel, Basel, Switzerland
f Stroke Unit, Stadtspital Triemli, Zurich, Switzerland
g Department of Neurology, University Hospital Basel, Basel, Switzerland
h Department of Research, Reha Rheinfelden, Rheinfelden, Switzerland
i Department of Neurology and Stroke Centre, Cantonal Hospital of Aarau,
Aarau, Switzerland
j Department of Neurology and Stroke Centre,
University Hospital Basel and University of Basel, Basel, Switzerland
Summary
This position paper on the detection of atrial fibrillation after ischaemic
stroke is a statement of the “Heart and Brain” committee of the Swiss Stroke Society.
This position paper summarises present knowledge on the detection of atrial fibrillation
after ischaemic stroke or transient ischaemic attack. An interdisciplinary standard
for monitoring on the stroke unit and after discharge is proposed respecting recent
developments and Swiss particularities. The main evolution in the field is that
the role of atrial fibrillation screening after stroke or transient ischaemic attack
has shifted from understanding the index stroke to preventing the next stroke; it
therefore should also be performed in patients with certain other stroke aetiologies,
e.g. symptomatic carotid artery stenosis. The duration of atrial fibrillation monitoring
should be based on an individualised risk assessment incorporating clinical characteristics
as well as cardiac and laboratory biomarkers. Given the paucity of randomised controlled
data on this topic, this position paper intends to give practical advice to healthcare
professionals involved in stroke care in Switzerland based on a consensus between
experts in the field.
Abbreviations
- MR-proANP:
-
mid-regional pro-atrial natriuretic peptide
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- TIA:
-
transient ischaemic attack
Introduction
Ischaemic stroke is a major cause of disability and mortality worldwide [1]. Atrial
fibrillation is the most common
cardiac arrhythmia, affecting up to 2% of the European population [2]. Up to a quarter
of ischaemic strokes can be attributed to atrial fibrillation,
and atrial fibrillation increases the risk of ischaemic stroke significantly [3].
Besides the 25% of ischaemic stroke caused
by atrial fibrillation, an increasing number of patients with ischaemic stroke have
atrial fibrillation (whether causally related or not) [4]. Incidence and prevalence
of atrial fibrillation vary by sex [5], age, race/ethnicity, geographical region and
intensity of screening. Accordingly, the incidence of atrial fibrillation is higher
in men than in women, higher in Western European countries than in Eastern European
countries [6] and increases rapidly with age:
at the age of 80 years, over 10% of the population are affected by atrial fibrillation
with atrial fibrillation starting at an older age in women [7, 8]. Atrial fibrillation-associated
strokes are
particularly severe [9], have a high recurrence
risk [10–12] and a worse prognosis than ischaemic
strokes of other aetiologies [13].
In patients with stroke and no other clear aetiology, the benefit of atrial
fibrillation monitoring and detection is obvious, because oral anticoagulation reduces
recurrent stroke in atrial fibrillation patients with a relative risk reduction
of up to 60–70% [14]. However, recent data
shows that the incidence of atrial fibrillation does not differ between cryptogenic
stroke patients [15] and stroke caused by
large-artery or small-vessel disease [16]
– presumably because shared cardiovascular risk factors cause both arteriosclerotic
disease as well as atrial fibrillation. Hence, another relevant percentage of patients
with ischaemic stroke have atrial fibrillation – without atrial fibrillation necessarily
being the cause of the index ischaemic stroke – and atrial fibrillation screening
and treatment may also benefit these patients because it may prevent future stroke
of cardioembolic origin. Effective anticoagulation in the event of an ischaemic
stroke in patients with atrial fibrillation is associated with a reduced stroke
severity and better outcome compared to patients without effective anticoagulation
[17, 18].
However, atrial fibrillation can be difficult to detect, as it is often asymptomatic
and sporadic [6]. Various monitoring strategies
are available to detect subclinical atrial fibrillation, including in-hospital and
outpatient monitoring with the use of external and implantable recorders as well
as wearables and app-based technology [6, 19].
This guideline aims to provide recommendations on screening for atrial fibrillation
in patients with ischaemic stroke or transient ischaemic attack (TIA) in order to
enable effective secondary prevention through anticoagulation and novel treatment
strategies.
Epidemiology in Switzerland
Around 1–2% of the population is affected by atrial fibrillation – the most
common cardiac arrhythmia – translating to around 150,000 individuals in Switzerland
[20].
Atrial fibrillation cases will likely double in the upcoming decades [21]. Every year,
about 16,000 strokes occur in
Switzerland, with around 4000 of them attributable to atrial
fibrillation [22]. Half of this latter
group has known atrial fibrillation (and often inadequate treatment – especially
in women [23]), whereas the other half is
newly diagnosed with atrial fibrillation after stroke [24]. The absolute number of
strokes is higher in women than in men
in Switzerland while the relative proportion of stroke events per age group is lower
in women [22].
Embolic stroke of undetermined source
Due to the better
safety profile of direct oral anticoagulants (DOAC), it had been hoped that atrial
fibrillation screening would no longer be necessary and that all patients with an
embolic ischaemic stroke pattern and without any other obvious stroke cause would
benefit from DOAC treatment. However, the trials failed to show a benefit of DOAC
therapy ex juvantibus [25]. Certain hypothesis-generating embolic stroke of undetermined source subgroups
(impaired renal function [25], patients aged
>75 years [26] and left ventricular dysfunction
[27], or dilated left atrium[28]) could benefit from empirical DOAC therapy.
However, the ARCADIA trial did not show a benefit of apixaban over antiplatelet
therapy [29] even in those patients with the
highest quartile of left atrial myopathy [30].
Further adequately powered randomised trials addressing these subpopulations are needed.
In the meantime, an embolic stroke
of undetermined source without detected atrial fibrillation is currently insufficient
to initiate DOAC treatment.
Purpose of the position paper
The purpose
of this position paper was primarily to translate the most recent and rapidly evolving
evidence on this topic into country-specific recommendations reflecting peculiarities
and conditions of the Swiss healthcare system. We aimed to provide concise yet comprehensive
practical advice for atrial fibrillation screening after ischaemic stroke or TIA.
Secondly, we aimed to cover knowledge gaps and blind spots of recommendations of
the available international guidelines. The position paper is intended to provide
guidance to physicians involved in the care of stroke patients. In line with the
nature of a position paper, the labelling of evidence levels has been omitted, as
it mainly represents the expert opinions based on lower evidence levels and clinical
experience. Therefore, it is not intended
to replace any international guidelines but rather complement them.
Search strategy, consensus and composition of the module
working group
The recommendations presented are evidence-based and supported by a literature
review. For this purpose, the members of the working group reviewed international
guidelines on atrial fibrillation monitoring after stroke, including those by the
European Stroke Organisation (ESO), the European Society of Cardiology, the American
Heart Association/American Stroke Association, as well as the certification criteria
of international stroke societies. Additionally, original articles not yet incorporated
in the international guidelines were included. Recommendations were reviewed and
discussed among the full committee to ensure diverse perspectives. In case of discrepancy,
recommendations were voted on to reach consensus. The references provided are representative
and not exhaustive.
The writing
group for this position paper was appointed by the Swiss Stroke Society and included
three stroke neurologists (MK, MA, TRM) and two cardiac electrophysiologists (PK,
LR).
Atrial fibrillation definitions
We followed
the European Society of Cardiology definition of atrial fibrillation, namely an
episode of atrial fibrillation on a standard 12-lead ECG recording or a single-lead
ECG tracing
of >30 seconds’ duration with no discernible repeating P waves and irregular RR intervals
(when atrioventricular
conduction is not impaired) [6]. For implantable cardiac monitors, a
minimum duration of 6 minutes is required for the diagnosis of
subclinical atrial fibrillation. For the diagnosis of clinical atrial fibrillation
(whether symptomatic or asymptomatic), a documentation of atrial fibrillation on a
12-lead
ECG or on a rhythm strip of ≥30 seconds’ duration is necessary. Wearables using
automated algorithms to assess heart beat variability, e.g. via photoplethysmography,
are currently insufficient to make a final diagnosis of atrial fibrillation without
ECG proof. However, the diagnosis can be made if at least a single-lead ECG recording
of sufficient quality showing atrial fibrillation is available and is reviewed according
to the criteria mentioned above by an expert in ECG interpretation.
Wearable single-lead devices and novel detection devices
The development
of mobile health technologies for atrial fibrillation detection is progressing rapidly,
with hundreds of apps and more than 400 wearable activity monitors currently available
[6]. These devices mostly analyse beat-to-beat variability
and are useful as a first, coarse screening step. If these devices recognise pulse
irregularities of sufficient duration, atrial fibrillation may be the cause. However,
pulse irregularities may also have other causes, like frequent premature atrial
or ventricular complexes, or simply represent recording artefacts. Whereas wearable
single-lead devices that use photoplethysmography do not allow to diagnose atrial
fibrillation, devices that record a single-lead ECG of sufficient quality (such
as some smartwatches and other wearable single-lead devices) can be used for atrial
fibrillation diagnosis. To make the atrial fibrillation diagnosis, the same criteria
apply as mentioned above for the diagnosis of clinical atrial fibrillation. Namely,
at least a single-lead ECG tracing of >30 seconds’ duration with no discernible
repeating P waves and irregular RR intervals has to be documented, or a standard
12-lead ECG showing atrial fibrillation. These single-lead ECG tracings potentially
showing atrial fibrillation should always be reviewed by a physician with sufficient
experience in rhythm analysis to confirm the diagnosis of atrial fibrillation, as
these ECG tracings can be very tricky to assess. In case of doubt or if the quality
of the rhythm strip is insufficient for a definite diagnosis, atrial fibrillation
diagnosis should be rejected.
One drawback of these devices is that they are currently mainly used by the
younger generations and their use is less popular among the elderly stroke population.
Nevertheless, wearable single-lead devices may provide an alternative and an accessible
way of atrial fibrillation screening.
Key concepts
Beyond aetiology
Aetiological
stroke work-up, as traditionally practiced, aimed to identify the underlying cause
of an index stroke in order to tailor secondary prevention strategies specific to
that cause. In the context of an ageing patient population with generally higher
vascular risk, multiple potential underlying causes of stroke often coexist, with
variable attributable risk over time. Focusing solely on identifying the most likely
stroke mechanism for an event and assigning a mutually exclusive stroke
aetiology does not necessarily determine potential future stroke mechanisms and
offer the best protection. In the STROKE-AF trial [16], atrial fibrillation screening
by implantable cardiac monitors
had a similar diagnostic yield in patients with stroke attributable to large-artery
disease or small-vessel disease as compared to the original trials done in cryptogenic
stroke populations [15]. Thus, atrial fibrillation
screening seems equally important in ischaemic stroke cases where mechanisms other
than atrial fibrillation are likely involved to effectively prevent future events.
Another argument for performing rhythm monitoring regardless of stroke
aetiology stems from the randomised MonDAFIS study [31], where an additional Holter
ECG for up to seven days as compared
to standard of care was linked to a lower all-cause mortality, potentially driven
by detection of other relevant abnormal ECG findings and adequate (non)pharmacological
management [32].
We therefore recommend basing decisions on the duration of atrial fibrillation
screening on the individual patient risk of incident atrial fibrillation regardless
of the presumed index stroke aetiology. Specifically, this could mean that patients
with a symptomatic carotid stenosis with high individual risk for atrial fibrillation
might qualify for prolonged cardiac monitoring.
Structured rhythm rounds
For those patients, where telemetry is performed in the hospital, a structured rhythm
round
as part of stroke unit care might improve the atrial fibrillation detection rate
[33]. This includes screening the 24-hour
heart rate spectrum for drops or increases >20 beats per minute in heart
rate with consecutive evaluation of the corresponding ECG strips. Second, changes
in the amplitude of heart rate variation can be identified and similarly evaluated.
Third, all tachycardia >120 beats per minute or bradycardia <40
beats per minute events are evaluated. Fourth, automatically detected
episodes of arrhythmia (tachy-/bradycardia, flat line, ventricular arrhythmia) can
be verified. Fifth, the 24-hour “beat-to-beat” registration overview can be screened
for irregularities in RR intervals and atrial fibrillation episodes. An automated
analysis may be helpful in this context, but requires a manual and expert physician
validation of the ECG findings [34, 35]. If
the first monitoring is done using ambulatory Holter ECG, the same high-quality
and standardised evaluation of the ECG should be ensured.
Atrial fibrillation burden
The term “atrial
fibrillation burden” in relation to continuous device-based monitoring refers to
either the longest observed atrial fibrillation episode or the percentage
of time spent in atrial fibrillation [36].
Atrial fibrillation burden can only be reported reliably with continuous long-term
monitoring. As a basic principle, the longer the duration of monitoring for atrial
fibrillation, the higher the yield of atrial fibrillation screening [31, 37–39]. Most
importantly, with shorter monitoring,
atrial fibrillation is mainly detected in patients with a high atrial fibrillation
burden. This also impacts the risk of recurrence, which was 5-fold higher if atrial
fibrillation was detected by standard ECG as opposed to a 14-day Holter ECG [40].
Vice versa, a long monitoring duration increases
the likelihood of diagnosing atrial fibrillation in patients with a low or very
low atrial fibrillation burden [36].
If atrial fibrillation is diagnosed with intermittent Holter monitoring,
it can be assumed that the atrial fibrillation burden is relatively high, and oral
anticoagulation is clearly indicated. For long-term continuous monitoring with implantable
cardiac monitors, the cut-off of atrial fibrillation burden at which anticoagulation
should be initiated remains controversial.
In primary prevention, evidence suggests that
atrial fibrillation episodes lasting more than 24 hours carry a relevant increase
in the risk of stroke and warrant anticoagulation treatment [41]. Similarly, the LOOP
study randomising individuals
with stroke risk factors to atrial fibrillation screening by implantable
cardiac monitors versus standard
of care concluded that despite higher atrial fibrillation detection rates, not all
screen-detected atrial fibrillation merits anticoagulation since anticoagulation
initiation did not significantly reduce the risk of stroke or systemic arterial
embolism [42]. This is in line with the fact
that the STROKESTOP study found that screening for atrial fibrillation with a 14-day
Holter ECG showed a small absolute risk reduction of about 1% over a follow-up of
7 years [43], presumably because only atrial
fibrillation with a higher burden was picked up by the shorter monitoring duration
as compared to the LOOP trial.
The recently published, event-driven NOAH AFNET 6 trial randomised patients
who had at least one additional risk factor for stroke and device-detected atrial
high-rate episodes of ≥6 minutes duration to edoxaban versus placebo or aspirin
if indicated. The median CHA2DS2-VASc score was 4 and 10%
of patients had previous stroke or TIA. The incidence of stroke was low at about
1% per patient-year in both groups but the composite safety event, including death
and major bleeding, occurred more frequently in the edoxaban group [44]. A subanalysis
of patients with atrial high-rate
episodes >24 hours duration did not find an interaction between episode duration
and anticoagulation therapy [45].
In the ARTESIA trial, patients with subclinical atrial fibrillation lasting
6 minutes to 24 hours detected only by long-term continuous monitoring with pacemakers
or defibrillators were randomised to anticoagulation with apixaban or aspirin treatment.
The mean CHA2DS2-VASc score was 3.9 and 9% of patients had
previous stroke, TIA or systemic embolism. The rate of stroke or systemic embolism
was low in both groups and reduced from 1.21% per patient-year in the aspirin group
to 0.78% in the apixaban group with more severe strokes occurring in the aspirin
group [46]. However, major bleeding increased
from 0.94% per patient-year in the aspirin group to 1.71% in the apixaban group.
This was mainly driven by an increase in gastrointestinal bleeding whereas fatal
or intracranial bleeding was not different among groups. Of note, atrial fibrillation
episodes of >24 hours duration occurred in 24% of patients a mean of 18 months
after inclusion, and mandated study termination in these patients. Another 34% of
patients discontinued trial medication prematurely.
A meta-analysis of the NOAH AFNET 6 trial and ARTESIA trial confirmed that
oral anticoagulation with edoxaban or apixaban reduces the risk of stroke in patients
with device-detected atrial fibrillation but increases the risk of major bleeding
(i.e. mainly gastrointestinal bleeding) [47].
Subanalyses on patients with previous stroke or TIA have not yet been published.
Of note, in both trials most devices were pacemakers, defibrillators and cardiac
resynchronisation devices and only a very small proportion were implantable
cardiac monitors.
In secondary prevention,
and in particular after
an ischaemic stroke, the cut-off
to justify the initiation
of DOAC therapy is an ongoing debate and might be shorter than for primary prevention.
Given the fact that there is an interaction of the CHA2DS2-VASc
score and the risk of stroke
in atrial fibrillation patients, the threshold for initiating anticoagulation should
incorporate the cardiovascular risk profile as reflected by the CHA2DS2-VASc
score, the longest detected
atrial fibrillation episode, as well as individual patient factors and preferences
(table 1) [48]. For example, CT-based models
like the S2TOP-BLEED+ score [49]
or MRI-based models like the MICON score [50]
can help estimate ischaemic and haemorrhagic risks after ischaemic stroke depending
on different antithrombotics. If only shorter
episodes are recorded, atrial fibrillation monitoring should be continued as the
atrial fibrillation burden may evolve and oral anticoagulation treatment become
necessary in the future.
Table 1Suggested atrial fibrillation duration
registered on implantable cardiac monitors that should trigger the
evaluation of anticoagulation therapy by a qualified physician.
| Setting |
CHADS-VASc score |
Suggested
atrial fibrillation duration by implantable cardiac monitor reasonable to trigger
initiation of anticoagulation |
Notes |
| Primary prevention |
0–2 |
24 hours |
|
| 3 or higher |
6 min – 24 hours |
Anticoagulation
can be considered in patients with high ischaemic risk and low bleeding
risk [47, 51] |
|
>24 hours |
|
| Secondary
prevention |
0–1 |
|
Not possible
after an ischaemic stroke or transient ischaemic attack |
| 2 |
1 – 6 hours |
|
| 3 or higher |
Minimum
6 minutes |
|
Key clinical variables and biomarkers for individual risk stratification
Based on pathophysiological considerations, various factors have been proposed
to either increase the yield of atrial fibrillation detection rates during prolonged
monitoring or to identify atrial fibrillation cases associated with a higher risk
of stroke recurrence. These include clinical variables, blood-based biomarkers,
ECG and echocardiography parameters as well as brain-imaging characteristics. Most
of the current supporting evidence was derived either from cryptogenic stroke or
embolic stroke of undetermined source cohorts and less from the whole ischaemic
stroke population.
Clinical variables
The most prominent predictors for the detection of atrial fibrillation following
a stroke are older age and more severe stroke [52].
Furthermore, established cardiovascular risk factors such as hypertension are known
to be associated with an increased risk of atrial fibrillation detection. We suggest
incorporating evidence for clinical manifest heart failure with or without reduced
ejection fraction and arteriosclerotic disease (peripheral or coronary heart disease)
[52].
ECG markers
For clinical practice we suggest a cut-off of >500 supraventricular extrasystoles
over 24 hours or any atrial runs ≥20 beats per minute as a reasonable marker
to classify high-risk patients [53–55].
In addition, P-terminal force in lead V1 (PTFV1), the product of the amplitude
and duration of a terminal negative deflection of a biphasic P-wave in lead V1 >4000
µV × ms and an
ECG marker of left atrium abnormality, might also advocate for prolonged heart rhythm
monitoring [56].
Cardiac imaging
The presence of spontaneous echo contrast
or direct evidence of thrombi in the atrium can be indicative of underlying atrial
fibrillation [57]. Moreover, valvular abnormalities,
particularly rheumatic mitral valve stenosis or severe mitral and tricuspid valve
insufficiency, are recognised as risk factors for atrial fibrillation development
[57]. In addition to these factors, left atrial
enlargement is a promising biomarker for stratifying patients at high and low risk
for atrial fibrillation [58, 59]. A left atrial
volume index incorporating body size might be an even more reliable marker than
left atrial diameter alone and further measurements of left atrial function have
been linked to higher risk of atrial fibrillation detection [60–62]. Whereas most
of the evidence is available
by markers assessed by echocardiography, those biomarkers are theoretically also
available on cardioaortic CT and MRI, however the cut-offs are less established
with those novel imaging options.
Brain imaging
From a theoretical perspective, it remains plausible that specific lesion
patterns observed on MRI – such as evidence of large wedge-shaped territorial infarcts,
chronic cortical and cerebellar infarcts or cortical infarction in multiple vascular
territories – may indicate a higher likelihood of atrial fibrillation detection
[63]. However, the evidence in this regard
remains conflicting. For instance, a subanalysis of the CRYSTAL-AF study found no
significant association between acute lesion size, location or number of lesions
and the detection of subclinical atrial fibrillation [64]. Conversely, a recent subanalysis
of the MonDAFIS study showed
multiple lesions and an extensive spatial distribution of lesions as factors associated
with a higher rate of atrial fibrillation detection [65]. Also, chronic cortical lesions
were associated with atrial fibrillation
[66]. Furthermore, it is important to note
that atrial fibrillation can be detected in a substantial proportion of lacunar
strokes [16, 67]. Consequently, we do not
recommend identifying low-risk patients solely based on imaging patterns.
Blood-based biomarkers
Among several blood-based biomarkers, the natriuretic peptides, i.e. atrial
and brain-derived natriuretic peptide and especially their cleaved by-product MR-proANP
along with NT-proBNP, are the most robust and studied biomarkers for atrial fibrillation
risk assessment after stroke. Whereas the proposed cut-offs vary slightly (especially
for NT-proBNP), we suggest
that MR-proANP levels below 92 pmol/l [68]
or NT-proBNP levels below 200 pg/ml [69, 70],
can be utilised to classify patients into a low-risk group while MR-proANP levels
above 200 pmol/l [68, 71–73] or NT-proBNP
levels above 400 ng/l [19, 74] can be utilised
to define high-risk patients. It is recommended to obtain either of these biomarkers
in the acute setting; however only for MR-proANP have the cut-offs been validated
in all stroke subtypes within the first five days of symptom onset. Since the biomarkers
may be influenced by renal function, body mass index, age and sex [75], the suggested
values should be interpreted
as guidance and not as absolute cut-offs – further studies in this direction are
needed.
Atrial fibrillation detection risk scores
Several risk scores to predict newly detected atrial fibrillation after stroke
have been developed, but methodological weaknesses as well as restriction to specific
stroke subpopulations are problematic. Moreover, the performance of risk scores
in discriminating correctly was variable and the clinical utility remains uncertain
[76].
The suggested biomarkers for risk stratification and thresholds are summarised
in figure 1. Given the limited evidence to justify the superiority of any single
marker, we propose a simplified algorithm aimed at stratifying patients into low,
moderate and high risk for atrial fibrillation.
Flow chart
In this flow chart, we propose a pragmatic algorithm for atrial fibrillation
monitoring after ischaemic stroke or TIA. The individual components of the decision
algorithm (low or high pre-test probability for detecting atrial fibrillation) are
based on observational studies. The biomarkers are collinear and the proposed decision-making
process as a whole has not yet been validated. Ongoing studies will clarify whether
intensified rhythm monitoring in patients with recent ischaemic stroke leads to
a decrease in recurrent thromboembolism (e.g. NCT04371055).
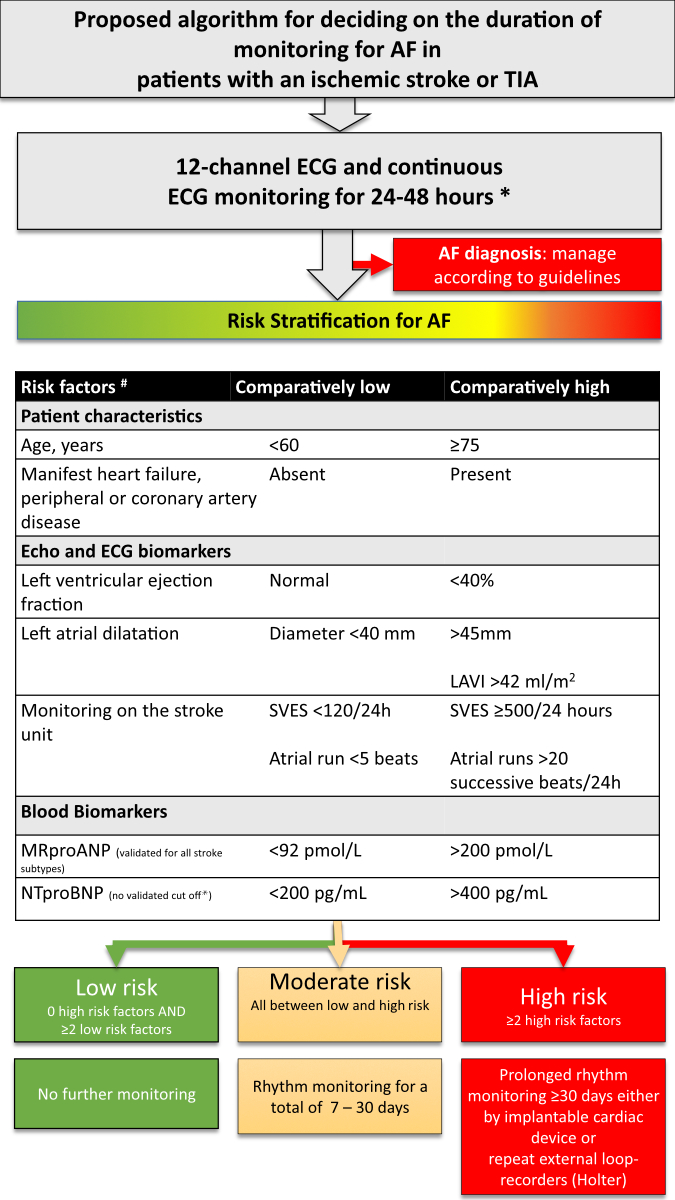
Figure 1Algorithm for determining the duration of ECG monitoring after
ischaemic stroke or transient ischaemic attack based on a risk stratification for
atrial fibrillation (AF) and applicable to all aetiologies of the index event. *
In patients with a clear different aetiology and no shared cardiovascular risk factors
(e.g. cervical artery dissection), it is reasonable to deviate from the recommended
minimum. The 48h ECG monitoring is preferably done on the stroke unit, alternatively
by ambulatory Holter ECG. # The available biomarkers should be used without
the need to obtain all biomarkers. LAVI: left atrial volume index; MR-proANP: mid-regional
pro-atrial natriuretic peptide; NT-proBNP: N-terminal pro-B-type natriuretic
peptide; SVES: supraventricular extrasystole; TIA: transient ischaemic attack.
In accordance
with the recommendations of professional societies [6, 19, 77–80], all patients with
ischaemic stroke should receive a
standard 12-lead ECG on admission, followed by continuous ECG monitoring for a minimum
of 48 hours (preferably ECG monitoring on the stroke
unit, alternatively by ambulatory Holter ECG). In patients with a clear different
aetiology and no shared cardiovascular risk factors (e.g. cervical artery dissection),
it is reasonable to deviate from the recommended minimum. An analysis software for
continuous ECG analysis can optimise detection rates of atrial fibrillation
on the stroke unit and should be used if possible. We recommend extended rhythm monitoring
up to 30 days
and long-term monitoring according to the individual risk stratification, provided
that the patient is suitable for anticoagulation. The sequential approach is recommended
given the fact that atrial fibrillation will be picked up by a standard 12-lead
ECG on admission in about 8%, by stroke unit monitoring in about 4%, by short external
continuous monitoring (Holter ECG) in about 8% and by prolonged external loop recorders
in another 4% of patients [39]. Hence, costly
and invasive testing can be avoided if the first tests already confirm the diagnosis
of atrial fibrillation. As opposed to the ESO guideline on atrial fibrillation screening
after stroke or TIA, we strongly recommend the use of biomarkers for selection of
patients for prolonged monitoring (see guidance in figure 1). The ESO recommendation
to avoid the use of biomarkers for excluding patients from prolonged monitoring
and the suggestion to use implantable devices instead of non-implantable devices
is in our opinion inappropriate for Switzerland given the availability, costs, logistic
and patient barriers of invasive ECG monitoring.
Monitoring should be carried
out as soon as possible after the stroke, as atrial fibrillation recurrence
is highest early after an ischaemic stroke. We do not recommend using prolonged
monitoring if risk stratification indicates a low risk for atrial fibrillation.
Expert recommendations for current knowledge gaps
We suggest making no distinction for atrial fibrillation monitoring between
patients with confirmed ischaemic stroke, patients with a clear-cut TIA and patients
with a non-consensus TIA if relevant differential diagnoses of a TIA do not seem
likely. This is due to the fact that also non-consensus TIAs bear a high risk of
recurrence of major cardiovascular events including stroke [81].
The recommendations given apply regardless of the biological sex, since so
far no relevant differences for atrial fibrillation monitoring and benefit of anticoagulation
have been reported. However, many referenced studies either did not explore sex
differences or had insufficient representation of women. Additionally, there is
a notable absence of data concerning the influence of sociocultural gender on atrial
fibrillation monitoring.
From a pathophysiological
perspective, it might be reasonable not to differentiate between patients with manifest
ischaemic stroke and patients with incidentally discovered post-ischaemic brain
lesions with regards to atrial fibrillation monitoring, if no other more likely
aetiology of the lesion (prior cardioaortic intervention, neuroinflammatory disease,
etc.) is present. However, evidence for this approach is lacking and studies ongoing
(NCT04449523).
Early rhythm
control for patients with a diagnosis of atrial fibrillation in the past 12 months
has proven beneficial for lowering the risk of cardiovascular outcomes as compared
to usual care. Patients with atrial fibrillation and (recent) stroke were underrepresented
in the pivotal early rhythm control trial [82].
However, subgroup analysis suggested an even bigger benefit of rhythm control in
patients with prior stroke [83]. Another study
from Korea confirmed the feasibility and potential efficacy of rhythm control in
stroke patients [84].
Thus, we suggest
to consider early rhythm control interventions in patients with atrial fibrillation
diagnosed after stroke. Such a strategy might include initiation of oral antiarrhythmics
on the stroke unit. Interdisciplinary discussion with cardiology regarding an evaluation
of pulmonary vein isolation >3 months after the stroke would be advisable.
In patients
with an intermediate likelihood of patent foramen ovale (PFO)-related stroke in
whom percutaneous PFO closure is considered, at least a 7-day Holter ECG should
be done before closure to exclude paroxysmal atrial fibrillation. If atrial fibrillation
is detected in these patients, we recommend initiating long-term anticoagulation,
and to reassess the indication for PFO closure with a patient-specific decision
on whether to proceed with the intervention. There is uncertainty regarding the
benefit of PFO closure on top of anticoagulation treatment since only one trial
(CLOSE) compared PFO closure to anticoagulation [85].
There is an
ongoing debate on whether atrial fibrillation detected after stroke has a differential
risk for recurrent events as compared to atrial fibrillation known before stroke
[24, 86, 87]. There is also evidence suggesting
that certain stroke locations (e.g. insular cortex lesions) can trigger atrial fibrillation
through neurogenic mechanisms [88]. We currently
do not recommend differentiating between the two entities regarding the decision
to initiate anticoagulation until higher-quality evidence concerning these subgroups
is available.
Key take-home messages
- The role of atrial fibrillation screening has expanded
from understanding the index stroke to preventing the next stroke. This might
be equally important for stroke mechanisms other than cardioembolism in patients
with a cardiovascular risk profile (e.g. lacunar stroke or symptomatic carotid stenosis).
- The decision on the optimal atrial fibrillation screening
strategy and duration of ECG monitoring should be based on individual risk classification.
We recommend utilising demographic/clinical features and available biomarkers
to classify patients into low-, moderate- and high-risk atrial fibrillation categories
(figure 1).
- While patients with
ischaemic stroke or transient ischaemic attack (TIA) should receive 24–48 hours
of continuous ECG monitoring (either on the Stroke
Unit or by telemetry monitoring thereafter), prolonged cardiac monitoring including implantable cardiac monitors should be considered in
patients with high risk of atrial fibrillation (regardless of aetiology).
- The yield and accuracy of ambulatory atrial fibrillation diagnosis depends
on the device and duration of monitoring; in general, it increases from
wearable single-lead devices to external loop-recorders (ELR) to implantable cardiac
monitors.
- In case of atrial fibrillation
diagnosis through continuous monitoring by an implantable cardiac monitor, the burden of atrial fibrillation should
be incorporated in an individual risk-benefit decision on initiation of direct oral
anticoagulation weighing the individual ischaemic and bleeding risk using established risk models.
- Atrial fibrillation
diagnosed by single-lead recording, e.g. from wearable single-lead monitors, needs
to be verified by a physician with sufficient experience in
rhythm analysis to confirm the diagnosis of atrial fibrillation. In case of doubt
or if the quality of the rhythm strip is insufficient for a definite diagnosis,
the atrial fibrillation diagnosis should be rejected.
- In patients with an intermediate likelihood of patent foramen ovale related
stroke in whom percutaneous PFO closure is considered, at least a 7-day Holter ECG
should be done before closure to exclude paroxysmal atrial fibrillation.
Thomas
Meinel, MD, PhD
Inselspital
Bern
University Hospital
Freiburgstrasse
10
CH-3010 Bern
thomas.meinel[at]insel.ch
References
1. Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, et al.; GBD 2019
Stroke Collaborators. Global, regional, and national burden of stroke and its risk
factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019.
Lancet Neurol. 2021 Oct;20(10):795–820. doi: https://doi.org/10.1016/S1474-4422(21)00252-0
2. Al-Khayatt BM, Salciccioli JD, Marshall DC, Krahn AD, Shalhoub J, Sikkel MB. Paradoxical
impact of socioeconomic factors on outcome of atrial fibrillation in Europe: trends
in incidence and mortality from atrial fibrillation. Eur Heart J. 2021 Feb;42(8):847–57.
doi: https://doi.org/10.1093/eurheartj/ehaa1077
3. Yaghi S, Kamel H. Stratifying stroke risk in atrial fibrillation beyond clinical risk
scores. Stroke. 2017 Oct;48(10):2665–70. doi: https://doi.org/10.1161/STROKEAHA.117.017084
4. Yiin GS, Li L, Bejot Y, Rothwell PM. Time Trends in Atrial Fibrillation-Associated
Stroke and Premorbid Anticoagulation: Population-Based Study and Systematic Review.
Stroke. 2019 Jan;50(1):21–7. doi: https://doi.org/10.1161/STROKEAHA.118.022249
5. Volgman AS, Benjamin EJ, Curtis AB, Fang MC, Lindley KJ, Naccarelli GV, et al.; American
College of Cardiology Committee on Cardiovascular Disease in Women. Women and atrial
fibrillation. J Cardiovasc Electrophysiol. 2021 Oct;32(10):2793–807. doi: https://doi.org/10.1111/jce.14838
6. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al.;
ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management
of atrial fibrillation developed in collaboration with the European Association for
Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of
atrial fibrillation of the European Society of Cardiology (ESC) Developed with the
special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur
Heart J. 2021 Feb;42(5):373–498. doi: https://doi.org/10.1093/eurheartj/ehaa612
7. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of
diagnosed atrial fibrillation in adults: national implications for rhythm management
and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation
(ATRIA) Study. JAMA. 2001 May;285(18):2370–5. doi: https://doi.org/10.1001/jama.285.18.2370
8. Linde C, Bongiorni MG, Birgersdotter-Green U, Curtis AB, Deisenhofer I, Furokawa T,
et al. Sex differences in cardiac arrhythmia: A consensus document of the european
heart rhythm association, endorsed by the heart rhythm society and Asia pacific heart
rhythm society. Europace. 2018;20(10):1565-1565ao. doi: https://doi.org/10.1093/europace/euy067
9. Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, et al. Stroke severity
in atrial fibrillation. The Framingham Study. Stroke. 1996 Oct;27(10):1760–4. doi: https://doi.org/10.1161/01.STR.27.10.1760
10. Hart RG, Eikelboom JW. Reducing the risk of recurrent stroke in patients with AF.
Lancet Neurol. 2012 Jun;11(6):479–81. doi: https://doi.org/10.1016/S1474-4422(12)70096-0
11. Seiffge DJ, Werring DJ, Paciaroni M, Dawson J, Warach S, Milling TJ, et al. Timing
of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation.
Lancet Neurol. 2019 Jan;18(1):117–26. doi: https://doi.org/10.1016/S1474-4422(18)30356-9
12. Seiffge DJ, De Marchis GM, Koga M, Paciaroni M, Wilson D, Cappellari M, et al.; RAF,
RAF-DOAC, CROMIS-2, SAMURAI, NOACISP, Erlangen, and Verona registry collaborators.
Ischemic Stroke despite Oral Anticoagulant Therapy in Patients with Atrial Fibrillation.
Ann Neurol. 2020 Feb;87(5):677–87. doi: https://doi.org/10.1002/ana.25700
13. Steger C, Pratter A, Martinek-Bregel M, Avanzini M, Valentin A, Slany J, et al. Stroke
patients with atrial fibrillation have a worse prognosis than patients without: data
from the Austrian Stroke registry. Eur Heart J. 2004 Oct;25(19):1734–40. doi: https://doi.org/10.1016/j.ehj.2004.06.030
14. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke
in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007 Jun;146(12):857–67.
doi: https://doi.org/10.7326/0003-4819-146-12-200706190-00007
15. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al.; CRYSTAL
AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J
Med. 2014 Jun;370(26):2478–86. doi: https://doi.org/10.1056/NEJMoa1313600
16. Bernstein RA, Kamel H, Granger CB, Piccini JP, Sethi PP, Katz JM, et al.; STROKE-AF
Investigators. Effect of long-term continuous cardiac monitoring vs usual care on
detection of atrial fibrillation in patients with stroke attributed to large- or small-vessel
disease: the stroke-af randomized clinical trial. JAMA -. JAMA. 2021 Jun;325(21):2169–77.
doi: https://doi.org/10.1001/jama.2021.6470
17. Meinel TR, Branca M, De Marchis GM, Nedeltchev K, Kahles T, Bonati L, et al.; Investigators
of the Swiss Stroke Registry. Prior Anticoagulation in Patients with Ischemic Stroke
and Atrial Fibrillation. Ann Neurol. 2021 Jan;89(1):42–53. doi: https://doi.org/10.1002/ana.25917
18. Xian Y, O’Brien EC, Liang L, Xu H, Schwamm LH, Fonarow GC, et al. Association of preceding
antithrombotic treatment with acute ischemic stroke severity and in-hospital outcomes
among patients with atrial fibrillation. JAMA -. JAMA. 2017 Mar;317(10):1057–67. doi: https://doi.org/10.1001/jama.2017.1371
19. Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann J, et al. Searching
for Atrial Fibrillation Poststroke: A White Paper of the AF-SCREEN International Collaboration.
Circulation. 2019 Nov;140(22):1834–50. doi: https://doi.org/10.1161/CIRCULATIONAHA.119.040267
20. Samim D, Choffat D, Vollenweider P, Waeber G, Marques-Vidal P, Méan M. Prevalence
of atrial fibrillation : the Swiss population-based CoLaus|PsyCoLaus study. Herz.
2023 Feb;48(1):48–54. doi: https://doi.org/10.1007/s00059-021-05090-7
21. Di Carlo A, Bellino L, Consoli D, Mori F, Zaninelli A, Baldereschi M, et al.; National
Research Program: Progetto FAI. La Fibrillazione Atriale in Italia. Prevalence of
atrial fibrillation in the Italian elderly population and projections from 2020 to
2060 for Italy and the European Union: the FAI Project. Europace. 2019 Oct;21(10):1468–75.
doi: https://doi.org/10.1093/europace/euz141
22. Meyer K, Simmet A, Arnold M, Mattle H, Nedeltchev K. Stroke events, and case fatalities
in Switzerland based on hospital statistics and cause of death statistics. Swiss Med
Wkly. 2009 Feb;139(5-6):65–9.
23. Thompson LE, Maddox TM, Lei L, Grunwald GK, Bradley SM, Peterson PN, et al. Sex differences
in the use of oral anticoagulants for atrial fibrillation: A report from the National
Cardiovascular Data Registry (NCDR®) PINNACLE registry. J Am Heart Assoc. 2017 Jul;6(7):e005801.
doi: https://doi.org/10.1161/JAHA.117.005801
24. Lyrer F, Zietz A, Seiffge DJ, Koga M, Volbers B, Wilson D, et al.; NOACISP-LONGTERM,
Erlangen Registry, CROMIS-2, SAMURAI-NVAF and Verona Registry collaborators. Atrial
Fibrillation Detected before or after Stroke: role of Anticoagulation. Ann Neurol.
2023 Jul;94(1):43–54. doi: https://doi.org/10.1002/ana.26654
25. Hariharan NN, Patel K, Sikder O, Perera KS, Diener HC, Hart RG, et al. Oral anticoagulation
versus antiplatelet therapy for secondary stroke prevention in patients with embolic
stroke of undetermined source: A systematic review and meta-analysis. Eur Stroke J.
2022 Jun;7(2):92–8. doi: https://doi.org/10.1177/23969873221076971
26. Diener HC, Sacco RL, Easton JD, Granger CB, Bar M, Bernstein RA, et al. Antithrombotic
Treatment of Embolic Stroke of Undetermined Source: RE-SPECT ESUS Elderly and Renally
Impaired Subgroups. Stroke. 2020 Jun;51(6):1758–65. doi: https://doi.org/10.1161/STROKEAHA.119.028643
27. Birnbaum LA, Kamel H, Sheth KN, Sharma R. Left Ventricular Dysfunction Among Patients
With Embolic Stroke of Undetermined Source and the Effect of Rivaroxaban vs Aspirin
A Subgroup Analysis of the NAVIGATE ESUS Randomized Clinical Trial. 2021;10065:1–7.
28. Healey JS, Gladstone DJ, Swaminathan B, Eckstein J, Mundl H, Epstein AE, et al. Recurrent
Stroke With Rivaroxaban Compared With Aspirin According to Predictors of Atrial Fibrillation:
Secondary Analysis of the NAVIGATE ESUS Randomized Clinical Trial. JAMA Neurol. 2019 Jul;76(7):764–73.
doi: https://doi.org/10.1001/jamaneurol.2019.0617
29. ESOC 2023 Abstract Book. Eur Stroke J. 2023;8(2 suppl):3–669.
30. World Stroke Congress. WSC23 Late Breaking Abstracts. Int J Stroke. 2023;18(3 suppl):421–58.
31. Haeusler KG, Kirchhof P, Kunze C, Tütüncü S, Fiessler C, Malsch C, et al.; MonDAFIS
Investigators. Systematic monitoring for detection of atrial fibrillation in patients
with acute ischaemic stroke (MonDAFIS): a randomised, open-label, multicentre study.
Lancet Neurol. 2021 Jun;20(6):426–36. doi: https://doi.org/10.1016/S1474-4422(21)00067-3
32. Olma MC, Tütüncü S, Fiessler C, Kunze C, Krämer M, Steindorf-Sabath L, et al.; MonDAFIS
Investigators [Link]. In-Hospital ECG Findings, Changes in Medical Management, and
Cardiovascular Outcomes in Patients With Acute Stroke or Transient Ischemic Attack.
J Am Heart Assoc. 2023 Jan;12(2):e027149. doi: https://doi.org/10.1161/JAHA.122.027149
33. Kallmünzer B, Breuer L, Hering C, Raaz-Schrauder D, Kollmar R, Huttner HB, et al. A
structured reading algorithm improves telemetric detection of atrial fibrillation
after acute ischemic stroke. Stroke. 2012 Apr;43(4):994–9. doi: https://doi.org/10.1161/STROKEAHA.111.642199
34. Rizos T, Güntner J, Jenetzky E, Marquardt L, Reichardt C, Becker R, et al. Continuous
stroke unit electrocardiographic monitoring versus 24-hour Holter electrocardiography
for detection of paroxysmal atrial fibrillation after stroke. Stroke. 2012 Oct;43(10):2689–94.
doi: https://doi.org/10.1161/STROKEAHA.112.654954
35. Uphaus T, Grings A, Gröschel S, Müller A, Weber-Krüger M, Wachter R, et al. Automatic
detection of paroxysmal atrial fibrillation in patients with ischaemic stroke: better
than routine diagnostic workup? Eur J Neurol. 2017 Jul;24(7):990–4. doi: https://doi.org/10.1111/ene.13326
36. Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, et al.; American Heart
Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing;
Council on Quality of Care and Outcomes Research; and Stroke Council. Atrial Fibrillation
Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement
From the American Heart Association. Circulation. 2018 May;137(20):e623–44. doi: https://doi.org/10.1161/CIR.0000000000000568
37. Haeusler KG, Tütüncü S, Schnabel RB. Detection of Atrial Fibrillation in Cryptogenic
Stroke. Curr Neurol Neurosci Rep. 2018 Aug;18(10):66. doi: https://doi.org/10.1007/s11910-018-0871-1
38. Wachter R, Gröschel K, Gelbrich G, Hamann GF, Kermer P, Liman J, et al.; Find-AF(randomised)
Investigators and Coordinators. Holter-electrocardiogram-monitoring in patients with
acute ischaemic stroke (Find-AFRANDOMISED): an open-label randomised controlled trial. Lancet Neurol. 2017 Apr;16(4):282–90.
doi: https://doi.org/10.1016/S1474-4422(17)30002-9
39. Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis
of atrial fibrillation after stroke and transient ischaemic attack: a systematic review
and meta-analysis. Lancet Neurol. 2015 Apr;14(4):377–87. doi: https://doi.org/10.1016/S1474-4422(15)70027-X
40. Alvarado-Bolaños A, Ayan D, Khaw AV, Mai LM, Mandzia JL, Bogiatzi C, et al. Differences
in Stroke Recurrence Risk Between Atrial Fibrillation Detected on ECG and 14-Day Cardiac
Monitoring. Stroke. 2023 Aug;54(8):2022–30. doi: https://doi.org/10.1161/STROKEAHA.123.043672
41. Van Gelder IC, Healey JS, Crijns HJ, Wang J, Hohnloser SH, Gold MR, et al. Duration
of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT.
Eur Heart J. 2017 May;38(17):1339–44. doi: https://doi.org/10.1093/eurheartj/ehx042
42. Svendsen JH, Diederichsen SZ, Højberg S, Krieger DW, Graff C, Kronborg C, et al. Implantable
loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study):
a randomised controlled trial. Lancet. 2021 Oct;398(10310):1507–16. doi: https://doi.org/10.1016/S0140-6736(21)01698-6
43. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical
outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre,
parallel group, unmasked, randomised controlled trial. Lancet. 2021 Oct;398(10310):1498–506.
doi: https://doi.org/10.1016/S0140-6736(21)01637-8
44. Kirchhof P, Toennis T, Goette A, Camm AJ, Diener HC, Becher N, et al.; NOAH-AFNET
6 Investigators; NOAH-AFNET6 sites and investigators. Anticoagulation with Edoxaban
in Patients with Atrial High-Rate Episodes. N Engl J Med. 2023 Sep;389(13):1167–79.
doi: https://doi.org/10.1056/NEJMoa2303062
45. Becher N, Toennis T, Bertaglia E, Blomström-Lundqvist C, Brandes A, Cabanelas N, et
al. Anticoagulation with edoxaban in patients with long atrial high-rate episodes
≥24 h. Eur Heart J. 2024 Mar;45(10):837–49. doi: https://doi.org/10.1093/eurheartj/ehad771
46. Healey JS, Lopes RD, Granger CB, Alings M, Rivard L, McIntyre WF, et al.; ARTESIA
Investigators. Apixaban for Stroke Prevention in Subclinical Atrial Fibrillation.
N Engl J Med. 2024 Jan;390(2):107–17. doi: https://doi.org/10.1056/NEJMoa2310234
47. McIntyre WF, Benz AP, Becher N, Healey JS, Granger CB, Rivard L, et al. Direct Oral
Anticoagulants for Stroke Prevention in Patients With Device-Detected Atrial Fibrillation:
A Study-Level Meta-Analysis of the NOAH-AFNET 6 and ARTESiA Trials. Circulation. 2024 Mar;149(13):981–8.
doi: https://doi.org/10.1161/CIRCULATIONAHA.123.067512
48. Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke risk as
a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation. 2019 Nov;140(20):1639–46.
doi: https://doi.org/10.1161/CIRCULATIONAHA.119.041303
49. Hilkens NA, Li L, Rothwell PM, Algra A, Greving JP. Refining prediction of major bleeding
on antiplatelet treatment after transient ischaemic attack or ischaemic stroke. Eur
Stroke J. 2020 Jun;5(2):130–7. doi: https://doi.org/10.1177/2396987319898064
50. Best JG, Ambler G, Wilson D, Lee KJ, Lim JS, Shiozawa M, et al.; Microbleeds International
Collaborative Network. Development of imaging-based risk scores for prediction of
intracranial haemorrhage and ischaemic stroke in patients taking antithrombotic therapy
after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual
patient data from cohort studies. Lancet Neurol. 2021 Apr;20(4):294–303. doi: https://doi.org/10.1016/S1474-4422(21)00024-7
51. Rossini R, Peirone A. When to set anticoagulant therapy in asymptomatic AF? looking
for a cut-off duration. Eur Hear Journal, Suppl. 2022;24(Si):I143–7.
52. Cameron A, Cheng HK, Lee RP, Doherty D, Hall M, Khashayar P, et al. Biomarkers for
Atrial Fibrillation Detection After Stroke: Systematic Review and Meta-analysis. Neurology.
2021 Nov;97(18):e1775–89. doi: https://doi.org/10.1212/WNL.0000000000012769
53. Binici Z, Intzilakis T, Nielsen OW, Køber L, Sajadieh A. Excessive supraventricular
ectopic activity and increased risk of atrial fibrillation and stroke. Circulation.
2010 May;121(17):1904–11. 10.1161/CIRCULATIONAHA.109.874982
54. Thijs VN, Brachmann J, Morillo CA, Passman RS, Sanna T, Bernstein RA, et al. Predictors
for atrial fibrillation detection after cryptogenic stroke: results from CRYSTAL AF.
Neurology. 2016 Jan;86(3):261–9. doi: https://doi.org/10.1212/WNL.0000000000002282
55. Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS, et al.; EMBRACE
Steering Committee and Investigators. Atrial premature beats predict atrial fibrillation
in cryptogenic stroke: results from the EMBRACE trial. Stroke. 2015 Apr;46(4):936–41.
doi: https://doi.org/10.1161/STROKEAHA.115.008714
56. Wolder LD, Graff C, Baadsgaard KH, Langgaard ML, Polcwiartek C, Ji-Young Lee C, et
al. Electrocardiographic P terminal force in lead V1, its components, and the association
with stroke and atrial fibrillation or flutter. Heart Rhythm. 2023 Mar;20(3):354–62.
doi: https://doi.org/10.1016/j.hrthm.2022.11.010
57. Saric M, Armour AC, Arnaout MS, Chaudhry FA, Grimm RA, Kronzon I, et al. Guidelines
for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism.
J Am Soc Echocardiogr. 2016 Jan;29(1):1–42. doi: https://doi.org/10.1016/j.echo.2015.09.011
58. Perlepe K, Sirimarco G, Strambo D, Eskandari A, Karagkiozi E, Vemmou A, et al. Left
atrial diameter thresholds and new incident atrial fibrillation in embolic stroke
of undetermined source. Eur J Intern Med. 2020 May;75:30–4. 10.1016/j.ejim.2020.01.002
59. Schwamm LH, Kamel H, Granger CB, Piccini JP, Katz JM, Sethi PP, et al.; STROKE AF
Investigators. Predictors of Atrial Fibrillation in Patients With Stroke Attributed
to Large- or Small-Vessel Disease: A Prespecified Secondary Analysis of the STROKE
AF Randomized Clinical Trial. JAMA Neurol. 2023 Jan;80(1):99–103. doi: https://doi.org/10.1001/jamaneurol.2022.4038
60. Baturova MA, Sheldon SH, Carlson J, Brady PA, Lin G, Rabinstein AA, et al. Electrocardiographic
and Echocardiographic predictors of paroxysmal atrial fibrillation detected after
ischemic stroke. BMC Cardiovasc Disord. 2016 Nov;16(1):209. doi: https://doi.org/10.1186/s12872-016-0384-2
61. Waldenhjort D, Sobocinski Doliwa P, Alam M, Frykman-Kull V, Engdahl J, Rosenqvist M,
et al. Echocardiographic measures of atrial function may predict atrial fibrillation
in stroke patients. Scand Cardiovasc J. 2016 Aug;50(4):236–42. doi: https://doi.org/10.1080/14017431.2016.1175657
62. Stahrenberg R, Edelmann F, Haase B, Lahno R, Seegers J, Weber-Krüger M, et al. Transthoracic
echocardiography to rule out paroxysmal atrial fibrillation as a cause of stroke or
transient ischemic attack. Stroke. 2011 Dec;42(12):3643–5. doi: https://doi.org/10.1161/STROKEAHA.111.632836
63. Favilla CG, Ingala E, Jara J, Fessler E, Cucchiara B, Messé SR, et al. Predictors
of finding occult atrial fibrillation after cryptogenic stroke. Stroke. 2015 May;46(5):1210–5.
doi: https://doi.org/10.1161/STROKEAHA.114.007763
64. Bernstein RA, Di Lazzaro V, Rymer MM, Passman RS, Brachmann J, Morillo CA, et al. Infarct
Topography and Detection of Atrial Fibrillation in Cryptogenic Stroke: results from
CRYSTAL AF. Cerebrovasc Dis. 2015;40(1-2):91–6. doi: https://doi.org/10.1159/000437018
65. Pimentel BC, Ingwersen T, Haeusler KG, Schlemm E, Forkert ND, Rajashekar D, et al. Association
of stroke lesion shape with newly detected atrial fibrillation - Results from the
MonDAFIS study. Eur Stroke J. 2022 Sep;7(3):230–7. doi: https://doi.org/10.1177/23969873221100895
66. Vynckier J, Kaesmacher J, Wardlaw JM, Roten L, Beyeler M, Belachew NF, et al. Phenotypes
of Chronic Covert Brain Infarction in Patients With First-Ever Ischemic Stroke: A
Cohort Study. Stroke. 2022 Feb;53(2):558–68. doi: https://doi.org/10.1161/STROKEAHA.121.034347
67. Demeestere J, Fieuws S, Lansberg MG, Lemmens R. Detection of Atrial Fibrillation Among
Patients With Stroke Due to Large or Small Vessel Disease: A Meta-Analysis. J Am Heart
Assoc. 2016 Sep;5(9):e004151. doi: https://doi.org/10.1161/JAHA.116.004151
68. Schweizer J, Arnold M, König IR, Bicvic A, Westphal LP, Schütz V, et al. Measurement
of Midregional Pro-Atrial Natriuretic Peptide to Discover Atrial Fibrillation in Patients
With Ischemic Stroke. J Am Coll Cardiol. 2022 Apr;79(14):1369–81. doi: https://doi.org/10.1016/j.jacc.2022.01.042
69. Wachter R, Lahno R, Haase B, Weber-Krüger M, Seegers J, Edelmann F, et al. Natriuretic
peptides for the detection of paroxysmal atrial fibrillation in patients with cerebral
ischemia—the Find-AF study. PLoS One. 2012;7(4):e34351. doi: https://doi.org/10.1371/journal.pone.0034351
70. Fonseca AC, Brito D, Pinho e Melo T, Geraldes R, Canhão P, Caplan LR, et al. N-terminal
pro-brain natriuretic peptide shows diagnostic accuracy for detecting atrial fibrillation
in cryptogenic stroke patients. Int J Stroke. 2014 Jun;9(4):419–25. doi: https://doi.org/10.1111/ijs.12126
71. Arnold M, Nakas C, Luft A, Christ-Crain M, Leichtle A, Katan M. Independent Prognostic
Value of MRproANP (Midregional Proatrial Natriuretic Peptide) Levels in Patients With
Stroke Is Unaltered Over Time. Stroke. 2020 Jun;51(6):1873–5. doi: https://doi.org/10.1161/STROKEAHA.120.029333
72. De Marchis GM, Schneider J, Weck A, Fluri F, Fladt J, Foerch C, et al. Midregional
proatrial natriuretic peptide improves risk stratification after ischemic stroke.
Neurology. 2018 Feb;90(6):e455–65. doi: https://doi.org/10.1212/WNL.0000000000004922
73. Katan M, Fluri F, Schuetz P, Morgenthaler NG, Zweifel C, Bingisser R, et al. Midregional
pro-atrial natriuretic peptide and outcome in patients with acute ischemic stroke.
J Am Coll Cardiol. 2010 Sep;56(13):1045–53. doi: https://doi.org/10.1016/j.jacc.2010.02.071
74. Llombart V, Antolin-Fontes A, Bustamante A, Giralt D, Rost NS, Furie K, et al. B-type
natriuretic peptides help in cardioembolic stroke diagnosis: pooled data meta-analysis.
Stroke. 2015 May;46(5):1187–95. doi: https://doi.org/10.1161/STROKEAHA.114.008311
75. Luchner A, Behrens G, Stritzke J, Markus M, Stark K, Peters A, et al. Long-term pattern
of brain natriuretic peptide and N-terminal pro brain natriuretic peptide and its
determinants in the general population: contribution of age, gender, and cardiac and
extra-cardiac factors. Eur J Heart Fail. 2013 Aug;15(8):859–67. doi: https://doi.org/10.1093/eurjhf/hft048
76. Kishore AK, Hossain MJ, Cameron A, Dawson J, Vail A, Smith CJ. Use of risk scores
for predicting new atrial fibrillation after ischemic stroke or transient ischemic
attack-A systematic review. Int J Stroke. 2022 Jul;17(6):608–17. doi: https://doi.org/10.1177/17474930211045880
77. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D,
et al. Guideline for the prevention of stroke in patients with stroke and transient
ischemic attack; A guideline from the American Heart Association/American Stroke Association.
Stroke. 2021 Jul;52(7):e364–467. doi: https://doi.org/10.1161/STR.0000000000000375
78. Schlaganfall-gesellschaft Ö. Positionspapier - Update 2018 Detektion von Vorhofflimmern
beim kryptogenen Hirninfarkt. 2018. p. 1–16. Available from: https://www.ögsf.at/wp-content/uploads/2016/11/Positionspapier-2018_OEGSF_neurologisch.pdf
79. Rubiera M, Aires A, Antonenko K, Lémeret S, Nolte CH, Putaala J, et al. European Stroke
Organisation (ESO) guideline on screening for subclinical atrial fibrillation after
stroke or transient ischaemic attack of undetermined origin. Eur Stroke J. 2022 Sep;7(3):VI.
doi: https://doi.org/10.1177/23969873221099478
80. Häusler KG, Gröschel K, Köhrmann M, Schnabel RB, Anker SD, Brachmann J, et al. Position
Paper on Atrial Fibrillation Detection after Ischemic Stroke: Working Group Heart
and Brain of the German Cardiac Society (DGK) and the German Stroke Society (DSG).
Aktuelle Neurol. 2018;45(2):93–106. Available from: https://www.thieme-connect.de/products/ejournals/pdf/10.1055/s-0043-118476.pdf
81. Tuna MA, Rothwell PM; Oxford Vascular Study. Diagnosis of non-consensus transient
ischaemic attacks with focal, negative, and non-progressive symptoms: population-based
validation by investigation and prognosis. Lancet. 2021 Mar;397(10277):902–12. doi: https://doi.org/10.1016/S0140-6736(20)31961-9
82. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al.; EAST-AFNET 4
Trial Investigators. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation.
N Engl J Med. 2020 Oct;383(14):1305–16. doi: https://doi.org/10.1056/NEJMoa2019422
83. Jensen M, Suling A, Metzner A, Schnabel RB, Borof K, Goette A, et al. Early rhythm-control
therapy for atrial fibrillation in patients with a history of stroke: a subgroup analysis
of the EAST-AFNET 4 trial. Lancet Neurol. 2023 Jan;22(1):45–54. doi: https://doi.org/10.1016/S1474-4422(22)00436-7
84. Park J, Shim J, Lee JM, Park JK, Heo J, Chang Y, et al.; RAFAS Investigators*. Risks
and Benefits of Early Rhythm Control in Patients With Acute Strokes and Atrial Fibrillation:
A Multicenter, Prospective, Randomized Study (the RAFAS Trial). J Am Heart Assoc.
2022 Feb;11(3):e023391. doi: https://doi.org/10.1161/JAHA.121.023391
85. Turc G, Calvet D, Guérin P, Sroussi M, Chatellier G, Mas JL; CLOSE Investigators.
Closure, anticoagulation, or antiplatelet therapy for cryptogenic stroke with patent
foramen ovale: systematic review of randomized trials, sequential meta-analysis, and
new insights from the CLOSE study. J Am Heart Assoc. 2018 Jun;7(12):e008356. doi: https://doi.org/10.1161/JAHA.117.008356
86. Sposato LA, Chaturvedi S, Hsieh CY, Morillo CA, Kamel H. Atrial Fibrillation Detected
After Stroke and Transient Ischemic Attack: A Novel Clinical Concept Challenging Current
Views. Stroke. 2022 Mar;53(3):e94–103. doi: https://doi.org/10.1161/STROKEAHA.121.034777
87. Fridman S, Jimenez-Ruiz A, Vargas-Gonzalez JC, Sposato LA. Differences between Atrial
Fibrillation Detected before and after Stroke and TIA: A Systematic Review and Meta-Analysis.
Cerebrovasc Dis. 2022;51(2):152–7. doi: https://doi.org/10.1159/000520101
88. Scheitz JF, Erdur H, Haeusler KG, Audebert HJ, Roser M, Laufs U, et al. Insular cortex
lesions, cardiac troponin, and detection of previously unknown atrial fibrillation
in acute ischemic stroke: insights from the troponin elevation in acute ischemic stroke
study. Stroke. 2015 May;46(5):1196–201. doi: https://doi.org/10.1161/STROKEAHA.115.008681
