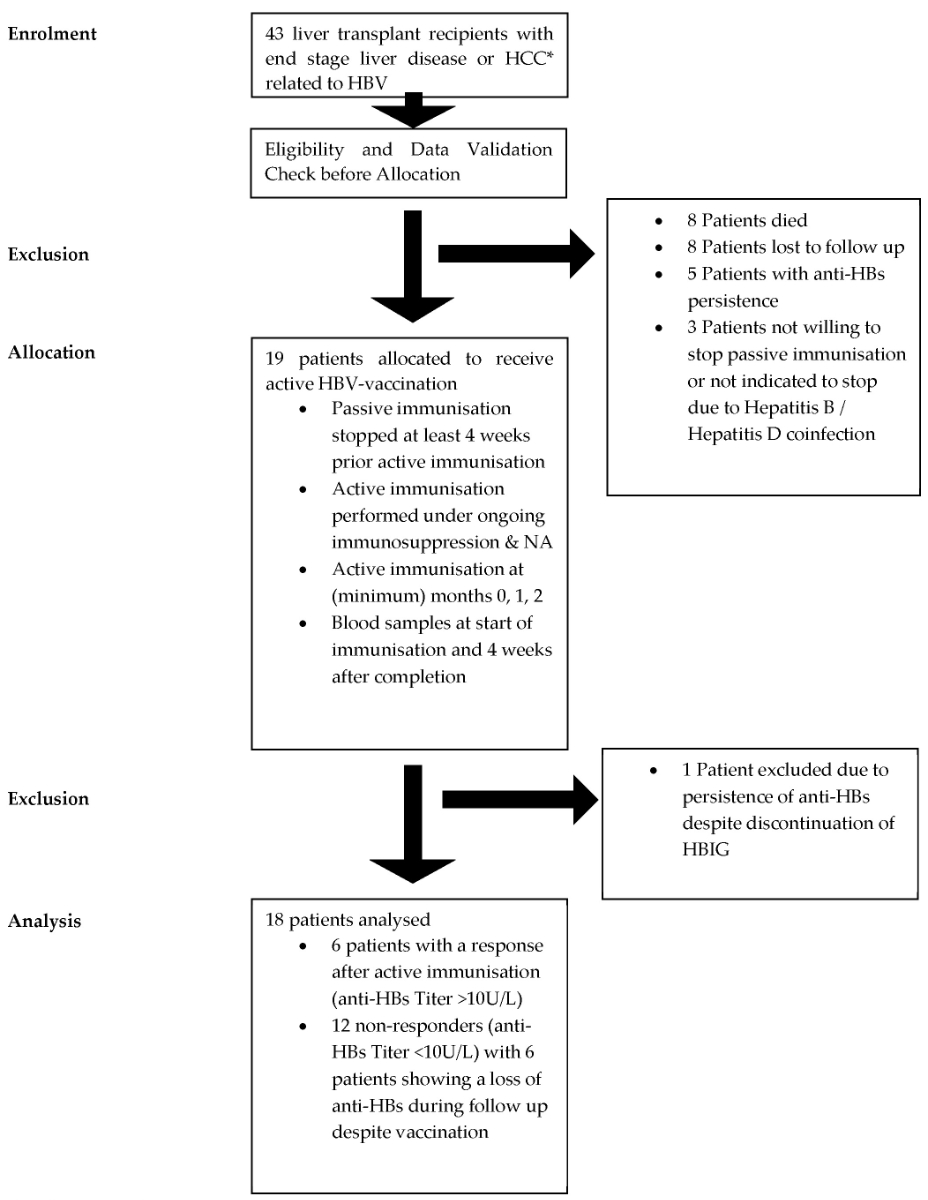
Figure 1Study protocol outline. * HCC: hepatocellular carcinoma; HBIG: hepatitis B immunoglobulin; HBV: hepatitis B virus; NA: nucleos(t)ide analogue.
DOI: https://doi.org/https://doi.org/10.57187/s.4116
In liver transplantation for diseases related to HBV, preventing HBV recurrence is crucial. In a landmark study in 1991, Samuel et al. reported that post-liver transplantation administration of hepatitis B immunoglobulin (HBIG) reduced the graft infection rate from 75% to 33% and increased 3-year survival from 54% to 83% [1]. Since this study’s publication, the standard post-liver transplant care for patients with HBV infection has been parenteral hepatitis B immunoglobulin [2]. After the approval of the first nucleos(t)ide analogue, lamivudine, and later the more effective third-generation nucleos(t)ide analogues, tenofovir and entecavir, the combination regimen of nucleos(t)ide analogues with hepatitis B immunoglobulin became standard. Various studies have shown that combination regimens significantly reduce the risk of graft re-infection to below 5% and improve patient survival rates [3, 4].
For many years, a combination of hepatitis B immunoglobulin and high-barrier nucleos(t)ide analogues such as entecavir and tenofovir has been the standard treatment [2, 5, 6]. However, because of the high costs and logistical challenges associated with long-term hepatitis B immunoglobulin use, alternative approaches such as vaccination and hepatitis B immunoglobulin-free regimens have been investigated. Recent guidelines by the WHO emphasise the need for simplified and expanded access to HBV treatments, underscoring the importance of innovative strategies in managing HBV infection [7]. Although prophylactic HBV vaccines have significantly reduced the prevalence of HBV-related diseases [8], the effectiveness of these vaccines in liver transplant recipients remains under debate [9, 10]. Furthermore, recent research has highlighted the importance of therapeutic vaccines and their potential to induce a comprehensive immune response, which could be beneficial in the post-transplantation context [11–13]. This study gathered information on a potential response or lack thereof and addressed potential adverse events associated with active immunisation after discontinuing hepatitis B immunoglobulin in conjunction with nucleos(t)ide analogue therapy in liver transplant recipients previously infected with hepatitis B.
A total of 43 liver transplantation recipients with end-stage liver disease or hepatocellular carcinoma related to HBV infection and active infection at the time of transplantation were identified. The intervention was applied in liver transplant recipients who underwent transplantation between 1994 and 2021 at the University Hospital of Bern. Participants were recruited at the University Hospital of Bern between January 2022 and December 2023. Eligibility was restricted to liver transplant recipients with HBV-related disease who were receiving hepatitis B immunoglobulin and nucleos(t)ide analogue therapy at the time of study entry. An active HBV immunisation protocol was initiated in these patients, who, after discontinuing hepatitis B immunoglobulin in 2022–2023, were receiving ongoing nucleos(t)ide analogue therapy and immunosuppression, as per clinical guidelines. Blood samples for evaluating immunisation response were collected at baseline and 4 weeks after vaccination, with follow-up extending for at least 12 months.
In this open-label cohort study, passive immunisation with hepatitis B immunoglobulin was discontinued at least 4 weeks before the commencement of active immunisation. All patients had negative anti-HBs and viral load at the time of hepatitis B immunoglobulin cessation. The hepatitis B immunoglobulin regimen varied between the participants and was at the treating physician’s discretion. Active immunisation was carried out a minimum of 4 weeks after stopping passive immunisation, under ongoing immunosuppression and antiviral therapy with nucleos(t)ide analogue. Blood samples were collected at the beginning of immunisation and 4 weeks after completion. All vaccinations were administered intramuscularly (i.m.), following standard immunisation protocols for liver transplant recipients. Not all patients received their vaccines at the University Hospital of Bern; some participants were vaccinated by their general practitioner.
Data were obtained from patient medical records and laboratory reports. Key variables, such as HBV DNA levels, anti-HBs titres, and demographic data, were assessed through standardised serological testing at baseline and follow-up visits. The immunisation response was measured through anti-HBs titres recorded in laboratory assessments. To minimise selection bias, all eligible patients during the study period were included based on predefined criteria. Observer bias was reduced by relying on objective laboratory measurements for key outcomes and using standardised questions for patient-reported adverse events. Adverse events following vaccination were systematically assessed during clinical visits using structured patient interviews. Patients were specifically asked about any symptoms or discomfort experienced after each vaccination. The questions focused on local reactions (e.g. pain, redness, or swelling at the injection site), systemic symptoms (e.g. fever, fatigue, or nausea), and the presence of severe or unusual side effects. Duration, severity, and the need for medical treatment were also recorded. This approach relied on self-reported data, which, while limited in detecting subclinical adverse events, provided a practical and patient-centred method for adverse event documentation.
The primary outcome was HBV relapse following the discontinuation of hepatitis B immunoglobulin. The secondary outcomes were the response rate to active immunisation and reported adverse events associated with active immunisation.
Following the active immunisation protocol, patients were monitored for a minimum of 12 additional months. Patients were classified as responders if they achieved anti-HBs levels of >10 IU/l at the completion of active immunisation. Non-responders (anti-HBs <10 IU/l) were not reintroduced to passive immunisation but were monitored for hepatitis B recurrence. HBV recurrence was defined as the recurrence of HBsAg and/or HBV DNA. During the follow-up visits, additional laboratory value tests (including haemogramme, liver values, and kidney retention parameters) were obtained. Patients with incomplete data and those lost to follow-up were excluded from the final analysis to ensure data consistency across reported outcomes. Missing data are indicated in table 1, where applicable.
Table 1Comparison between responders and non-responders. Because of the descriptive nature of this study and the small sample size, no inferential statistics were performed, and p-values have been omitted. The number of participants with missing data is indicated as n-X.
| Variable | Non-responder (n = 12) | Responder (n = 6) | |
| Sex male (%) | 9 (75.0) | 4 (66.7) | |
| Before liver transplantation | |||
| Hepatocellular carcinoma (%) | 5 (41.7) | 2 (33.3) | |
| MELD Score (median [IQR]) | 13.00 [9.00, 16.50] n-1 | 16.00 [9.00, 17.00] n-1 | |
| HBsAg positive (%) | 12 (100.0) | 4 (66.7) n-1 | |
| Anti-HBs positive (%) | 0 (0.0) n-1 | 1 (16.7) n-1 | |
| HBeAg positive (%) | 1 (8.3) n-1 | 1 (16.7) n-3 | |
| HBV DNA positive (%) | 7 (58.3) n-2 | 1 (16.7) n-2 | |
| At time of vaccination | |||
| Age in years (median [IQR]) | 60.00 [51.00, 66.25] | 61.00 [50.50, 69.25] | |
| Duration from liver transplantation to first vaccination in days (median [IQR]) | 2555.00 [1368.75, 6218.00] | 6387.50 [4562.50, 7665.00] | |
| Number of vaccines (median [IQR]) | 3.00 [3.00, 4.00] | 3.50 [3.00, 4.00] | |
| Anti-HBs positive (%) | 5 (41.7) | 1 (16.7) | |
| BMI (median [IQR]) | 27.60 [23.95, 29.00] n-1 | 28.10 [24.90, 29.00] n-1 | |
| Smoking (%) | 1 (8.3) | 0 (0.0) | |
| Diabetes mellitus (%) | 5 (41.7) | 2 (33.3) | |
| Mono-immunosuppression (%) | 10 (83.3) | 4 (66.7) | |
| Nucleos(t)ide analogue (%) | NA | NA | |
| Tenofovir alafenamide | 7 (58.3) | 2 (33.3) | |
| Tenofovir disoproxil fumarate | 1 (8.3) | 0 (0.0) | |
| Entecavir | 1 (8.3) | 1 (16.7) | |
| Lamivudine | 3 (25.0) | 3 (50.0) | |
| Creatinine in μmol/l (median [IQR]) | 103.00 [87.25, 115.50] n-2 | 96.00 [87.50, 152.50] | |
| Leucocyte × 109/l (median [IQR]) | 4.99 [4.17, 5.49] n-2 | 7.07 [4.12, 7.69] | |
HBV: hepatitis B virus; MELD: Model for End-Stage Liver Disease; BMI: body mass index.
No formal study protocol was registered for this observational study. The study was conducted following international ethical guidelines and with approval from the Cantonal Ethics Commission of Bern, Switzerland (approval number: 2021-00246). No protocol deviations occurred during the study period. The STROBE cohort study reporting guidelines were used to aid in the drafting of this manuscript [14].
Categorical variables are expressed as numbers and percentages. Quantitative variables, such as age and anti-HBs titres, were analysed descriptively and are presented as medians with interquartile ranges. No categorisation of continuous variables was performed due to the small sample size. Data analysis was conducted using Microsoft Excel and R (version 4.2.1), both widely available and supported software packages. No custom analytical code was created for this study.
The study flow diagram shown in figure 1 illustrates the progression from initial patient identification of liver transplant recipients with HBV-related disease to the final analysis of vaccination outcomes. Following the enrolment of 43 liver transplant recipients with end-stage liver disease or hepatocellular carcinoma (HCC) related to HBV, an eligibility and data validation step was conducted to confirm that the patients met the inclusion criteria and that baseline data were accurate. After validation, 19 eligible patients were allocated to receive active HBV vaccination. The figure also details patient exclusions due to death, loss to follow-up, persistent anti-HBs, or patient refusal to discontinue passive immunisation. The final analysis involved 18 patients who completed the study.

Figure 1Study protocol outline. * HCC: hepatocellular carcinoma; HBIG: hepatitis B immunoglobulin; HBV: hepatitis B virus; NA: nucleos(t)ide analogue.
Twenty-four patients were excluded for the following reasons: death before study enrolment (eight patients), loss to follow-up (eight patients), anti-HBs positivity at the time of transplantation (five patients), and unwillingness to discontinue hepatitis B immunoglobulin (three patients). Additionally, one patient was excluded due to persistent anti-HBs despite cessation of passive immunisation. Consequently, 18 patients were selected to receive active immunisation after discontinuing passive immunisation while continuing immunosuppression and antiviral nucleos(t)ide analogue therapy (figure 1).
A total of 18 patients were enrolled in this study. The indications for liver transplantation included decompensated hepatitis B-related cirrhosis in seven patients (39%), decompensated cirrhosis with hepatitis B/D coinfection in four patients (22%), and hepatocellular carcinoma (with or without cirrhosis) in seven patients (39%) who were HBV-positive. At the time of liver transplantation, only one patient was positive for HBeAg. Eight patients had detectable HBV DNA before transplantation. One patient additionally had alcoholic steatohepatitis, and another had metabolic dysfunction-associated steatohepatitis (MASLD).
The characteristics of each group, including clinical and virological features, are presented in table 1. Most patients were male (72%). The median age at the time of vaccination was 60 years (51, 66.25) in the non-responder group and 61 years (50.50, 69.25) in the responder group. Most patients in both groups were under monotherapy immunosuppression (83.3% in the non-responder group and 66.7% in the responder group). The median number of vaccinations was similar in both groups (median: 3 in non-responders and 3.5 in responders). One participant had positive anti-HBs at the time of transplantation, potentially indicating seroconversion. However, this participant lost anti-HBs by the time of vaccination and was included in the analysis.
All patients completed a minimum of three vaccinations. Eight patients received more than three vaccine shots (maximum six vaccine shots) in cases of non-response, at the discretion of the treating physician. In all but one patient, a double dose of 40 μg was administered (in two patients, a combination of 20 μg and 40 μg doses was used). At the time of vaccination, six patients had positive anti-HBs (anti-HBs values: 50 IU/l, 83 IU/l, 84 IU/l, 139 IU/l, 235 IU/l, and 283 IU/l). This was attributed to a short interval between hepatitis B immunoglobulin cessation (or, in one case, measurement of anti-HBs while the patient was still receiving hepatitis B immunoglobulin) and anti-HBs measurement, rather than to seroconversion prior to active vaccination. Consequently, these patients were included in the analysis, especially as only one of the six participants showed seroconversion after active vaccination; the other five lacked anti-HBs after vaccination. Upon completion of the vaccination regimen, six patients responded to the active immunisation, resulting in a response rate of 33.3% (6/18). The double-dose vaccine was well tolerated, with no reported side effects. During the study, no recurrence of HBV occurred in either group.
Differences between responders and non-responders were described based on demographic and clinical characteristics without inferential statistics, given the study's small sample size. Descriptive comparisons highlight variations in age, BMI, and immunosuppression status, as detailed in table 1. Statistical significance testing was not performed, and the results should be interpreted with caution.
In this study involving 18 participants, no recurrence of HBV was observed (negative HBsAg and HBV DNA) after at least 12 months of follow-up following the cessation of hepatitis B immunoglobulin. This outcome included patients who received liver transplants for hepatocellular carcinoma (7 patients) and those with positive HBV DNA at the time of transplantation (8 patients), despite the higher recurrence rates typically reported in these groups [15, 16]. Although third-generation nucleos(t)ide analogues such as entecavir or tenofovir, which have high resistance barriers, have been shown to decrease HBV recurrence rates, residual risk remains [17]. Consequently, active HBV immunisation (vaccination) after parenteral hepatitis B immunoglobulin cessation is an appealing approach to provide additional immunity.
The efficacy of HBV vaccination remains controversial because of variable response rates in patients who are immunosuppressed. In immunocompetent adults, particularly those under 40 years of age, the HBV vaccine demonstrates a 95% seroconversion rate, indicating high effectiveness [18, 19]. The recommended vaccination schedule consists of a 20-µg dose administered at 0, 1, and 6 months [20, 21]. Some studies have shown a greater immune response with a double dose (40 µg) in patients with impaired immune systems [22]. Therefore, our study primarily employed a double-dose vaccine regimen, with 15 of 18 patients receiving 40 µg doses and the remaining patients receiving a combination of 20 µg and 40 µg doses; only one patient received the standard 20 µg dose.
In this study, a relatively low response rate of 33.3%, comparable to that reported in other studies [23–25], was observed after a median of three vaccine shots, with most patients receiving the 40-µg double dose. A cut-off of >10 IU/l for anti-HBs was selected to define a serologic response, as this threshold is widely regarded as protective in immunocompromised populations [26–28]. Measurements were consistently reported in IU/l, following standard laboratory practices at the University Hospital of Bern. In the literature, factors linked to reduced vaccination response include smoking, male sex, obesity, and age over 40 years [29]. In our study, no differences were observed in vaccine response regarding BMI, smoking, diabetes, the interval between vaccination and liver transplantation, or age. However, due to the small sample size, the lack of significant differences for these variables must be interpreted with caution, and causality cannot be confirmed.
The time from liver transplantation to the first active HBV vaccination varied significantly among patients, with a median interval of 2555.00 days for some and 6387.50 days for others. This prolonged interval reflects the clinical caution traditionally exercised in discontinuing hepatitis B immunoglobulin. The shift towards hepatitis B immunoglobulin-free regimens and active immunisation has only gained traction in recent years, influencing the timing of vaccination initiation.
The production of antibodies to HBV is primarily driven by the immune system’s response to hepatitis B surface antigen (HBsAg). Consequently, transplant recipients under immunosuppression exhibit a decreased response rate following active immunisation [30, 31]. There was no difference in vaccine response rates between different immunosuppression regimens (monotherapy immunosuppression vs combination therapy), with 83.3% of non-responders on monotherapy immunosuppression compared to 66.7% of responders.
In conclusion, in this prospective cohort study with a short follow-up period, we observed that among 18 patients who underwent liver transplantation due to HBV infection who discontinued parenteral hepatitis B immunoglobulin while continuing nucleos(t)ide analogue therapy, no recurrence of HBV was documented. Furthermore, the response rate of 33.3% following active HBV vaccination was low, despite the administration of a double-dose (40 µg) vaccination regimen in most patients. Lastly, no adverse events were reported after immunisation.
The limitations of this study are the small sample size and short follow-up, especially considering that some studies have demonstrated a decline in immunity over time [32]. Due to the small sample size (and lack of a control group), the results must be interpreted with caution, as causality cannot be proven, and therefore, the results are not generalisable. Nonetheless, this study cohort presented a similar response rate to active immunisation compared to similar studies with no adverse events reported from the patients. HBV genotyping was not performed as part of this study, and therefore, no genotype data are available for analysis. This limitation restricts any genotype-specific conclusions regarding immunisation response in our cohort. Another potential limitation is the questioning of patients regarding adverse events after vaccination. This method relied on self-reported data and was not supplemented by formal clinical assessments, which may have limited the detection of subclinical adverse events. However, no patients reported any significant adverse effects during the study period.
In this prospective cohort study with a short follow-up period, we observed that among 18 patients who underwent liver transplantation for HBV infection and discontinued parenteral hepatitis B immunoglobulin while continuing nucleos(t)ide analogue therapy, no recurrence of HBV was documented. Furthermore, the response rate of 33.3% following active HBV vaccination was low, despite the administration of a double dose (40 µg) vaccination regimen in most patients. Lastly, no adverse events were reported after immunisation.
Further studies are needed to assess potential markers for predicting a vaccination response and to optimise vaccination strategies in this population. Additionally, future studies should evaluate whether nucleos(t)ide analogue withdrawal is feasible in vaccine responders.
De-identified study data, including the data dictionary, will be made available on the Open Science Framework (OSF). The shared data will include raw data and the statistical analysis plan. The data will be accessible starting on 1 July 2025 for a period of five years. Access will be granted to researchers who submit a reasonable request and whose proposed analyses are ethically justifiable. Requests can be directed to the corresponding author via email.
This study received no funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. EA received support from Gilead Sciences, unrelated to this article, for attending meetings or travel as part of the EASL Congress 2023 and 2024. No other potential conflict of interest related to the content of this manuscript was disclosed.
1. Samuel D, Muller R, Alexander G, Fassati L, Ducot B, Benhamou JP, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993 Dec;329(25):1842–7. doi: https://doi.org/10.1056/NEJM199312163292503
2. Duvoux C, Belli LS, Fung J, Angelico M, Buti M, Coilly A, et al. 2020 position statement and recommendations of the European Liver and Intestine Transplantation Association (ELITA): management of hepatitis B virus-related infection before and after liver transplantation. Aliment Pharmacol Ther. 2021 Sep;54(5):583–605. doi: https://doi.org/10.1111/apt.16374
3. Burra P, Germani G, Adam R, Karam V, Marzano A, Lampertico P, et al. Liver transplantation for HBV-related cirrhosis in Europe: an ELTR study on evolution and outcomes. J Hepatol. 2013 Feb;58(2):287–96. doi: https://doi.org/10.1016/j.jhep.2012.10.016
4. Loomba R, Rowley AK, Wesley R, Smith KG, Liang TJ, Pucino F, et al. Hepatitis B immunoglobulin and Lamivudine improve hepatitis B-related outcomes after liver transplantation: meta-analysis. Clin Gastroenterol Hepatol. 2008 Jun;6(6):696–700.
5. Burra P, Burroughs A, Graziadei I, et al.; European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu. EASL Clinical Practice Guidelines: liver transplantation. J Hepatol. 2016 Feb;64(2):433–85.
6. Lampertico P, Agarwal K, Berg T, Buti M, Janssen HL, Papatheodoridis G, et al.; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017 Aug;67(2):370–98.
7. World Health Organization. WHO Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015.
8. Mahmood F, Xu R, Awan MU, Song Y, Han Q, Xia X, et al. HBV Vaccines: advances and Development. Vaccines (Basel). 2023 Dec;11(12):1862. doi: https://doi.org/10.3390/vaccines11121862
9. Angelico M, Di Paolo D, Trinito MO, Petrolati A, Araco A, Zazza S, et al. Failure of a reinforced triple course of hepatitis B vaccination in patients transplanted for HBV-related cirrhosis. Hepatology. 2002 Jan;35(1):176–81.
10. Rosenau J, Hooman N, Hadem J, Rifai K, Bahr MJ, Philipp G, et al. Failure of hepatitis B vaccination with conventional HBsAg vaccine in patients with continuous HBIG prophylaxis after liver transplantation. Liver Transpl. 2007 Mar;13(3):367–73.
11. Su J, Brunner L, Ates Oz E, Sacherl J, Frank G, Kerth HA, et al. Activation of CD4 T cells during prime immunization determines the success of a therapeutic hepatitis B vaccine in HBV-carrier mouse models. J Hepatol. 2023 Apr;78(4):717–30.
12. Bienzle U, Günther M, Neuhaus R, et al. Immunization With an Adjuvant Hepatitis B Vaccine After Liver Transplantation for Hepatitis B-Related Disease. Hepatology. 2003 Oct;38(4):811–9.
13. Sánchez-Fueyo A, Rimola A, Grande L, Costa J, Mas A, Navasa M, et al. Hepatitis B immunoglobulin discontinuation followed by hepatitis B virus vaccination: A new strategy in the prophylaxis of hepatitis B virus recurrence after liver transplantation. Hepatology. 2000 Feb;31(2):496–501. doi: https://doi.org/10.1002/hep.510310233
14. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008 Apr;61(4):344–9.
15. Faria LC, Gigou M, Roque-Afonso AM, Sebagh M, Roche B, Fallot G, et al. Hepatocellular carcinoma is associated with an increased risk of hepatitis B virus recurrence after liver transplantation. Gastroenterology. 2008 Jun;134(7):1890–9.
16. Marzano A, Gaia S, Ghisetti V, Carenzi S, Premoli A, Debernardi-Venon W, et al. Viral load at the time of liver transplantation and risk of hepatitis B virus recurrence. Liver Transpl. 2005 Apr;11(4):402–9.
17. Manini MA, Whitehouse G, Bruce M, Passerini M, Lim TY, Carey I, et al. Entecavir or tenofovir monotherapy prevents HBV recurrence in liver transplant recipients: A 5-year follow-up study after hepatitis B immunoglobulin withdrawal. Dig Liver Dis. 2018 Sep;50(9):944–53.
18. Rosenau J, Hooman N, Hadem J, Rifai K, Bahr MJ, Philipp G, et al. Failure of hepatitis B vaccination with conventional HBsAg vaccine in patients with continuous HBIG prophylaxis after liver transplantation. Liver Transpl. 2007 Mar;13(3):367–73.
19. Sester M, Gärtner BC, Girndt M, Sester U. Vaccination of the solid organ transplant recipient. Transplant Rev (Orlando). 2008 Oct;22(4):274–84.
20. Schillie SF, Murphy TV. Seroprotection after recombinant hepatitis B vaccination among newborn infants: a review. Vaccine. 2013 May;31(21):2506–16. doi: https://doi.org/10.1016/j.vaccine.2012.12.012
21. Fitzgerald B, Mac Kenzie WR, Rasmussen SA, et al. Morbidity and Mortality Weekly Report. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. Centers for Disease Control and Prevention. MMWR Editorial and Production Staff (Serials) MMWR Editorial Board. 2018. Recommendations and Reports. 2018;67(1):1-31. Available from: https://www.cdc.gov/mmwr/volumes/67/rr/pdfs/rr6701-H.PDF
22. Günther M, Neuhaus R, Bauer T, Jilg W, Holtz JA, Bienzle U. Immunization with an adjuvant hepatitis B vaccine in liver transplant recipients: antibody decline and booster vaccination with conventional vaccine. Liver Transpl. 2006 Feb;12(2):316–9.
23. Yang A, Guo Z, Ren Q, Wu L, Ma Y, Hu A, et al. Active immunization in patients transplanted for hepatitis B virus related liver diseases: A prospective study. PLoS One. 2017 Nov;12(11):e0188190.
24. Lu SC, Jiang T, Lai W, Liu Y, Zhang J, Zeng DB, et al. Reestablishment of active immunity against HBV graft reinfection after liver transplantation for HBV-related end stage liver disease. J Immunol Res. 2014;2014:764234.
25. Jo HS, Khan JF, Han JH, Yu YD, Kim DS. Efficacy and Safety of Hepatitis B Virus Vaccination Following Hepatitis B Immunoglobulin Withdrawal After Liver Transplantation. Transplant Proc. 2021 Dec;53(10):3016–21.
26. Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999 Feb;179(2):489–92.
27. Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al.; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014 Feb;58(3):e44–100.
28. Stephens JW, Bernhardt JM, Director M, et al. Morbidity and Mortality Weekly Report Recommendations and Reports A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of Adults INSIDE: Continuing Education Examination Editorial and Production Staff. 2006.
29. Yang S, Tian G, Cui Y, Ding C, Deng M, Yu C, et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep. 2016 Jun;6(1):27251.
30. Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8(2):e56974.
31. Gangappa S, Kokko KE, Carlson LM, Gourley T, Newell KA, Pearson TC, et al. Immune responsiveness and protective immunity after transplantation. Transpl Int. 2008 Apr;21(4):293–303. doi: https://doi.org/10.1111/j.1432-2277.2007.00631.x
32. Loinaz C, de Juanes JR, Gonzalez EM, López A, Lumbreras C, Gómez R, et al. Hepatitis B vaccination results in 140 liver transplant recipients. Hepatogastroenterology. 1997;44(13):235–8.