Update on prevention and antimicrobial prophylaxis of infective endocarditis
DOI: https://doi.org/https://doi.org/10.57187/s.4169
Maryam
Pavlicek-Bahloa,
Barbara Hasseb,
Michelle
Frankc,
Anna
Conend,
Matthaios Papadimitriou-Olivgerisef,
Benoit
Gueryf,
Alain M. Bernheimg,
Philipp K. A. Agyemanh,
Walter
Knirschi,
Michael M. Bornsteinj,
Matthias
Greutmannc,
Parham
Sendik
a Department of Cardiology, Bern
University Hospital (Inselspital), University of Bern, Bern, Switzerland
b Department of Infectious Diseases and Hospital
Epidemiology, University Hospital Zurich, Zurich, Switzerland
c University Heart Centre, Department of
Cardiology, University Hospital of Zurich, Zurich, Switzerland
d Clinic of Infectious Diseases
and Infection Prevention, Cantonal Hospital Aarau, Aarau, Switzerland
e Infectious Diseases Service,
Cantonal Hospital of Sion and Institut Central des Hôpitaux (ICH), Sion,
Switzerland
f Infectious Diseases Service, Department of
Medicine, University of Lausanne and Lausanne University Hospital, Lausanne, Switzerland
g Department of Cardiology,
Triemli Hospital Zurich, Zurich, Switzerland
h Division of Paediatric Infectious Diseases, Inselspital, Bern
University Hospital, University of Bern, Bern, Switzerland
i Pediatric Cardiology, Pediatric
Heart Centre, University Children's Hospital, Zurich, Switzerland
j Department of Oral Health and Medicine, University Centre for
Dental Medicine UZB, University of Basel, Basel, Switzerland
k Institute
for Infectious Diseases, University of Bern, Bern, Switzerland
Summary
The Swiss
expert group published revised guidelines on the prevention and antibiotic
prophylaxis against infective endocarditis in 2021. In this viewpoint article,
the group reports on their experiences two years after implementing the new
prevention concept, which included information flyers and antimicrobial
prophylaxis cards. Challenges included communicating the concept and indications
for antimicrobial prophylaxis to both high-risk patients and providers.
Introduction
The indication and adequate use of antibiotic prophylaxis against
infective endocarditis is frequently discussed in clinical practice. The
growing use of implantable cardiac devices, advanced techniques in minimally
invasive heart valve implantation, and the increasing number of adults with congenital
heart disease have led to an increased number of patients at risk, emphasising
the importance of preventing infective endocarditis. In 2021, the Swiss expert
group published revised guidelines on the prevention and antibiotic prophylaxis
against infective endocarditis [1]. This article reviews the cornerstones of
these guidelines, highlights prevention strategies, and provides a 2024 update
on the most recent adaptations [2].
The aim of antibiotic prophylaxis against infective endocarditis is to
prevent bacterial attachment to the endocardium following transient bacteraemia
after invasive procedures in high-risk patients. Earlier guidelines recommended
antibiotic prophylaxis for almost all patients with any cardiac condition that
could predispose them to infective endocarditis, and prior to a variety of invasive
procedures. However, transient bacteraemia frequently occurs during daily
activities, such as brushing teeth, flossing, or chewing, making it impossible
to prevent all instances. Broad and uncritical use of antibiotic prophylaxis
increases the risk of adverse events and contributes to rising antimicrobial
resistance.
In 2007, the American Heart Association (AHA) restricted antibiotic
prophylaxis use to high-risk patients only [3]. In 2008, the United Kingdomʼs National
Institute
for Health and Care Excellence (NICE) advised against any antibiotic
prophylaxis, regardless of risk category [4]. In subsequent years, an increase
in infective endocarditis cases was observed, though without evidence of
causality or an associated increase in mortality [5, 6]. The 2015 European
Society of Cardiology (ESC) guidelines aligned with the second set of infective
endocarditis AHA recommendations published in 2007 [7]. In 2019, a Swiss expert
committee on infective endocarditis prevention revised the existing
recommendations from 2008 [8], adapting the 2015 ESC guidelines [7] while considering
issues relevant to the Swiss healthcare system. These recommendations were
published in 2021 [1, 2] and were launched alongside an information campaign in
collaboration with the Swiss Heart Foundation for both healthcare providers and
patients [9]. In 2024, minor updates were implemented following the release of
the 2023 ESC guidelines [10].
Reminder: What was new in 2021?
Heart disease
Antibiotic prophylaxis against infective endocarditis has been
recommended since 2008 for high-risk patients only. However, from 2021, antibiotic
prophylaxis is no longer recommended for patients with uncorrected ventricular
septum defect or uncorrected patent ductus arteriosus Botalli. For heart
transplant recipients, with or without valvulopathy, a case-based discussion is
recommended [1, 2]. The number of individuals with heart transplants is low in
Switzerland [11]. The existing
evidence on this topic remains limited [12]. A list of high-risk conditions is provided
in figure 1.
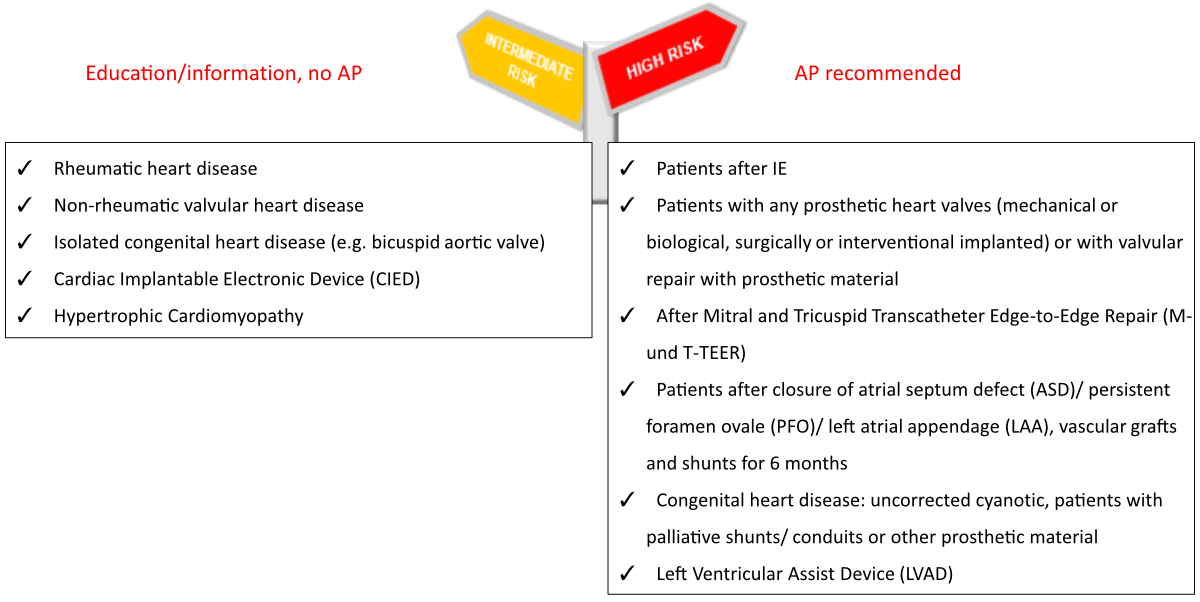
Figure 1Patient risk categorisation
according to the Swiss Infective Endocarditis Expert Group 2024 and adapted
from the 2023 European Society of Cardiology (ESC) guidelines on infective
endocarditis [7]. For heart transplant recipients, with or without valvulopathy,
a
case-based discussion is recommended [1, 2]. AP: antibiotic
prophylaxis.
Invasive
procedures
Based on
the frequency of questions arising in clinical practice and for the purposes of
teaching infective endocarditis prevention, invasive procedures with a risk of bacteraemia
were divided into
two categories, namely dental and non-dental procedures. Recommendations on antibiotic
prophylaxis for dental procedures are noted on an infective endocarditis
prophylaxis card, which is handed to patients at high risk for infective
endocarditis. The current card replaces the previous cards that had different colours.
Recommendations
on antibiotic prophylaxis for non-dental interventions are displayed in
publications for healthcare professionals [1, 2] and on websites [11].
Education
One of the
most central elements of the infective endocarditis prevention strategy is the
education of all patients at any level of risk for infective
endocarditis. Therefore, the Swiss infective endocarditis expert group, in
collaboration with the Swiss Heart Foundation, released a flyer for all at-risk
patients in 2021 (figures 2 and 3). However, antibiotic prophylaxis is only recommended
for high-risk patients. Moreover, raising awareness of the signs and symptoms
of infective endocarditis triggers patients to seek medical help earlier in the
course of the disease. New infective endocarditis cards were introduced,
replacing the old ones, to help physicians identify at-risk patients and
increase patient awareness.

Figure 2Introduction of new endocarditis
cards for high-risk patients,
provided by the Swiss Heart Association (available in German, French, Italian and
English at www.swissheart.ch).
Figure 3Introduction of new flyers for all at-risk patients,
provided by the Swiss Heart Association (available in German, French, Italian and
English at www.swissheart.ch).
How good is the information and communication strategy?
As outlined
earlier, the prevention campaign was directed at all patients at risk
for infective endocarditis. The information strategy included the following key
elements: (a) knowledge transfer to increase awareness of good dental and skin
hygiene and (b) education about symptoms consistent with infective endocarditis
and the appropriate steps to take when these symptoms occur (i.e., contact a doctor).
Two years after the release of the Swiss guidelines, the expert group, together
with the Swiss Heart Foundation, reviewed and evaluated how well the
information and communication strategy was distributed (Meeting at the Swiss
Heart Foundation, Bern, Switzerland, December 5, 2022). The impression of the
members was that the flyer was not as frequently distributed to all
patients with any level of risk for infective endocarditis as expected.
Although patients at the highest risk for infective endocarditis often received
the flyer and the antibiotic prophylaxis card (i.e., they required antibiotic prophylaxis
before dental intervention), those at intermediate (also called moderate) risk
for infective endocarditis were missed. Consequently, providers were urged to
distribute the flyer to all at-risk patients. This message will need to be
continuously reinforced at education sessions, conferences, and meetings that
include the topic of infective endocarditis across Switzerland.
Update 2023 from the European Society of Cardiology and adaptations to
the Swiss guidelines
In 2023, the European Society of Cardiology released new guidelines on infective
endocarditis [10], eight years after the previous update [7]. Antibiotic
prophylaxis for high-risk patients only and a patient-centred management
approach continue to be the two cornerstones. The task force of the European
Society of Cardiology members strengthened some preexisting specifications and
elevated the classes of recommendations for several topics.
Dental procedures
Although antibiotic prophylaxis was “considered” for high-risk patients
in the 2015 guidelines (Class IIa recommendation), the wording was changed to
“indicated” (Class I) in the 2023 guidelines. Furthermore, there is stronger
evidence that patients with previous infective endocarditis are at the highest
risk (new evidence level B) [13].
A new recommendation includes antibiotic prophylaxis for patients with a
left ventricular assist device at destination therapy (Class I, evidence level
C), and may be considered for heart transplant recipients (Class IIa). This was
mentioned in the previous Swiss guidelines, though without a class of
recommendation or level of evidence.
Because of the increasing number of transcatheter edge-to-edge valve
repair cases, the European Society of Cardiology proposed antibiotic
prophylaxis in these patients up to
six months following their repair (figure
1). The benefits of this strategy appear to outweigh the risks of antibiotic
side effects in this patient group, characterised by higher ages and morbidity and
relatively low life expectancy. However, this matter is debated and should be
decided on a case-by-case basis.
Non-invasive dental procedures
Recent studies from Sweden and England using nationwide databases have
shown a link between non-dental invasive procedures and the risk of infective
endocarditis. A Swedish study [14] compared infective endocarditis risk during
two periods: 12 weeks before endocarditis and the same period one year earlier.
In 7013 infective endocarditis patients, coronary artery bypass grafting, skin
and wound procedures, transfusions, dialysis, bone marrow puncture, and some
endoscopies, particularly bronchoscopy, were strongly associated with increased
infective endocarditis risk. An English study [15] compared infective
endocarditis hospital admissions in the 3 months before infective endocarditis
(case period) with the preceding 12 months (control period), involving almost
15,000 admissions. It found associations between infective endocarditis and non-dental
invasive procedures, such as permanent pacemaker and defibrillator implantations,
gastrointestinal endoscopies, bone marrow biopsies, bronchoscopies, and blood
transfusions. Based on this evidence, the European Society of Cardiology
decided that a class III recommendation against antibiotic prophylaxis for
high-risk patients undergoing non-dental medical procedures was no longer
appropriate, supporting instead a class IIb recommendation. Our group feel that
the evidence is too low, and we do not recommend antibiotic prophylaxis for non-dental
invasive procedures. However, physicians should be aware of these findings and
take special care with high-risk patients, adhering to infection control
measures.
Outlook
We are
convinced that our efforts to improve patient education and prevention
strategies are heading in the right direction; however, further promotion is
necessary. All disciplines involved must work together. Over the last three
years, since the publication of the Swiss Update Initiative in 2021, the most
challenging aspect of our campaign has been the dissemination of information to
providers who have direct patient contact. Figure 4 shows the number of endocarditis
cards distributed by the Swiss Heart Foundation from 2021–2023.
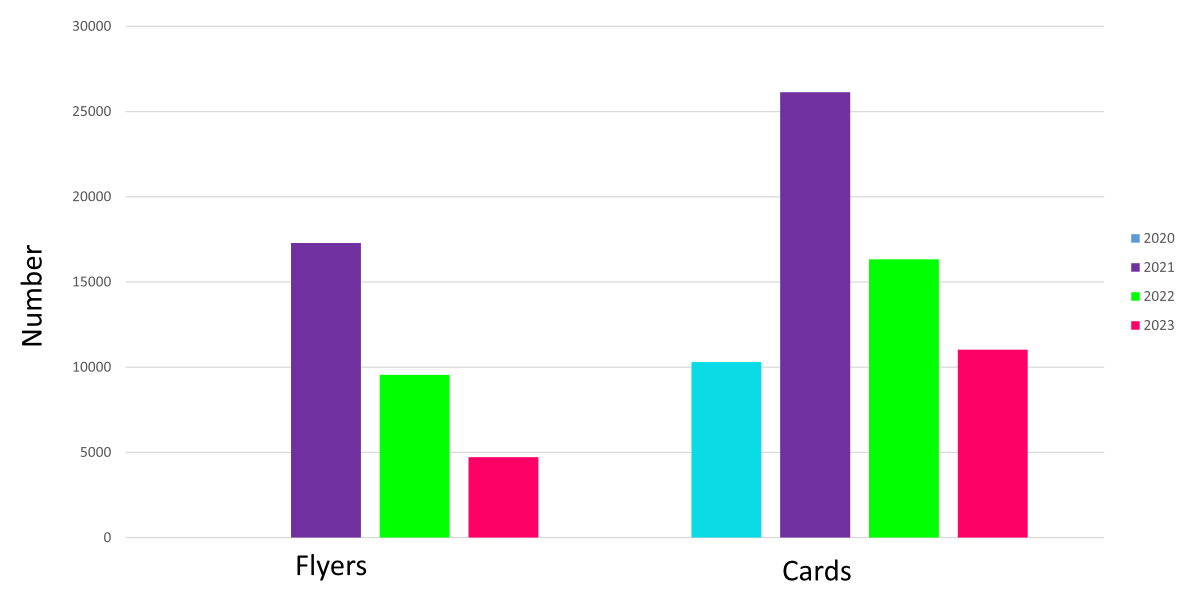
Figure 4Distribution of endocarditis flyers
and cards by the Swiss Heart Foundation from 2020 to 2023. Flyers have been available
since 2021, meaning there was no distribution in 2020 (i.e., the missing blue
column on the left). A new version of the endocarditis cards and flyers was printed
in 2021 and then distributed gardually over the following years. This explains the
high number of flyers and cards distributed in 2021 and the subsequent decline in
2022–2023.
Infective
endocarditis is a systemic disease with a non-specific clinical presentation.
First contact with medical personnel can involve various specialists, with
cardiologists often being last. Therefore,
education concerning early diagnosis must be provided. Many disciplines are
involved in the management and prevention of infective endocarditis. A broad
dissemination campaign targeting healthcare professionals, in particular
general practitioners and medical practice assistants, is essential to reach as
many people as possible to promote consideration of infective endocarditis
early in the diagnostic process. Another goal is to increase the awareness of
risks and hygiene precautions for all
at-risk patients. These goals underscore the importance of educating both
patients and doctors.
Prof. Parham Sendi, MD
Institute
for Infectious Diseases
University of Bern
Hochschulstrasse 6
CH-3012 Bern
parham.sendi[at]ifik.unibe.ch
References
1. Sendi P, Hasse B, Frank M, Flückiger U, Boggian K, Guery B, et al.; Swiss Medical
Weekly. Infective endocarditis: prevention and antibiotic prophylaxis. Swiss Med Wkly.
2021 Feb;151(708):w20473. doi: https://doi.org/10.4414/smw.2021.20473
2. ssi.guidelines.ch. Swiss Society for Infectious Diseases; c2024. Available from: https://ssi.guidelines.ch/guideline/2979/
3. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al.; American
Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; American
Heart Association Council on Cardiovascular Disease in the Young; American Heart Association
Council on Clinical Cardiology; American Heart Association Council on Cardiovascular
Surgery and Anesthesia; Quality of Care and Outcomes Research Interdisciplinary Working
Group. Prevention of infective endocarditis: guidelines from the American Heart Association:
a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and
Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the
Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia,
and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation.
2007 Oct;116(15):1736–54. doi: https://doi.org/10.1161/CIRCULATIONAHA.106.183095
4. Centre for Clinical Practice at NICE (UK). Prophylaxis against infective endocarditis:
Antimicrobial prophylaxis against infective endocarditis in adults and children undergoing
interventional procedures. London: National Institute for Health and Clinical Excellence
(UK); c2024. Available from: https://www.nice.org.uk/Guidance/cg64
5. Geach T. Epidemiology: infective endocarditis rises as prophylactic antibiotic use
falls. Nat Rev Cardiol. 2015 Jan;12(1):5. doi: https://doi.org/10.1038/nrcardio.2014.200
6. Thornhill MH, Gibson TB, Cutler E, Dayer MJ, Chu VH, Lockhart PB, et al. Antibiotic
Prophylaxis and Incidence of Endocarditis Before and After the 2007 AHA Recommendations.
J Am Coll Cardiol. 2018 Nov;72(20):2443–54. doi: https://doi.org/10.1016/j.jacc.2018.08.2178
7. Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H, et al.; ESC Scientific
Document Group. 2023 ESC Guidelines for the management of endocarditis. Eur Heart
J. 2023 Oct;44(39):3948–4042. 10.1093/eurheartj/ehad193 10.1093/eurheartj/ehad193
8. Jaussi A, Flückiger U. Revidierte schweizerische Richtlinien für die Endokarditis-Prophylaxe.
Kardiovaskuläre Medizin. 2008;11(12):392–400.
9. Schweizerische Herzstiftung. Endokarditis. c2024. Available from: https://swissheart.ch
10. Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H, Caselli S, Doenst T,
Ederhy S, Erba PA, Foldager D, Fosbøl EL, Kovac J, Mestres CA, Miller OI, Miro JM,
Pazdernik M, Pizzi MN, Quintana E, Rasmussen TB, Ristić AD, Rodés-Cabau J, Sionis A,
Zühlke LJ, Borger MA; ESC Scientific Document Group. 2023 ESC Guidelines for the management
of endocarditis. Eur Heart J. 2023 Pct 14:44(39):3948-4042.
11. Bundesamt für Gesundheit. Kennzahlen zur Transplantation und zum Empfang von Organen.
c2024. Available from: https://www.bag.admin.ch/bag/de/home/zahlen-und-statistiken/zahlen-fakten-zu-transplantationsmedizin/zahlen-fakten-zur-spende-und-transplantation-von-organen/kennzahlen-transplantation-und-empfang-von-organen.html
12. Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al.; International
Society of Heart and Lung Transplantation Guidelines. The International Society of
Heart and Lung Transplantation Guidelines for the care of heart transplant recipients.
J Heart Lung Transplant. 2010 Aug;29(8):914–56. doi: https://doi.org/10.1016/j.healun.2010.05.034
13. Thornhill MH, Jones S, Prendergast B, Baddour LM, Chambers JB, Lockhart PB, et al. Quantifying
infective endocarditis risk in patients with predisposing cardiac conditions. Eur
Heart J. 2018 Feb;39(7):586–95. doi: https://doi.org/10.1093/eurheartj/ehx655
14. Janszky I, Gémes K, Ahnve S, Asgeirsson H, Möller J. Invasive Procedures Associated
With the Development of Infective Endocarditis. J Am Coll Cardiol. 2018 Jun;71(24):2744–52.
doi: https://doi.org/10.1016/j.jacc.2018.03.532
15. Thornhill MH, Crum A, Campbell R, Stone T, Lee EC, Bradburn M, et al. Temporal association
between invasive procedures and infective endocarditis. Heart. 2023 Jan;109(3):223–31.
doi: https://doi.org/10.1136/heartjnl-2022-321519



