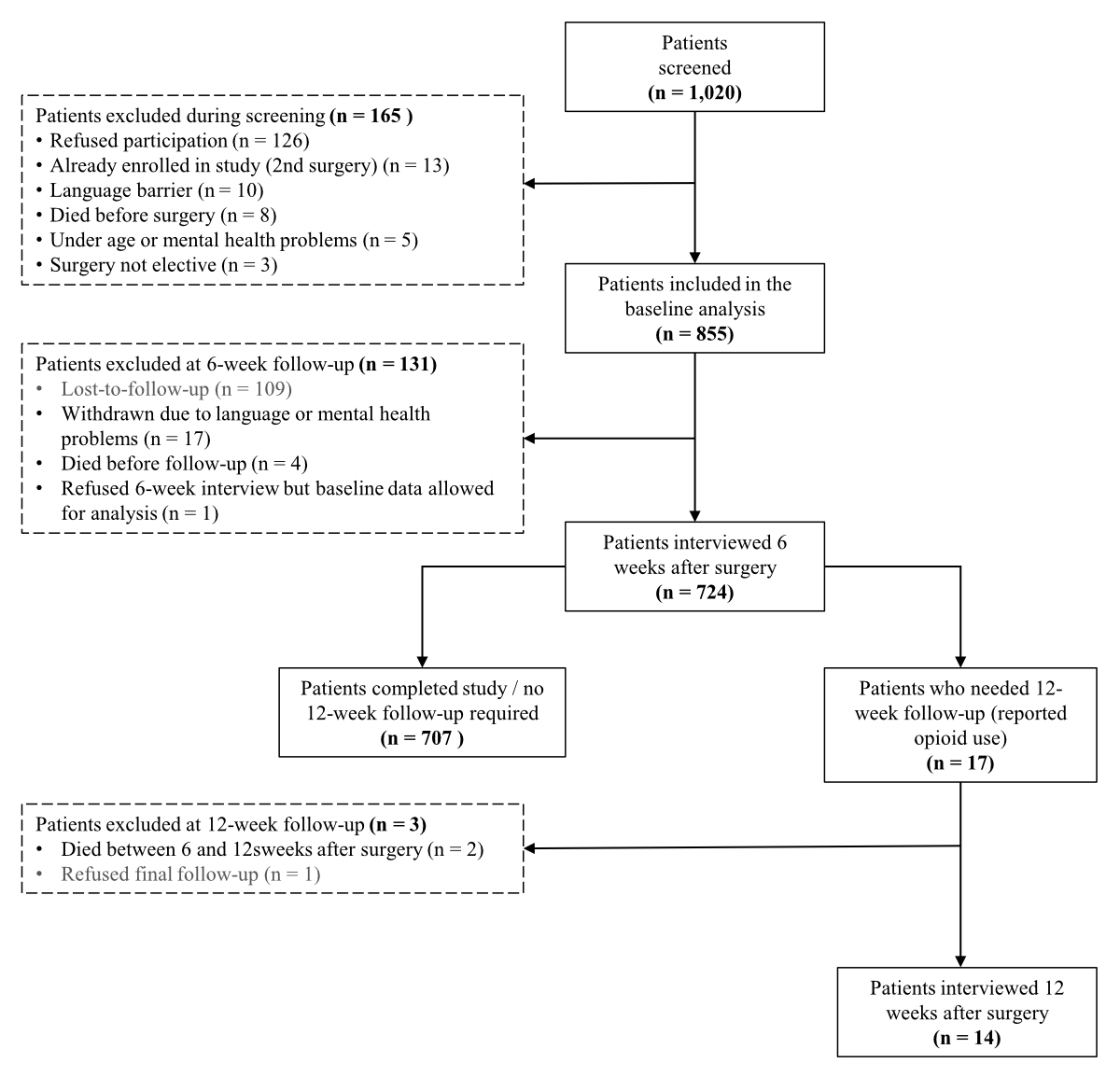
Figure 1Flow diagram of patient screening and inclusion.
DOI: https://doi.org/https://doi.org/10.57187/s.4152
Opioids are essential in the management of acute and chronic pain, including use in anaesthesia and for treating postoperative pain. However, excessive exposure may lead to persistent or prolonged opioid use [1, 2]. In October 2017, the Department of Health and Human Services declared the opioid overdose epidemic a public health emergency [3, 4] in the United States of America. At that time, the number of deaths (17,029) from a prescription opioid overdose in the US was nearly five times higher than the number reported in 1999 [5]. Three important medical factors that apparently contributed to increased opioid use and patient expectations of zero pain levels were: (1) the American Pain Society’s “Pain as the fifth vital sign” campaign launched in 1996 [6], (2) more comprehensive pain management approaches, and (3) aggressive pharmaceutical marketing and sales strategies [1].
Although data reported by European countries indicate more restrictive prescribing behaviour than the one in the US [7], an international comparison published in 2018 found that Swiss opioid consumption had risen between 1985 and 2015 from 18 to 421 mg per person/year [8]. According to this report, Switzerland had the seventh highest per capita consumption in 2015. The prescription rates for pain medication, including strong opioids, have increased over the last ten years [9]. Furthermore, a recent study of reports from the Swiss national poisons centre found a 177% increase in the rate of opioid-related poisoning calls [10]. In contrast, a single-centre retrospective chart review found that opioids prescribed in the emergency department decreased from 2013 to 2017 [11], which may have been in response to increased global awareness of the risks of opioid misuse.
Undergoing surgery is one of the most common indications for initiating opioids, with some studies reporting that 3–8% of opioid-naive patients consume opioids 12 months postoperatively [12]. Although many patients are choosing to take fewer opioids than originally prescribed for short-term postoperative pain, the leftover pills may be prone to drug diversion if not properly stored or discarded [13]. In response to these trends and the fear of contributing to the problem, anaesthetists have been changing their practice, including intraoperative care, by using opioid-sparing or even opioid-free anaesthesia [12, 14].
Nevertheless, it remains unclear whether an association exists between postoperative prescription practices and opioid misuse in Switzerland. Further evidence is needed to determine whether similar strategies for tackling the US opioid epidemic, such as opioid-free anaesthesia, are warranted. Our study’s primary aim was to investigate the extent of persisting opioid use among patients undergoing intermediate-to-major elective surgery at a Swiss cantonal hospital, and the secondary aim was to identify factors predictive of persistent use.
This single-centre prospective cohort study was conducted at a cantonal hospital in northeast Switzerland that serves approximately 150,000 inhabitants in a rather rural community. The hospital treats approximately 15,000 inpatient cases and performs close to 9000 anaesthetics per year. Physicians in this canton can obtain authorisation to operate private pharmacies, allowing them to dispense medications, including opioids, directly from their offices. A public pharmacy is located on the hospital premises, where many patients fill their prescriptions.
This project was approved by the local ethics committee (Ethics Committee East Switzerland, EKOS approval number 2022-00503, 31 March 2022) and conducted in accordance with the principles of the Declaration of Helsinki. To assess eligibility, we searched the electronic medical records to identify patients with a signed general consent form – whether granting or refusing consent – that the institution routinely obtains for further use of data for research purposes. At this point, we excluded patients who had completed the general consent form but refused use of their data for research purposes. Only patients who had provided written consent or those whose consent forms were missing were contacted by telephone to be informed about the study. Verbal consent was requested at each telephone contact (6 and 12 weeks postoperatively) and the response was subsequently documented in the study’s electronic data capture system. Verbal refusal to participate took precedence over pre-existing, written general consent. The study was registered at the German Clinical Trials Register (DRKS00029189).
All consecutive patients aged 18 years or over undergoing, on an elective basis, (1) primary unilateral hip arthroplasty; (2) intermediate spinal surgery; (3) intermediate-to-major visceral surgery such as cholecystectomy, hemicolectomy, bariatric surgery; (4) partial or complete prostatectomy; (5) a caesarean delivery; or (6) major hand surgery (planned duration of surgery >60 minutes), were assessed for inclusion. We included this range of intermediate-to-major surgery types as it encompassed commonly performed procedures (hip arthroplasty, prostatectomy, spinal surgery, visceral surgery), those typically associated with higher pain risks (extended hand surgery) and those with reportedly elevated rates of persistent opioid use (caesarean section). Additionally, this range provided a representative cross-section of the patient population, capturing diverse characteristics across the surgical groups, and ensuring an adequate number of cases annually.
The study enrolment period covered one calendar year (1 June 2022 to 31 May 2023) and was designed to minimise bias from potential seasonal variations, given that the procedures were elective. We excluded patients who verbally refused to participate, denied further use of medical data for research purposes (declined written general consent form of our institution), were cognitively impaired or mentally incapable of completing the interviews, were unable to communicate in the German language or who had been previously enrolled in this study. On the basis of the number of operations performed in previous years, we estimated that approximately 1000 patients would be screened for enrolment. Ours was a convenience sample, not determined using an a priori power analysis.
Baseline data were collected during pre-admission anaesthesia visits while clinical data, documented from hospital admission to discharge, were extracted from medical and anaesthesia records. Additionally, structured telephone interviews were conducted by physicians at 6 weeks post-surgery (± 1 week). Only patients who reported still taking opioids at this point were contacted again for an interview at 12 weeks post-surgery (± 1 week). No more than three attempts were made to contact each study participant by telephone for follow-up interviews after surgery. If attempts was unsuccessful, the patient was classified as “lost to follow-up”.
During these telephone calls, the interviewers used a structured questionnaire (in German, see supplementary file at https://doi.org/10.57187/s.4152) and responses were entered directly into the electronic data capture system. Two trained anaesthetists carried out the interviews. When first calling the patient at six weeks following surgery, the interviewers explained the aim and design of the study, and the patient was asked for verbal consent to participate before starting the interview. A follow-up appointment was scheduled if the patient needed more time to consider involvement in the study. In addition, patients were given the opportunity to ask questions. Additional verbal consent was requested for the follow-up interview at 12 weeks for eligible patients.
The structured telephone interviews, which consisted of 10 to 27 questions (depending on responses), lasted approximately 5 to 15 minutes. Similar variables were collected at both the 6- and 12-week interviews (opioid use and amount, source of prescription, pain levels and alternative pain-relieving strategies). The tool was pre-tested for validity with hospital administrative staff and then modified accordingly.
Basic demographic data (age, sex, height, weight), clinical characteristics (ASA [American Society of Anesthesiologists] physical status, major comorbidities defined as ASA physical status ≥3), concomitant medications, and pain levels at rest and during movement measured using visual analogue scales (VAS; 10-point scales with 10 denoting maximum pain) were collected at the pre-anaesthesia clinical visit and from hospital admission data. Postoperative complications/adverse events grade ≥3 according to the Clavien-Dindo classification [15] and hospitalisation data (surgery type and duration, anaesthesia type, opioids administered during the anaesthesia episode, length of hospitalisation) were extracted from the anaesthesia chart and electronic medical records. The use of opioids before surgery, as well as the type and dosage of opioid prescriptions given at hospital discharge, were collected directly from the hospital’s electronic information system. Opioids administered during anaesthesia episodes, particularly fentanyl and the short-acting opioid remifentanil, were found in the anaesthesia chart.
The primary endpoint of this study was the rate of persistent opioid use, defined as using opioids after 6 or 12 weeks. While persistent use is more commonly defined at 12 weeks, we broadened our case definition to include 6-week use to increase sensitivity and identify more cases. While this deviated from the original protocol, this modification was considered necessary due to the low rate of use in the population. Secondary endpoints were patient-reported pain levels (VAS scores) and overall pain management strategies. We analysed the following additional factors that may have influenced persistent opioid use: the percentage of patients who reported filling the prescription at the hospital, the total morphine milligram equivalent dose [16] and the type of opioid dispensed. In addition, opioids prescribed by physicians outside our institution were assessed by asking the patients about this during the telephone interview; the information could then be compared to the information gathered about opioid use from hospital prescriptions. Lastly, patients were asked about how they handled unused pills.
For this longitudinal study, we performed mostly descriptive analyses for categorical and continuous variables. Normality tests were done using the Shapiro-Wilk test, and data were presented as either mean/standard deviation or median / interquartile range (IQR). The pain levels (VAS scores) before and after surgery were compared using the Wilcoxon signed-rank test. In addition, we assessed the association of preoperative dosage with the likelihood of persistent use (6 or 12 weeks after surgery) using bivariate logistic regression and compared the median morphine milligram equivalent values using the Mann-Whitney U test. Bivariate logistic regression was also used to measure the association between pre- and postoperative pain levels and persistent opioid use. Furthermore, two different multivariate logistic regression models were used to assess the association of potential predictors on the dependent outcome (persistent opioid use): model I predictors were age, sex, ASA score, preoperative pain levels at rest; model II predictors were age, sex, ASA score, preoperative morphine milligram equivalent values. Due to the potential multicollinearity between preoperative pain and morphine milligram equivalent values, these variables were analysed in separate models. All tests were two-sided, and the level of statistical significance was set at <0.05. The electronic data capture system was developed using FileMaker Pro (version 20.3.2.201, Claris International Inc., Cupertino, CA, USA), and Stata version 15.0 (StataCorp, College Station, TX, US) was used to carry out the analyses.
Of the 1020 patients screened for inclusion in the study according to their surgery, 855 were included in the main analysis. The flow diagram depicted in figure 1 provides the number of patients screened and reasons for exclusion at each study phase. At the 6-week follow-up, 724 (85%) patients completed the interview, and 14 of the 17 (82%) eligible patients completed the 12-week interview.

Figure 1Flow diagram of patient screening and inclusion.
Of the 855 patients included in the main analysis, the largest proportion (30%) underwent intermediate-to-major visceral surgery. Table 1 presents demographic and patient characteristics according to surgery type. The median age of the entire cohort was 62 years (IQR 45–73), 52% were male and the median BMI was 27 kg/m² (IQR 24–32). The median ASA score was 3 (IQR 3–3, range 1–4), 77% (n = 656) had major comorbidities and 66% (n = 564) were taking concomitant medication. Among the 564 patients taking concomitant medication, the median number of medications was 2 (range 0–14). Complications occurred in 51 (6%) patients. More than two-thirds of the complications resulted in additional surgery.
Table 1Patient and surgical characteristics according to surgery type (n = 855).
| Parameters | Total | Major hand | Caesarean delivery | Prostatectomy | Spinal | Intermediate-to-major visceral | Hip arthroplasty | ||||||||
| n | % | 855 | 100% | 153 | 18% | 115 | 13% | 80 | 9% | 71 | 8% | 258 | 30% | 178 | 21% | |
| Age at surgery in years* | 62 | 45–73 | 59 | 47–69 | 33 | 30–36 | 69 | 66–76 | 67 | 56–79 | 60 | 47–73 | 71 | 63–78 | |
| Body mass index in kg/m2* | 27 | 24–32 | 25.5 | 23–29 | 29 | 27–33 | 26 | 24–29 | 27 | 25–31 | 28 | 24–37 | 27 | 25–32 | |
| Missing, n | 4 | 1 | 1 | – | – | 2 | – | ||||||||
| Sex, n | % | Male | 443 | 52% | 88 | 58% | – | – | 80 | 100% | 29 | 41% | 134 | 52% | 112 | 63% |
| Female | 412 | 48% | 65 | 42% | 115 | 100% | – | – | 42 | 59% | 124 | 48% | 66 | 37% | |
| ASA* | 3 | 3–3 | 3 | 2–3 | 2 | 2–3 | 3 | 3–3 | 3 | 3–3 | 3 | 3–3 | 3 | 3–3 | |
| Major comorbidities present, n | 656 | 77% | 102 | 67% | 31 | 27% | 73 | 91% | 63 | 89% | 229 | 89% | 158 | 89% | |
| Number of comorbidities* | 1 | 1–3 | 1 | 0–2 | 0 | 0–1 | 2 | 1–3 | 2 | 1–4 | 2 | 1–3 | 2 | 1–3 | |
| Concomitant medications used, n | % | 564 | 66% | 89 | 58% | 24 | 21% | 65 | 81% | 63 | 89% | 176 | 68% | 147 | 83% | |
| Number of concomitant medications* | 2 | 0–4 | 1 | 0–3 | 0 | 0–0 | 2 | 1–4 | 4 | 2–7 | 2 | 0–4 | 3 | 1–5 | |
| Length of hospitalisation, in days* | 4 | 3–7 | 2 | 0–2 | 4 | 4–5 | 3 | 2–4 | 7 | 5–10 | 4 | 3–8 | 7 | 6–8 | |
| Duration of surgery, in minutes* | 68 | 48–109 | 84 | 63–108 | 50 | 45–57 | 56 | 42–78 | 112 | 60–152 | 107 | 73–162 | 48 | 40–60 | |
| Anaesthesia type, n | % | General | 611 | 71% | 85 | 56% | 0 | 0% | 69 | 86% | 71 | 100% | 222 | 86% | 168 | 94% |
| Regional | 200 | 23% | 64 | 42% | 115 | 100% | 11 | 14% | 0 | 0% | 1 | 0% | 9 | 5% | |
| Combination | 40 | 5% | 4 | 3% | 0 | 0% | 0 | 0% | 0 | 0% | 35 | 14% | 1 | 1% | |
| Remifentanil used, n | % | 600 | 70% | 88 | 58% | 2 | 2% | 63 | 79% | 67 | 94% | 227 | 88% | 153 | 86% | |
| Remifentanil amount* | 0.92 | 0.58–1.46 | 0.77 | 0.47–1.4 | 0.6 | 0.6–0.6 | 0.57 | 0.36–0.99 | 1 | 0.56–1.72 | 1.24 | 0.84–2.04 | 0.69 | 0.48–0.98 | |
| Postoperative complications occurred, n | % | 51 | 6% | 3 | 2% | 0 | 0% | 3 | 4% | 4 | 6% | 38 | 15% | 10 | 6% | |
| Patient died, n | % | 6 | 1% | 0 | 0% | 0 | 0% | 1 | 1% | 2 | 3% | 3 | 1% | 0 | 0% | |
ASA: American Society of Anesthesiologists physical status.
* Median and interquartile range
The median length of hospitalisation was 4 days (IQR 3–7). Regarding anaesthesia type, 72% were general, 23% regional and 5% combined. The median duration of the surgeries was 68 minutes (IQR 48–109). Remifentanil was used in 70% of the cases (median dose 0.92 mg, IQR 0.58–1.46). The median intraoperative fentanyl dose was 0.4 mg (IQR 0.3–0.5), morphine was used in 39 cases (4.6%) and methadone was administered in 154 (18%) patients. Fifty-six patients were taking opioids before surgery, and the median dosage was 30 morphine milligram equivalent (IQR 15–60). Other pain medications taken before surgery included paracetamol (11%), metamizole (4%) and nonsteroidal anti-inflammatory drugs (3%), while 82% reported not taking any form of medication to relieve pain. At the time of discharge, 40 (4.7%) patients received an opioid prescription.
A total of 724 patients completed the 6-week follow-up interview. Of the 40 patients who received an opioid prescription at hospital discharge, 17 filled that prescription. An additional 9 patients received a prescription from an external source other than the hospital (i.e. primary care physicians), and 4 filled prescriptions from both sources. Thirty patients (4%) reported having taken opioids at some point from the time of discharge to the 6-week telephone interview (median morphine milligram equivalent 60, IQR 22–131), with a median length of consumption of 7 days (IQR 3–18). The opioid types in descending order of frequency were oxycodone (46%), fentanyl (22%), tramadol (14%), morphine sulphate (13%) and hydromorphone (5%). Thirteen of the patients reported having stopped taking the opioids at the time of the interview. Therefore, 17 (2%) patients were still taking opioids at the 6-week interview. Of the five patients who stated that they had leftover pills, none had disposed of them.
VAS during movement decreased from a median of 2 (IQR 0–6) preoperatively to 0 (IQR 0–2) at 6 weeks postoperatively (p <0.0001). Bivariate logistic regression tests showed that preoperative pain levels (at rest and during movement) were associated with persistent opioid use (odds ratio [OR] 1.27, 95% confidence interval [CI]: 1.11–1.46, p = 0.001; OR 1.3, 95% CI: 1.12–1.5, p = 0.001, respectively), as were 6-week postoperative pain levels (OR 1.96, 95% CI: 1.61–2.39, p <0.0001; OR 1.82, 95% CI: 1.52–2.18, p <0.0001, respectively). Forty-eight percent of the patients used alternative pain reduction methods (most commonly physical therapy) and 18% took other pain medications (e.g. paracetamol, nonsteroidal anti-inflammatory drugs, metamizole). The most commonly reported reason to stop taking pain medication was no need (63%), followed by success with other pain reduction methods (33%), side effects (3%) and fear of addiction (1%).
Of the 17 patients still taking opioids 6 weeks after surgery, 14 were successfully interviewed again at 12 weeks. Seven patients were still taking opioids at 12 weeks. Five of the seven patients were preoperative opioid users. The two opioid-naive patients who were taking opioids 12 weeks after surgery were: (1) an 83-year-old female taking hydromorphone after spinal surgery who reported pain during movement at level 8 on a 10-point scale, and (2) a 75-year-old male taking tramadol after hand surgery despite decreasing pain scores over time (VAS 8 preoperatively, 5 at 6 weeks and 3 at 12 weeks). Neither of these patients experienced a surgery-related complication. Table 2 summarises patient and clinical characteristics of the persistent opioid users. For this subgroup, the median morphine milligram equivalent at the last follow-up was 64 (IQR 60–120). One case was taking a high dosage (1200 morphine milligram equivalent/day) to treat chronic back pain from a traumatic injury sustained 20 years earlier, although the surgery type for this study was unrelated to this pre-existing condition.
Table 2Patient characteristics and outcomes of persistent opioid users three months after surgery (n = 7).
| Age | Sex | Surgery type | BMI | ASA | Comorbidities | Concomitant medications | Complications? | Preoperative opioid use? | Opioid type / morphine milligram equivalent preoperatively | Opioid type / morphine milligram equivalent at 12-week follow-up | VAS preoperatively (rest/moving) | VAS at 6-week follow-up (rest/moving) | VAS at 12-week follow-up (rest/moving) |
| 59 | Female | Visceral | 46 | 3 | 3 | 3 | No | Yes | Morphine sulfate (1600) | Morphine sulfate (1200) | 0/5 | 7/7 | 0/4 |
| 65 | Female | Visceral | 40 | 4 | 4 | 6 | Yes | Yes | Oxycodone (60) | Oxycodone (60) | 0/0 | 0/0 | 0/0 |
| 75 | Male | Hand | 28 | 3 | 3 | 6 | No | No | – | Tramadol (9.75) | 5/8 | 3/5 | 0/3 |
| 76 | Female | Hand | 34 | 3 | 4 | 7 | No | Yes | Fentanyl (180) | Fentanyl (120) | 5/6 | 0/3 | 0/0 |
| 83 | Female | Spinal | 24 | 3 | 3 | 1 | No | No | – | Hydromorphone (64) | 0/5 | 0/7 | 2/8 |
| 84 | Female | Spinal | 27 | 3 | 5 | 14 | No | Yes | Fentanyl / oxycodone (149) | Fentanyl / oxycodone (75) | 3/8 | 4/9 | 5/6 |
| 91 | Male | Hand | 23 | 3 | 4 | 9 | No | Yes | Fentanyl (60) | Fentanyl (60) | 7/9 | 3/5 | 2/4 |
ASA: American Society of Anesthesiologists classification; BMI: body mass index; VAS: visual analogue scale (0–10 with 10 denoting highest pain level).
When comparing the preoperative morphine milligram equivalent levels of the persistent opioid users (6 or 12 weeks after surgery) with non-persistent users, the median morphine milligram equivalent was 60 (IQR 30–180) versus 22.5 (IQR 15–30), respectively (p = 0.0155). The bivariate logistic regression analysis revealed a slight positive association between higher preoperative morphine milligram equivalent dosage and persistent postoperative opioid use (OR 1.024, 95% CI: 1.003–1.0456, p = 0.023), with a 2.4% increase in the likelihood of prolonged use per morphine milligram equivalent unit.
After controlling for age, sex, ASA score and preoperative pain levels (model I), we found that higher ASA (OR 11.8, 95% CI: 2.48–56.51, p = 0.002) and preoperative pain levels (OR 1.23, 95% CI: 1.05–1.43, p = 0.008) were associated with prolonged opioid use. However, in the subsequent multivariate logistic regression (model II, with predictors: age, sex, ASA score, preoperative morphine milligram equivalent values), only higher morphine milligram equivalent values were associated with persistent use (OR 1.02, 95% CI: 1.01–1.05, p = 0.041).
Patients undergoing certain types of major elective surgery at a Swiss cantonal hospital were interviewed about their opioid consumption up to 12 weeks after surgery. Less than 1% of the patients who were opioid-naive before surgery were still taking opioids 12 weeks after surgery, and most who were using opioids prior to surgery stopped before the 12-week follow-up. Furthermore, only 5% of patients left the hospital with an opioid prescription. Of the 724 patients included in the follow-up, 7 were still using opioids three months after surgery, 5 of whom were preoperative users. Pre- and postoperative pain levels were associated with persistent opioid use, and the median preoperative morphine milligram equivalent of persistent opioid users was significantly higher than non-persistent users.
Opioid use post-surgery was very low in our study, even lower than previously reported rates of new persistent opioid users (5–10%) in surgical patients. A 2017 study published in Journal of the American Medical Association, which included 36,177 patients grouped according to minor (80%) or major (20%) surgery, found that the new persistent opioid user rates were similar between the two surgical groups (5.9% and 6.5%, respectively) [17]. In our current study, only two opioid-naive patients were taking opioids 12 weeks after surgery. Although one of these patients reported chronic, elevated pain levels after spinal surgery, the other case experienced decreasing pain after hand surgery – and no surgery-related complications developed in either of these cases.
Reported discrepancies in prescribing patterns among countries may explain difference among studies and contribute to the complexity of extrapolating findings. A large multicentre study of 4690 patients undergoing intermediate surgery (appendectomy, inguinal hernia repair, cholecystectomy) found significant differences in opioid prescribing patterns at hospital discharge between the US and the other included countries (91% versus 5%, respectively) [18]. The prescription rate at our hospital was low (4.7%). Similar differences were reported in a multicountry comparison, including 129,379 patients in the US, 84,653 in Canada and 9802 in Sweden [7]. Eleven percent of patients from the Swedish study population who underwent any surgical procedure type filled an opioid prescription within the first seven days after discharge, compared to 76% in the United States and 79% in Canada. Another study conducted in Sweden found that 28% of bariatric surgery or cholecystectomy patients were prescribed opioids after discharge, and 5% were still receiving prescriptions 6 to 12 months after surgery [19]. All these prolonged-use patients had also been prescribed opioids before surgery, most often to manage back and joint pain. Only 7% of our study population reported preoperative opioid use, and of the 5% who left the hospital with an opioid prescription, one-third had undergone spinal surgery.
Similarly, we found restrictive prescribing and opioid use in our obstetric population, with only 1 of 115 patients who had a caesarean delivery receiving a hospital-issued prescription. In contrast, a US study including 308,226 deliveries (63% vaginal and 37% caesarean) found that 1.7% of women who delivered vaginally and 2.2% who had a caesarean section developed new persistent opioid use [20]. If the patients did not receive a peripartum opioid prescription, only 0.5% who had a vaginal delivery and 1.0% undergoing a caesarean delivery became new persistent opioid users.
Since a multitude of factors can lead to persistent opioid use, the potential contribution of anaesthetic agents should also be considered, particularly given that two prominent opioids – oxycodone and fentanyl – are widely used for anaesthesia and perioperative pain control. Our study population received opioids intraoperatively because of the potential advantages such as blunting surgical stress response, reducing the amount of general anaesthetics and neuromuscular blocking agents, and providing a drug level for effective postoperative pain management. Furthermore, to the best of our knowledge, the hospital’s standard practice for postoperatively prescribed opioid doses is moderate, primarily restricted to the first postoperative day.
Weiner [21] concluded in his 2020 editorial that new persistent opioid use represents a common but previously underappreciated surgical complication that warrants increased awareness. However, proper opioid stewardship [22] requires identifying high-risk cases and factors, and offering comprehensive support since persistent use likely has multiple causes beyond undergoing surgery or anaesthesia. In our study, pre- and postoperative pain levels, as well as median preoperative morphine milligram equivalent, were associated with persistent opioid use. A study based on insurance claims in Switzerland proposed a model for predicting the risk of developing long-term opioid use, which is based on demographic information, episode-specific information, comorbidities, and co-medication [23]. In their model, comorbidities were associated with a higher risk for long-term opioid use, along with factors such as being aged 80 years or over, having multiple prescribers, experiencing a previous episode within the past 6 months, and using co-medications. Interestingly, a higher initial opioid dose was associated with reduced risk of long-term opioid use.
The study has both strengths and limitations. First, we gathered both objective data from medical records and subjectively reported data through telephone interviews on a sensitive topic – potential prescription drug misuse. Although most of the routinely collected data in the medical records were available, the quality and consistency of documentation of variables such as postoperative complications and comorbidities likely varied across departments, potentially influencing our findings. We attempted to mitigate several possible sources of bias through our standardised interview protocol, pre-testing of data collection tools, training interviewers, incorporating short and clear questions, and making multiple attempts to contact patients at different times of the day. Nevertheless, the most noteworthy obstacles were non-response bias (lost to follow-up), social desirability bias (socially acceptable rather than truthful responses – such as the amount or handling of leftover tablets) and recall bias (memory errors, particularly dosages, medication start and end dates, leftover tablets, pain levels).
Although we found little evidence of persistent opioid use when we scanned the medical records of patients unavailable for follow-up (missing data from 16/40 hospital-issued prescriptions), this search strategy deviated from the original protocol and was limited given that many patients go to their primary care physicians for postoperative follow-up visits. Unless the patient returned to the hospital for further care, it is unlikely this information would be available in our medical records system. Similarly, some patients who reported not taking opioids at week six may have subsequently filled a prescription. Therefore, choosing not to conduct the longer term follow-up with all patients weakened the study’s sensitivity in identifying all potential cases of persistent use. Furthermore, the exclusion of non-German-speaking patients who may be vulnerable to lack of care and poor understanding of the instructed risks of opioids, may have resulted in a loss of valuable data and potential misrepresentation of the larger population catchment area. Despite successfully reaching and prospectively following many patients (85% response rate), close to 250 of the patients screened were “missed” (lost to follow-up, refused participation or had a language barrier) over the various study phases. These missed opportunities may have been prevented with a different study design.
Second, despite including a wide range of surgery types, we only analysed elective surgeries performed at one centre in a rural Swiss canton not known for having a major opioid problem. The homogeneity of the population catchment area (predominantly German-speaking, white residents) limits our ability to generalise the findings to all Swiss hospitals. Extrapolating our results to more diverse demographic and socioeconomic populations is challenging, particularly given that other studies have identified significant differences in opioid use based on race, socioeconomic status and sex [24]. Moreover, we used a convenience sample of the surgical population rather than perform a power analysis to determine the sample size for this study.
Third, our definition of prolonged opioid use after surgery differed from the commonly used definition: “filling of at least one opioid prescription between 90 and 180 days after surgery”. We used the 6-week postoperative telephone interview to identify potential long-term users needing further follow-up. Since the rate of patients with hospital-issued opioid prescriptions was relatively low, we expected our earlier contact would improve patients’ recall and likelihood of response.
Lastly, while we made efforts to control for potential confounding factors that could have influenced the outcome under investigation, certain limitations were inherent to our analytical approach. We did not transform the VAS or ASA values to categorical variables, which could distort the relationship between the outcome and the predictors in cases of extreme values or non-linearity. In the first multivariate logistic regression model, the wide confidence interval for the ASA score indicates a high degree of uncertainty. Furthermore, the magnitude of any associations should be interpreted with caution due to the small number of cases of the outcome (persistent opioid use).
We detected a preoperative opioid use rate of 7% and restrictive postoperative prescribing behaviour of healthcare practitioners in our setting. Specifically, 5% of the patients were given a prescription at hospital discharge, 2% were still taking opioids six weeks after surgery and only 1% of the patients who underwent intermediate-to-major elective surgery at a Swiss cantonal hospital continued opioid use 12 weeks postoperatively. This low rate of prolonged opioid use may be due to the restrictive prescription policy of the centre and local healthcare providers.
Individual deidentified participant data, as well as the study protocol and statistical analysis plan will be shared after publication with investigators whose proposed use of the data has been approved by an independent review committee upon request to the corresponding author.
The authors would like to thank Marion Schmid and Carmen Di Criscio for assisting with data extraction and entry.
This research project received no financial support from an external source.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
Preliminary results of this study have been presented as a poster at the ESAIC Euroanesthesia 2023 Congress: Poster 13AP06-1213AP06-12; Rixecker A, Breitenmoser F, Welter J, Dullenkopf A. A prospective, longitudinal cohort study of persistent postsurgical opioid use among patients treated at a Swiss hospital: a preliminary analysis. Eur J Anaesthesiol. 2023;40(e-Supplement 61):414.
1. Larach DB, Hah JM, Brummett CM. Perioperative Opioids, the Opioid Crisis, and the Anesthesiologist. Anesthesiology. 2022 Apr;136(4):594–608. doi: https://doi.org/10.1097/ALN.0000000000004109
2. Callinan CE, Neuman MD, Lacy KE, Gabison C, Ashburn MA. The Initiation of Chronic Opioids: A Survey of Chronic Pain Patients. J Pain. 2017 Apr;18(4):360–5. doi: https://doi.org/10.1016/j.jpain.2016.11.001
3. Humphreys K. Avoiding globalisation of the prescription opioid epidemic. Lancet. 2017 Jul;390(10093):437–9. doi: https://doi.org/10.1016/S0140-6736(17)31918-9
4. Barreveld AM, Mendelson A, Deiling B, Armstrong CA, Viscusi ER, Kohan LR. Caring for Our Patients With Opioid Use Disorder in the Perioperative Period: A Guide for the Anesthesiologist. Anesth Analg. 2023 Sep;137(3):488–507. doi: https://doi.org/10.1213/ANE.0000000000006280
5. Huang X, Keyes KM, Li G. Increasing Prescription Opioid and Heroin Overdose Mortality in the United States, 1999-2014: An Age-Period-Cohort Analysis. Am J Public Health. 2018 Jan;108(1):131–6. doi: https://doi.org/10.2105/AJPH.2017.304142
6. Max MB; American Pain Society Quality of Care Committee. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995 Dec;274(23):1874–80. doi: https://doi.org/10.1001/jama.1995.03530230060032
7. Ladha KS, Neuman MD, Broms G, Bethell J, Bateman BT, Wijeysundera DN, et al. Opioid Prescribing After Surgery in the United States, Canada, and Sweden. JAMA Netw Open. 2019 Sep;2(9):e1910734. doi: https://doi.org/10.1001/jamanetworkopen.2019.10734
8. Ruchat D, Suter MR, Rodondi PY, Berna C. [Opioid consumption from 1985 to 2015 : the situation in Switzerland, with an international comparison]. Rev Med Suisse. 2018 Jun;14(612):1262–6. doi: https://doi.org/10.53738/REVMED.2018.14.612.1262
9. Müller D, Scholz SM, Thalmann NF, Trippolini MA, Wertli MM. Increased Use and Large Variation in Strong Opioids and Metamizole (Dipyrone) for Minor and Major Musculoskeletal Injuries Between 2008 and 2018: An Analysis of a Representative Sample of Swiss Workers. J Occup Rehabil. 2024 Mar;34(1):157–68. doi: https://doi.org/10.1007/s10926-023-10115-5
10. Hooijman MF, Martinez-De la Torre A, Weiler S, Burden AM. Opioid sales and opioid-related poisonings in Switzerland: A descriptive population-based time-series analysis. Lancet Reg Health Eur. 2022 Jun;20:100437. doi: https://doi.org/10.1016/j.lanepe.2022.100437
11. Gaertner K, Wildbolz S, Speidel V, Exadaktylos AK, Hautz WE, Müller M. Prevalence and practice of opioid prescription at a Swiss emergency department: 2013-2017. Swiss Med Wkly. 2020 Apr;150(1516):w20202. doi: https://doi.org/10.4414/smw.2020.20202
12. Koepke EJ, Manning EL, Miller TE, Ganesh A, Williams DG, Manning MW. The rising tide of opioid use and abuse: the role of the anesthesiologist. Perioper Med (Lond). 2018 Jul;7(1):16. doi: https://doi.org/10.1186/s13741-018-0097-4
13. Kharasch ED, Clark JD, Adams JM. Opioids and Public Health: The Prescription Opioid Ecosystem and Need for Improved Management. Anesthesiology. 2022 Jan;136(1):10–30. doi: https://doi.org/10.1097/ALN.0000000000004065
14. Brandal D, Keller MS, Lee C, Grogan T, Fujimoto Y, Gricourt Y, et al. Impact of Enhanced Recovery After Surgery and Opioid-Free Anesthesia on Opioid Prescriptions at Discharge From the Hospital: A Historical-Prospective Study. Anesth Analg. 2017 Nov;125(5):1784–92. doi: https://doi.org/10.1213/ANE.0000000000002510
15. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004 Aug;240(2):205–13. doi: https://doi.org/10.1097/01.sla.0000133083.54934.ae
16. Spitalpharmazie Basel. Opioid-Rechner, Version 3.0, 2017. Available from: https://www.spitalpharmazie-basel.ch/dienstleistungen/opioide.php
17. Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017 Jun;152(6):e170504. doi: https://doi.org/10.1001/jamasurg.2017.0504
18. Kaafarani HM, Han K, El Moheb M, Kongkaewpaisan N, Jia Z, El Hechi MW, et al. Opioids After Surgery in the United States Versus the Rest of the World: The International Patterns of Opioid Prescribing (iPOP) Multicenter Study. Ann Surg. 2020 Dec;272(6):879–86. doi: https://doi.org/10.1097/SLA.0000000000004225
19. Larsson V, Nordenson C, Karling P. Long-term postoperative opioid prescription after cholecystectomy or gastric by-pass surgery: a retrospective observational study. Scand J Pain. 2021 Apr;21(3):569–76. doi: https://doi.org/10.1515/sjpain-2020-0150
20. Peahl AF, Dalton VK, Montgomery JR, Lai YL, Hu HM, Waljee JF. Rates of New Persistent Opioid Use After Vaginal or Cesarean Birth Among US Women. JAMA Netw Open. 2019 Jul;2(7):e197863. doi: https://doi.org/10.1001/jamanetworkopen.2019.7863
21. Weiner SG. Addressing the ignored complication: chronic opioid use after surgery. BMJ Qual Saf. 2021 Mar;30(3):180–2. doi: https://doi.org/10.1136/bmjqs-2020-011841
22. Ardeljan LD, Waldfogel JM, Bicket MC, Hunsberger JB, Vecchione TM, Arwood N, et al. Current state of opioid stewardship. Am J Health Syst Pharm. 2020 Apr;77(8):636–43. doi: https://doi.org/10.1093/ajhp/zxaa027
23. Held U, Forzy T, Signorell A, Deforth M, Burgstaller JM, Wertli MM. Development and internal validation of a prediction model for long-term opioid use-an analysis of insurance claims data. Pain. 2024 Jan;165(1):44–53.
24. Bonar EE, Coughlin L, Roche JS, Philyaw-Kotov ML, Bixler EA, Sinelnikov S, et al. Prescription opioid misuse among adolescents and emerging adults in the United States: A scoping review. Prev Med. 2020 Mar;132:105972. doi: https://doi.org/10.1016/j.ypmed.2019.105972
The questionnaire is available for download as a separate file at https://doi.org/10.57187/s.4152.