Hospital resource use and in-hospital mortality before and during the COVID-19 pandemic:
a nationwide cohort study
DOI: https://doi.org/https://doi.org/10.57187/s.4109
Rebecca Felder-Wieserab*,
Rahel Laagerac*,
Roshaani Rasiaha,
Claudia Gregorianoa,
Philipp Schuetzabd,
Alexander Kutzab
a Medical
University Department, Division of General Internal and Emergency Medicine,
Cantonal Hospital Aarau, Aarau, Switzerland
b Faculty
of Medicine, University of Basel, Basel, Switzerland
c University
Hospital of Child and Adolescent Psychiatry and Psychotherapy, University of
Bern, Bern, Switzerland
d Medical
University Department, Division of Endocrinology, Diabetes, and Metabolism,
Cantonal Hospital Aarau, Aarau, Switzerland
* These
authors contributed equally as first authors to this manuscript
Summary
INTRODUCTION:
The COVID-19 pandemic has placed an enormous strain on the
Swiss healthcare system. This study aims to assess the associations of the
pandemic on Switzerland’s hospital resource use and in-hospital mortality among
both COVID-19 and non-COVID-19 patients.
METHODS:
In this national cohort study, we analysed
administrative claims data for medical inpatients from 1 January 2018 to 31 December
2021, using mixed-effects segmented regression models. Hospitalisations were
divided into a control and an exposure group before (January 2018 to December 2019)
and during (January
2020 to December 2021) the pandemic. Before the pandemic, the division into the groups
was
performed by random split. We investigated trends in in-hospital mortality, hospital
length of stay, 30-day hospital readmission and facility discharge rates before
and during the COVID-19 pandemic, to assess the pandemic’s association with both
COVID-19 (exposure) and non-COVID-19 (control) patients.
RESULTS:
Among 1,510,836 included cases, 763,533 were hospitalised
before and 747,303 during the COVID-19 pandemic including 61,151 with a
diagnosis of COVID-19. Before the pandemic, there were no relevant changes in population-averaged
in-hospital mortality in the control group and the randomly defined exposure
group (−0.0263% and 0.0201% per month, respectively). During the pandemic, however,
mortality showed an increase among COVID-19 patients by 0.3553% per month (95% confidence
interval [CI]: 0.3546–0.3560; change in slope p <0.001; difference in slopes
p <0.001), while there was no relevant change in the pandemic control group
(slope: −0.0277% per month). Similarly, COVID-19 patients showed an increase in
hospital length of stay and discharge to a post-acute care facility, while the
trend for 30-day hospital readmission was decreased.
CONCLUSION: In this study, we observed an
association between the COVID-19 pandemic and hospital resource use in COVID-19
patients only, resulting in higher in-hospital mortality, longer lengths of hospital
stay and more frequent facility discharges. No relevant differences were seen
in the control group during both time periods.
Abbreviations
- COPD:
-
chronic obstructive pulmonary disease
- ICD-10 GM:
-
International Classification of
Diseases, version 10, German Modification
Introduction
During the COVID-19 pandemic, significant uncertainty
permeated daily hospital operations in Switzerland. While the first COVID-19
cases in Switzerland were registered in February 2020 [1], throughout the
spring of 2020, Europe experienced a decline in hospitalisation rates due to a
reduction in patient volume and the postponement of elective treatments [2–7]. Specifically,
a notable decrease in admissions for myocardial infarction, cerebral stroke and
cancer was observed, resulting in delays in diagnosis and initiation of therapy
[2, 6, 8–10]. The clinical and demographic profile of non-COVID-19 patients
admitted during the first wave of the pandemic was different to that of patients
admitted in the years before. They were older and the severity of the patient
status was increased [11].
The relatively calm period with the subsidy
programme transitioned to a period of patient overload in autumn 2020 [3]. Hospital
wards, especially intensive care units (ICU), were overwhelmed with COVID-19
patients requiring extensive inpatient care. Healthcare staff faced a lack of
knowledge regarding the aetiopathogenesis and lethality of the new virus, in addition
to rapidly evolving prevention and treatment strategies of uncertain efficacy. Moreover,
the care of COVID-19 patients demanded more time. Since then, hospitals have appeared
to operate at the limits of their capacity. Hospital beds had to be closed due
to staff shortages, and this strain was evident in daily life. Multiple studies
have demonstrated a correlation between COVID-19 surges and increased mortality
among hospitalised patients with COVID-19 [12–15]. Between March and August
2020, in 558 US hospitals 23% of COVID-19 deaths were linked to high hospital
strain due to the COVID-19 caseload [12]. Non-COVID-19 patients seemed to be
less affected by overburdening. A study in Alberta and Ontario reported similar
results for 30-day mortality and hospital length of stay (LOS) before and
during the pandemic in patients with a urinary tract infection, acute coronary
syndrome and stroke without COVID-19 [15]. Furthermore, a survey conducted in
Spain indicated a negative impact of the pandemic on healthcare quality for
both COVID-19 and non-COVID-19 patients, but more pronounced in the former group
[16]. Another study observed a negative effect in terms of encountered errors,
with an increased risk of medication errors and sepsis (particularly central line-associated
bloodstream infections) among hospitalised patients during the pandemic [17].
Still, Swiss data on hospital overload during
the COVID-19 pandemic, particularly regarding outcomes for non-COVID-19
patients, is limited. The terminology and definition of capacity strain varied across
published studies, complicating comparisons and leading to differing results [18].
Resource issues have been extensively studied in emergency departments and ICUs,
where associations between delayed care due to overcrowding, staff shortages,
decreased care efficiency and increased patient mortality have been identified [18–23].
However no such data among patients on medical acute care wards with and
without COVID-19 has been reported in Switzerland. We therefore aimed to
investigate whether and to what extent the COVID-19 pandemic was associated with
additional hospital resource use in patients with COVID-19 and in those
without.
Methods
Study design and data source
We conducted a population-based cohort
study of adults using a nationwide administrative claims database between 1 January
2018 and 31 December 2021, provided by the Swiss Federal Statistical Office
(Bundesamt für Statistik, Neuchâtel, Switzerland). The dataset included all
Swiss inpatient discharge records from hospitals for acute somatic care.
Individual-level data on patient demographics, healthcare utilisation, hospital
typology, medical diagnoses, clinical procedures and in-hospital patient
outcomes were provided. A multi-step anonymisation procedure ensured patient
confidentiality, and a unique anonymised patient identifier was used to
ascertain rehospitalisations. Medical diagnoses were coded using International
Classification of Disease version 10, German Modification (ICD-10-GM) codes
(http://www.who.int/classifications/icd/en/).
The institutional review board of Northwestern and Central Switzerland (EKNZ)
waived the need for an ethical authorisation due to the use of exclusively
anonymised data (EKNZ Project-ID: Req-2021-01397).
In this interrupted time-series design, we
compared outcomes between patients with and without a diagnosis of COVID-19.
The overall 48-month study period was divided into a 24-month pre-pandemic
phase, from January 2018 to December 2019, and a 24-month pandemic phase, from
January 2020 to December 2021. Before the pandemic, we randomly split the study
population into an “exposure” group (representing the pre-pandemic population
of the “exposure group” trajectory) and a “control” group (representing the
pre-pandemic population of the “control group” trajectory ); during the
pandemic, patients with a diagnosis of COVID-19 were allocated to the exposure
group and patients without a diagnosed COVID-19 infection to the control group.
Study population
For this analysis, we included all
hospitalisations from January 2018 to December 2021 on the medical ward. Hospitalisations
with a diagnosis of COVID-19 were identified using ICD-10-GM discharge codes
U07.1 and U07.2. Validation studies have demonstrated high sensitivity and
positive predictive value for these codes [24, 25]. Details on all ICD-10-GM
codes used in the analysis are provided in tables S1–S2. Comorbidities were assessed
using the Elixhauser Comorbidity Index [26], and frailty was quantified using
the Hospital Frailty Score [27]. We excluded children (age <18 years) and
patients with outlier hospitalisation stays longer than 100 days from the
analysis. This study adheres to the “Strengthening The Reporting of
Observational Studies in Epidemiology (STROBE)” statement [28].
Outcomes
The primary outcome was in-hospital
mortality among the exposure and control groups during the pre-pandemic and
pandemic periods. Secondary outcomes comprised trends in hospital length of
stay, 30-day hospital readmission and facility discharge, both between the two
groups and across the study phases. For facility discharge, we considered
nursing homes, residential homes for the elderly and rehabilitation facilities
based on claims data. The claims data also provided the number of days to the subsequent
hospitalisation, which was used to calculate the 30-day hospital readmission
rate. Rehospitalisations within 18 days after the initial hospitalisation for a
related reason were not considered readmissions; instead they were included in
the first hospitalisation. In accordance with the SwissDRG definition, every
readmission to the same hospital after 18 days from discharge or any
readmission into another hospital were defined as a new index hospitalisation [29].
Thus, a single patient may have more than one index admission during the study
period. Length of stay that crossed from one study phase to another or that
went past the study end date were attributed to the study phase applicable
during hospital admission.
Statistical analysis
Descriptive statistics were calculated for
patient demographics, including age, sex, nationality, patient comorbidity and
level of frailty. All baseline characteristics are expressed as median (interquartile
range [IQR]), standardised difference [Std. Diff.] or frequency (%). We
performed segmented regression analyses of interrupted time-series data from 1 January
2018 to 31 December 2021, to analyse in-hospital mortality, hospital length of
stay, 30-day hospital readmission and facility discharge. To model trends, we
used a multivariable mixed-effects linear regression, including study time, modelled
continuously as a linear spline with one knot location chosen at the start of
the pandemic phase (January 2020). The model included random effects for patient-specific
intercept and slope, allowing for individual trends for each patient, which
improved models’ fit over random intercept only models, based on log likelihood
ratio tests, with statistical significance set at p <0.05. At the patient level,
the model was adjusted for age, sex, housing conditions, Elixhauser Comorbidity
Index and the Hospital Frailty Risk Score. Time coefficients can be interpreted
as monthly mean change in percentage of mortality, 30-day hospital readmission
and facility discharge. For length of hospital stay, time coefficients are
provided in days. We excluded patients
who died during hospitalisation when assessing hospital readmission and
facility discharge.
In a subgroup analysis, we stratified the
results by the presence of the following diagnoses: cardiovascular disease (coronary
artery disease, heart failure, hypertension), chronic obstructive pulmonary
disease (COPD), diabetes mellitus, chronic kidney disease (CKD) and solid
cancer. Moreover, we conducted an analysis based on the size of the hospital
(small vs big hospital).
The
assumptions of the linear models, including linearity, normality of residuals
and homoscedasticity, were assessed using appropriate diagnostic plots and
statistical tests. No violations were identified, confirming the validity of
the model assumptions. There were no missing data for patient characteristics
and study outcomes. Statistical significance was based on 95% confidence
intervals (CIs) and all p-values were two-tailed. All statistical analyses were
performed using STATA version 18.0 (STATA Corp., College Station, TX, USA).
Results
Characteristics of the cohort
From 1 January 2018 to 31 December 2021, we
identified a total of 1,510,836 hospitalisations, 763,533 (50.5%) before and
747,303 (49.5%) during the COVID-19 pandemic (figure 1). Table 1 shows baseline
characteristics by study phase and exposure group. During the pre-pandemic
phase, there were no differences between the randomly split groups. During the
pandemic, exposure group hospitalisations (n=61,151) were significantly fewer
compared to controls (n = 686,152). The exposure group during the pandemic had
more male patients (57.6%) than before the pandemic (52.4%) and the control
group during the pandemic (53.1%). Most hospitalisations were for patients aged
over 69 years, but the exposure group had more patients aged 45–69 (41.4%) than
the control group (34.8%) or pre-pandemic group (35.3%). Most patients were
admitted from home, with fewer in the exposure group during the pandemic
(83.3%). The exposure group during the pandemic had fewer comorbidities, with a
lower median Elixhauser Comorbidity Index, fewer cardiovascular,
cerebrovascular, chronic obstructive pulmonary and solid cancer diseases, but
higher obesity rates. Despite fewer comorbidities, the exposure group during
the pandemic had higher frailty scores.
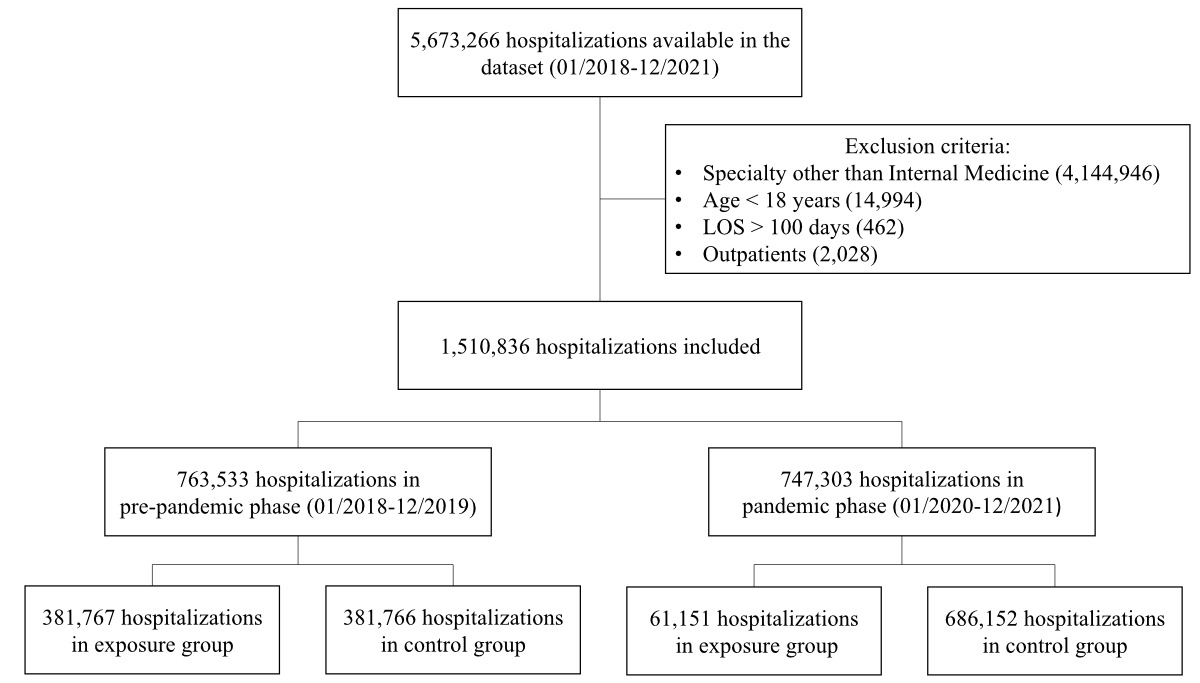
Figure 1Flowchart of included
hospitalisations. The exposure group included patients
diagnosed with COVID-19 or a randomly split pre-pandemic subset, while the
control group included those without COVID-19 or a randomly split pre-pandemic
subset. LOS: length of stay.
Table 1Baseline characteristics
stratified by study phase and group. The exposure group included patients
diagnosed with COVID-19 or a randomly split pre-pandemic subset, while the
control group included those without COVID-19 or a randomly split pre-pandemic subset.
Standardised difference is a measure of the size of the difference between two
group means relative to the variability within the groups.
|
Pre-pandemic phase |
Pandemic phase |
Standardised difference |
Standardised difference |
| Control |
Exposure |
Control |
Exposure |
Pre-pandemic vs pandemic exposure |
Pandemic control vs pandemic exposure |
| Hospitalisations, n |
381,766 |
381,767 |
686,152 |
61,151 |
|
|
| Demographics |
Male sex, n (%) |
200,402 (52.5%) |
200,007 (52.4%) |
364,039 (53.1%) |
35,211 (57.6%) |
0.104 |
0.091 |
| Age, n (%) |
|
|
|
|
0.128 |
0.139 |
| …19–44 |
42,458 (11.1%) |
42,396 (11.1%) |
74,449 (10.9%) |
6551 (10.7%) |
|
|
| …45–69 |
134,785 (35.3%) |
134,593 (35.3%) |
239,085 (34.8%) |
25,303 (41.4%) |
|
|
| …>69 |
204,523 (53.6%) |
204,778 (53.6%) |
372,618 (54.3%) |
29,297 (47.9%) |
|
|
| Swiss nationality, n (%) |
312,434 (81.8%) |
312,040 (81.7%) |
564,262 (82.2%) |
44,248 (72.4%) |
0.224 |
0.237 |
| Admission data |
Admission from home, n (%) |
332,717 (87.2%) |
333,024 (87.2%) |
594,260 (86.6%) |
50,919 (83.3%) |
0.112 |
0.093 |
| Tertiary care, n (%) |
|
|
|
|
0.073 |
0.070 |
| …University hospital |
68,618 (18.0%) |
68,283 (17.9%) |
127,089 (18.5%) |
11,159 (18.2%) |
|
|
| …Non-university hospital |
236,187 (61.9%) |
236,276 (61.9%) |
420,643 (61.3%) |
35,915 (58.7%) |
|
|
| …Secondary care hospital |
76,961 (20.2%) |
77,208 (20.2%) |
138,420 (20.2%) |
14,077 (23.0%) |
|
|
| Comorbidities, n (%) |
Hypertension |
184,509 (48.3%) |
184,054 (48.2%) |
338,015 (49.3%) |
28,465 (46.5%) |
0.033 |
0.054 |
| Coronary artery disease |
91,617 (24.0%) |
91,577 (24.0%) |
170,216 (24.8%) |
9079 (14.8%) |
0.233 |
0.252 |
| Heart failure |
52,575 (13.8%) |
53,064 (13.9%) |
102,959 (15.0%) |
5658 (9.3%) |
0.146 |
0.177 |
| Cerebrovascular disease |
30,774 (8.1%) |
30,341 (7.9%) |
62,418 (9.1%) |
2446 (4.0%) |
0.167 |
0.207 |
| Obesity |
7693 (2.0%) |
7728 (2.0%) |
17,751 (2.6%) |
2520 (4.1%) |
−0.122 |
−0.085 |
| Diabetes mellitus |
70,759 (18.5%) |
70,593 (18.5%) |
130,281 (19.0%) |
14,430 (23.6%) |
−0.126 |
−0.113 |
| Chronic obstructive pulmonary disease |
34,283 (9.0%) |
34,209 (9.0%) |
55,977 (8.2%) |
4026 (6.6%) |
0.089 |
0.060 |
| Chronic kidney disease |
74,743 (19.6%) |
75,154 (19.7%) |
140,016 (20.4%) |
10,456 (17.1%) |
0.067 |
0.085 |
| Solid cancer |
48,063 (12.6%) |
47,627 (12.5%) |
90,188 (13.1%) |
2746 (4.5%) |
0.290 |
0.309 |
| Liver disease |
15,931 (4.2%) |
15,907 (4.2%) |
31,849 (4.6%) |
2994 (4.9%) |
−0.035 |
−0.012 |
| Elixhauser Comorbidity Index, median
(IQR) |
2 (1; 4) |
2 (1; 4) |
3 (1; 4) |
2 (1; 4) |
0.116 |
0.173 |
| Frailty category |
|
|
|
|
0.123 |
0.084 |
| …<5 points |
271,217 (71.0%) |
271,061 (71.0%) |
477,153 (69.5%) |
40,143 (65.6%) |
|
|
| …5–15 points |
99,267 (26.0%) |
99,552 (26.1%) |
183,958 (26.8%) |
18,373 (30.0%) |
|
|
| …>15 points |
11,282 (3.0%) |
11,154 (2.9%) |
25,041 (3.6%) |
2635 (4.3%) |
|
|
Primary outcome before and during the COVID-19
pandemic
The primary outcome of in-hospital
mortality occurred in 35,483 (4.7%) patients before the pandemic and in 38,991 (5.2%)
patients during the pandemic, while more patients from the exposure group (10.96%)
rather than controls (4.7%) were affected. The results for in-hospital
mortality are summarised in table 2 and figure 2A. The increase in in-hospital
mortality during the pandemic was higher in the exposure group (slope: 0.3553,
95% CI: 0.3546–0.3560) compared to both the pandemic control group (slope: −0.0277,
95% CI: −0.0280 to −0.0273) and the pre-pandemic group (slope: 0.0201, 95% CI: 0.0199–0.0204)
(p <0.001 in both cases).
Table 2Multivariable
random slope mixed-effects model of changes in in-hospital mortality, hospital
length of stay, 30-day hospital readmission and facility discharge for exposure
and control groups between January 2018 and December 2021. The exposure group included
patients diagnosed with COVID-19 or a
randomly split pre-pandemic subset, while the control group included those
without COVID-19 or a randomly split pre-pandemic subset.
| Outcome and study phase* |
Coefficient (95% CI)** |
Slope (95% CI)*** |
p-value |
| Slope differs from zero |
Change in slope from prior slope |
Difference in slopes (Control vs Exposure
group) |
| In-hospital mortality |
| Control group (reference) |
Phase 1 (01/2018 to 12/2019) |
−0.0263 (−0.0266; −0.0261) |
−0.0263 (−0.0266; −0.0261) |
<0.001 |
NA |
NA |
| Phase 2 (01/2020 to 12/2021) |
−0.0013 (−0.0018; −0.0009) |
−0.0277 (−0.0280; −0.0273) |
<0.001 |
<0.001 |
NA |
| Exposure group |
Exposure group by time interaction during
phase 1 (01/2018 to 12/2019) |
0.0465 (0.0461; 0.0468) |
0.0201 (0.0199; 0.0204) |
<0.001 |
NA |
<0.001 |
| Exposure group by time interaction during
phase 2 (01/2020 to 12/2021) |
0.3365 (0.3356; 0.3374) |
0.3553 (0.3546; 0.3560) |
<0.001 |
<0.001 |
<0.001 |
| Hospital length of stay**** |
| Control group (reference) |
Phase 1 (01/2018 to 12/2019) |
−0.0396 (−0.0424; −0.0367) |
−0.0396 (−0.0424; −0.0367) |
<0.001 |
NA |
NA |
| Phase 2 (01/2020 to 12/2021) |
0.0091 (0.0054; 0.0127) |
−0.0305 (−0.0327; −0.0283) |
<0.001 |
<0.001 |
NA |
| Exposure group |
Exposure group by time interaction during
phase 1 (01/2018 to 12/2019) |
0.0401 (0.0363; 0.0440) |
0.0006 (−0.0023; 0.0034) |
0.6950 |
NA |
<0.001 |
| Exposure group by time interaction during
phase 2 (01/2020 to 12/2021) |
0.1267 (0.1190; 0.1344) |
0.1363 (0.1313; 0.1414) |
<0.001 |
<0.001 |
<0.001 |
| 30-day hospital readmission |
| Control group (reference) |
Phase 1 (01/2018 to 12/2019) |
−0.0068 (−0.0072; −0.0065) |
−0.0068 (−0.0072; −0.0065) |
<0.001 |
NA |
NA |
| Phase 2 (01/2020 to 12/2021) |
−0.0082 (−0.0088; −0.0076) |
−0.0150 (−0.0155; −0.0146) |
<0.001 |
<0.001 |
NA |
| Exposure group |
Exposure group by time interaction during
phase 1 (01/2018 to 12/2019) |
−0.0287 (−0.0292; −0.0282) |
−0.0355 (−0.0359; −0.0352) |
<0.001 |
NA |
<0.001 |
| Exposure group by time interaction during
phase 2 (01/2020 to 12/2021) |
−0.3639 (−0.3650; −0.3627) |
−0.4076 (−0.4085; −0.4067) |
<0.001 |
<0.001 |
<0.001 |
| Facility discharge |
| Control group (reference) |
Phase 1 (01/2018 to 12/2019) |
−0.0124 (−0.0127; −0.0121) |
−0.0124 (−0.0127; −0.0121) |
<0.001 |
NA |
NA |
| Phase 2 (01/2020 to 12/2021) |
0.0144 (0.0139; 0.0150) |
0.0020 (0.0016; 0.0025) |
<0.001 |
<0.001 |
NA |
| Exposure group |
Exposure group by time interaction during
phase 1 (01/2018 to 12/2019) |
0.0264 (0.0260; 0.0268) |
0.0140 (0.0137; 0.0143) |
<0.001 |
NA |
<0.001 |
| Exposure group by time interaction during
phase 2 (01/2020 to 12/2021) |
0.2360 (0.2350; 0.2371) |
0.2645 (0.2636; 0.2654) |
<0.001 |
<0.001 |
<0.001 |
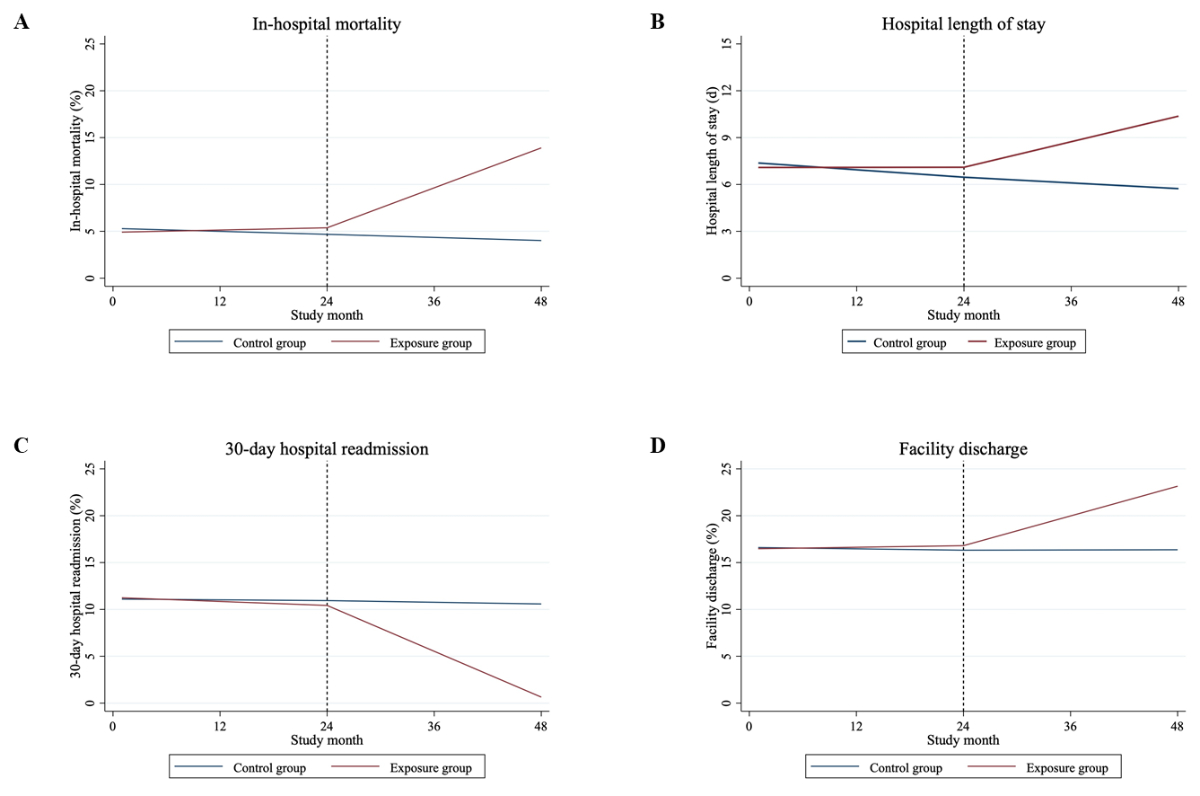
Figure 2Trends in in-hospital mortality (A),
hospital length of stay (B), 30-day hospital readmission (C) and facility
discharge (D) for exposure and control group before and during the COVID-19
pandemic. The exposure group included patients diagnosed with COVID-19 or a
randomly split pre-pandemic subset, while the control group included those
without COVID-19 or a randomly split pre-pandemic subset.
Secondary outcomes before and during the COVID-19
pandemic
The mean hospital length of stay was 6.7
days before the pandemic and 6.6 days during the pandemic. However, the mean hospital
length of stay for the exposure group was 9.6 days, higher than the control
group, which had a mean hospital length of stay of 6.4 days during the
pandemic. A longer hospital length of stay was observed in the exposure group
compared to the control group during the pandemic (p <0.001). The length of
stay for hospitalisations of the exposure group increased by 0.1363 days per
month (95% CI: 0.1313–0.1414) from January 2020 to December 2021. In contrast,
there was a slight decrease in the hospital length of stay for controls over
the same time period (slope: −0.0305 days per month, 95% CI: −0.0327 to −0.0283)
(table 2, figure 2B).
The secondary outcome 30-day hospital
readmission occurred in 84,202 (11.7%) patients before the pandemic and in
75,680 (10.8%) during the pandemic, whereas fewer patients from the exposure group
than the control group (5.1% vs 11.3%) were readmitted within 30 days after the
index hospitalisation. In the exposure group, we observed a decline in the
slope for 30-day hospital readmission during the pandemic (slope: −0.4076, 95%
CI: −0.4085 to −0.4067). The control group also experienced a decrease in the
slope during the pandemic (slope: −0.0150, 95% CI: −0.0155 to −0.0146),
although this decline was less pronounced compared to the exposure group
(difference in slopes, p <0.001) (table 2, figure 2C).
Before the pandemic, 124,369 (16.3%) patients
were discharged to an institution for further care, compared to 126,619 (16.9%)
during the pandemic. More patients from the exposure group (20.4%) compared to
the control group (16.6%) were discharged to a post-acute care facility. We
observed a significant increase in the proportion of facility discharge for
hospitalisations among the exposure group during the pandemic (slope: 0.2645, 95%
CI: 0.2636–0.2654) and thus, a change to the prior slope during the
pre-pandemic (p <0.001). The control group also showed an increase in slope
during the pandemic, although less pronounced (slope: 0.0020, 95% CI: 0.0016–0.0025;
difference between slopes during the pandemic, p <0.001) (table 2, figure 2D).
Subgroup analyses
Tables S3–S9 and figures S1–S7 present the findings
of the subgroup analysis. For in-hospital mortality, hospital length of stay
and facility discharge results remained mostly consistent; however, a steeper
increase in the exposure group (pre-existing illness plus COVID-19) compared to
the control group (pre-existing illness without COVID-19) was observed. The
slope for 30-day hospital readmission showed a relevant decline in patients
with an underlying health condition and COVID-19, while it was less pronounced
in the control group.
The analysis based on hospital size showed
similar findings as the primary outcome. Smaller hospitals showed a steeper
increase in facility discharge during the pandemic, while larger hospitals
showed a longer length of stay compared to smaller hospitals over the entire
period (tables S8–S9, figures S6–S7).
Discussion
This study, focusing on in-hospital patient
care in Switzerland before and during the COVID-19 pandemic, provides insights
into the association between the pandemic and in-hospital resource use among
individuals with and without a diagnosis of COVID-19. First, in-hospital
mortality increased significantly during the pandemic, with the exposure group
experiencing a notably higher mortality rate compared to controls. Second,
while the hospital length of stay for the exposure group increased markedly
during the pandemic, there was a substantial decline in 30-day hospital readmission
rates. Additionally, the proportion of facility discharges increased for the
exposure group during the pandemic, indicating a shift in discharge patterns.
A significant number of hospitals reported
shortages in COVID-19 testing supplies, the need to repurpose hospital spaces
into intensive care units (ICUs) and staffing shortages. While these issues
were prevalent around the world, there were regional differences due to the
initial heterogeneous spread of the pandemic. For instance, the repurposing of
hospital spaces to ICUs and higher staffing shortages in some regions of
Switzerland may reflect increased COVID-19 caseloads among certain hospital
areas, more limited space, fewer resources or lower staffing levels prior to
the pandemic compared to other institutions. Therefore, data is needed to determine
whether these operational strains may have contributed to higher rates of
adverse outcomes for patients without COVID-19.
Overall, we observed an increase in
in-hospital mortality, hospital length of stay and facility discharge among the
exposure group during the COVID-19 pandemic. These findings highlight the
additional use of hospital resources at that time among patients with COVID-19
and align with previous studies [12–15, 30]. University Hospital Basel reported
a stable in-hospital mortality rate of around 9.5% to 10.2% during the first
two waves, declining to 5.4% in the third wave due to factors such as
vaccination, therapeutic advancements, and shifts in patient demographics and
disease severity [31, 32]. This period also saw unchanged hospital length of
stay [31]. In contrast, Cantonal Hospital Aarau recorded higher initial in-hospital
mortality (19%) and longer hospital length of stay (8.9 days), with subsequent
waves showing a reduction in hospital length of stay to 6.5 days without
mortality change [33, 34]. Data from 20 Swiss hospitals during the second wave
revealed an in-hospital mortality rate of 14.5% [35], while a broader study across
14 hospitals, including all five Swiss university hospitals, found an
in-hospital mortality of 12.8% and an average hospital length of stay of 8 days
between February and July 2020 [36].
Global comparisons of in-hospital mortality
and hospital length of stay are challenging due to variations in testing
protocols, mortality ascertainment, societal age structures and healthcare
systems [37]. During the first wave of the COVID-19 pandemic, in-hospital
mortality was higher in Europe (22.9%) and America (22.2%) than in Asia (12.7%)
[38]. In Italy, Germany and the USA, in-hospital mortality rates were roughly
20% [32, 39–42]. Data from the UK showed higher in-hospital mortality rates of
about 30% [43, 44]. Conversely, Spain and China recorded lower first-wave in-hospital
mortality at 17% and 14%, respectively [45–47]. Subsequent waves saw
significantly reduced in-hospital mortality rates [32, 39, 46–50]. Second-wave
hospital length of stay data showed similar durations in Germany (8 days),
Spain (9 days) and the USA (8.9 days) [51–53], with Italy reporting a median hospital
length of stay of 6 days from February 2020 to March 2021 [54]. These findings
are summarised in table S10.
However, it remains unclear whether
COVID-19 patients themselves were directly affected by hospital overload and
what specific factors contributed to the increased resource use and mortality. Similarly,
our study design also does not allow for establishing causality. Nonetheless,
it is likely that several factors may have contributed to these findings,
including limited knowledge about the virus’ aetiopathogenesis and treatment
options, high frailty scores and frequent admissions from institutions.
Notably, the pandemic control group showed
no association with additional hospital resource use, and there were no
clinically meaningful changes compared to the pre-pandemic phase. While there
is no data from Switzerland on any in-hospital resource use among patients
without COVID-19 during the pandemic, in Canada patients with urinary tract
infection, acute coronary syndrome or stroke without COVID-19 did not show
increased 30-day mortality or hospital length of stay during COVID-19 surges. However,
patients with heart failure, COPD and asthma without COVID-19 exhibited higher
30-day mortality rates. Whether this was based on the competition for scarce
mechanical ventilation resources remains unclear [15]. Contrary to our
findings, several studies have reported higher in-hospital mortality for
non-COVID-19 patients during the beginning of the pandemic [55–58]. A survey of
hospital administrators revealed poor quality of care and worse disease outcomes
in inpatients without COVID-19 during pandemic hospital strain [59], even
though this finding was mainly observed in hospitals serving high proportions
of patients from racial or ethnic minority groups.
The decline in 30-day hospital readmission
in the exposure group during the COVID-19 pandemic is consistent with the
increase in facility discharges. Patients were often transferred to nursing homes
or rehabilitation centres for further care, reducing the need for readmission
within the first 30 days post-hospitalisation. Moreover, the COVID-19
population was younger with a lower burden of comorbidities, resulting in a
faster and more complete recovery. Frequent discharges to institutions have also
been previously described and represent an additional burden for nursing homes
and rehabilitation clinics [60]; this also prolonged the
hospital stay as due to overload patients had to wait for space.
In patients with pre-existing illnesses and
COVID-19, in-hospital mortality, hospital length of stay and facility discharge
increased to the same extent across all comorbidities during the pandemic. In
contrast, the population without COVID-19 showed no clinically meaningful
changes. These results in the exposure group align with previous findings [31,
61–63]. The mechanisms behind these outcomes remain unclear, with several possible
explanations. For instance, diabetes mellitus can impair immune function,
making it harder for patients to fight infections [64]. Many cancer treatments
also weaken the immune system, which complicates recovery even more. In patients
with underlying hypertension and other cardiovascular diseases, the risk of
severe outcomes may be increased by several mechanisms, including therapeutic
upregulation of angiotensin-converting enzyme 2, the host receptor for
SARS-CoV-2 [65]. Obesity, which tends to impair lung function and dysregulate
the immune system [66, 67], is another significant risk factor for a poorer
prognosis [68, 69]. Although we did not include obesity in our subanalysis due
to underreporting, our baseline characteristics indicated a higher percentage
of obesity in the COVID-19 population compared to other groups. These findings
suggest that patients with pre-existing conditions who contract COVID-19 are
particularly vulnerable to severe outcomes, necessitating additional hospital
resources and leading to higher rates of facility discharge. The increased
burden on nursing homes and rehabilitation centres underscores the need for
targeted strategies to manage these patients effectively during pandemics.
Limitations and strengths
These results must be interpreted in the
context of the study design. First, given that COVID-19 hospitalisations were
identified by ICD-10-GM codes used for billing purposes, misclassification and
underreporting are possible, especially during the beginning of the COVID-19 pandemic
when no specific ICD-10-GM codes were available. Second, confounding due to
population differences during the pandemic is likely. However, we do not
believe that the changes in trends among the groups are solely caused by different
baseline characteristics or secular trends, given the presence of a comparison
group. Third, because of the retrospective design of the study, no causal
inference is possible. Nonetheless, there are several notable strengths. This
analysis was based on nationwide hospital care data with high external
validity, strong statistical power and high generalisability across all regions
in Switzerland. Furthermore, our results provide robust insights into the
course of patients without COVID-19 during the pandemic, reassuring us of the
high quality of care within the Swiss healthcare system, even during the
challenging times of a pandemic.
Conclusion
In conclusion, our study assumes that the COVID-19 pandemic was associated with unfavourable
in-hospital outcomes, particularly among patients hospitalised with or for
COVID-19 and pre-existing conditions. However, we did not find evidence that
patients without COVID-19 experienced similar unfavourable outcomes. Despite
these challenges, the Swiss healthcare system maintained high-quality care for
non-COVID-19 patients, highlighting its resilience and adaptability during a
global health crisis.
Data sharing statement
Restrictions apply to the availability of
data generated or analysed during this study, in order to preserve patient
confidentiality or because they were used under licence. The corresponding
author will on request detail the restrictions and any conditions under which
access to some data may be provided.
Acknowledgments
We thank the Swiss Federal Office for
Statistics (Neuchâtel, Switzerland) for the acquisition and provision of data.
Author contributions: Data access: Dr. med.
univ. Felder-Wieser, Dr. med. Laager and Dr. med. Kutz had full access to all of the
data in the study and take responsibility for the integrity of the data and the
accuracy of the data analysis. Concept and design: Felder-Wieser, Laager, Kutz. Acquisition,
analysis and interpretation of data: Felder-Wieser, Laager, Rasiah, Kutz. Drafting
of
the manuscript: Felder-Wieser, Laager, Rasiah, Kutz. Critical revision of the
manuscript for important intellectual content: all authors. Statistical
analysis: Felder-Wieser, Laager, Rasiah, Kutz.
Dr. med. univ. Rebecca Felder-Wieser
University Clinic for Geriatric Medicine
Tièchestrasse 99
CH-8037 Zürich
rebecca.felder[at]stadtspital.ch
References
1. Schweizerischer Bundesrat. Coronavirus: Bundesrat erklärt die «ausserordentliche Lage»
und verschärft die Massnahmen. Der Bundesrat, Kommunikation Bundesamt für Gesundheit
(BAG). Bern: 2020. Available from: https://www.news.admin.ch/en/nsb?id=78454
2. Fournier JP, Amélineau JB, Hild S, Nguyen-Soenen J, Daviot A, Simonneau B, et al. Patient-safety
incidents during COVID-19 health crisis in France: an exploratory sequential multi-method
study in primary care. Eur J Gen Pract. 2021 Dec;27(1):142–51. doi: https://doi.org/10.1080/13814788.2021.1945029
3. Rachamin Y, Meyer MR, Rosemann T, Grischott T. Impact of the COVID-19 Pandemic on
Elective and Emergency Inpatient Procedure Volumes in Switzerland - A Retrospective
Study Based on Insurance Claims Data. Int J Health Policy Manag. 2023;12:6932.
4. Webb E, Hernández-Quevedo C, Williams G, Scarpetti G, Reed S, Panteli D. Providing
health services effectively during the first wave of COVID-19: A cross-country comparison
on planning services, managing cases, and maintaining essential services. Health Policy.
2022 May;126(5):382–90. doi: https://doi.org/10.1016/j.healthpol.2021.04.016
5. Winkelmann J, Webb E, Williams GA, Hernández-Quevedo C, Maier CB, Panteli D. European
countries’ responses in ensuring sufficient physical infrastructure and workforce
capacity during the first COVID-19 wave. Health Policy. 2022 May;126(5):362–72. doi: https://doi.org/10.1016/j.healthpol.2021.06.015
6. Wirth B, Stucki M, Joerg R, Thommen C, Höglinger M. Impact of the Covid-19 pandemic
on inpatient health care in Switzerland 2020-2021-A descriptive retrospective study
using admission data of all Swiss hospitals. PLoS One. 2024 Jul;19(7):e0306791. doi: https://doi.org/10.1371/journal.pone.0306791
7. Wu AW, Sax H, Letaief M, Bellandi T, Newman-Toker D, Paine LA, et al. COVID-19: the
dark side and the sunny side for patient safety. J Patient Saf Risk Manag. 2020;25(4):137–41.
10.1177/2516043520957116
8. Ferrara G, De Vincentiis L, Ambrosini-Spaltro A, Barbareschi M, Bertolini V, Contato E,
et al. Cancer diagnostic delay in northern and central Italy during the 2020 lockdown
due to the coronavirus disease 2019 pandemic: assessment of the magnitude of the problem
and proposals for corrective actions. Am J Clin Pathol. 2021 Jan;155(1):64–8. doi: https://doi.org/10.1093/ajcp/aqaa177
9. Jazieh AR, Akbulut H, Curigliano G, Rogado A, Alsharm AA, Razis ED, et al.; International
Research Network on COVID-19 Impact on Cancer Care. Impact of the COVID-19 pandemic
on cancer care: a global collaborative study. JCO Glob Oncol. 2020 Sep;6(6):1428–38.
doi: https://doi.org/10.1200/GO.20.00351
10. Wong LE, et al. Where are all the patients? Addressing Covid-19 fear to encourage
sick patients to seek emergency care. NEJM Catal. 2020;1(3):1–12.
11. Gasch-Illescas A, Calle-Serrano M, Vallejo-Vaz AJ, Praena-Fernández JM, Guerrero JA,
Calderón EJ, et al. Impact of the first wave of the COVID-19 pandemic on non-COVID
inpatient care in southern Spain. Sci Rep. 2023 Jan;13(1):1634. doi: https://doi.org/10.1038/s41598-023-28831-6
12. Kadri SS, Sun J, Lawandi A, Strich JR, Busch LM, Keller M, et al. Association between
caseload surge and COVID-19 survival in 558 US hospitals, March to August 2020. Ann
Intern Med. 2021 Sep;174(9):1240–51. doi: https://doi.org/10.7326/M21-1213
13. Gibbons PW, Kim J, Cash RE, He S, Lai D, Christian Renne B, et al. Influence of ICU
Surge and Capacity on COVID Mortality Across U.S. States and Regions During the COVID-19
Pandemic. J Intensive Care Med. 2023 Jun;38(6):562–5. doi: https://doi.org/10.1177/08850666231157338
14. Bravata DM, Perkins AJ, Myers LJ, Arling G, Zhang Y, Zillich AJ, et al. Association
of intensive care unit patient load and demand with mortality rates in US Department
of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021 Jan;4(1):e2034266.
doi: https://doi.org/10.1001/jamanetworkopen.2020.34266
15. McAlister FA, Chu A, Qiu F, Dong Y, van Diepen S, Youngson E, et al.; CORONA Collaboration.
Outcomes Among Patients Hospitalized With Non-COVID-19 Conditions Before and During
the COVID-19 Pandemic in Alberta and Ontario, Canada. JAMA Netw Open. 2023 Jul;6(7):e2323035.
doi: https://doi.org/10.1001/jamanetworkopen.2023.23035
16. Lopez-Picazo JJ, Vidal-Abarca I, Beteta D, López-Ibáñez M, García-Vázquez E. Impact
of the COVID-19 pandemic on the hospital: inpatient’s perceived quality in Spain.
J Patient Exp. 2021 Mar;8:2374373521998625. doi: https://doi.org/10.1177/2374373521998625
17. LeRose J, Sandhu A, Polistico J, Ellsworth J, Cranis M, Jabbo L, et al. The impact
of coronavirus disease 2019 (COVID-19) response on central-line-associated bloodstream
infections and blood culture contamination rates at a tertiary-care center in the
Greater Detroit area. Infect Control Hosp Epidemiol. 2021 Aug;42(8):997–1000. doi: https://doi.org/10.1017/ice.2020.1335
18. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guise JM. The association between hospital
capacity strain and inpatient outcomes in highly developed countries: a systematic
review. J Gen Intern Med. 2017 Jun;32(6):686–96. doi: https://doi.org/10.1007/s11606-016-3936-3
19. Bernstein SL, Aronsky D, Duseja R, Epstein S, Handel D, Hwang U, et al.; Society for
Academic Emergency Medicine, Emergency Department Crowding Task Force. The effect
of emergency department crowding on clinically oriented outcomes. Acad Emerg Med.
2009 Jan;16(1):1–10. doi: https://doi.org/10.1111/j.1553-2712.2008.00295.x
20. Carter EJ, Pouch SM, Larson EL. The relationship between emergency department crowding
and patient outcomes: a systematic review. J Nurs Scholarsh. 2014 Mar;46(2):106–15.
doi: https://doi.org/10.1111/jnu.12055
21. Kane RL, Shamliyan TA, Mueller C, Duval S, Wilt TJ. The association of registered
nurse staffing levels and patient outcomes: systematic review and meta-analysis. Med
Care. 2007 Dec;45(12):1195–204. doi: https://doi.org/10.1097/MLR.0b013e3181468ca3
22. Needleman J, Buerhaus P, Pankratz VS, Leibson CL, Stevens SR, Harris M. Nurse staffing
and inpatient hospital mortality. N Engl J Med. 2011 Mar;364(11):1037–45. doi: https://doi.org/10.1056/NEJMsa1001025
23. Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing
patterns and clinical outcomes in critically ill patients: a systematic review. JAMA.
2002 Nov;288(17):2151–62. doi: https://doi.org/10.1001/jama.288.17.2151
24. Kluberg SA, Hou L, Dutcher SK, Billings M, Kit B, Toh S, et al. Validation of diagnosis
codes to identify hospitalized COVID-19 patients in health care claims data. Pharmacoepidemiol
Drug Saf. 2022 Apr;31(4):476–80. doi: https://doi.org/10.1002/pds.5401
25. Wu G, D’Souza AG, Quan H, Southern DA, Youngson E, Williamson T, et al. Validity of
ICD-10 codes for COVID-19 patients with hospital admissions or ED visits in Canada:
a retrospective cohort study. BMJ Open. 2022 Jan;12(1):e057838. doi: https://doi.org/10.1136/bmjopen-2021-057838
26. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative
data. Med Care. 1998 Jan;36(1):8–27. doi: https://doi.org/10.1097/00005650-199801000-00004
27. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development
and validation of a Hospital Frailty Risk Score focusing on older people in acute
care settings using electronic hospital records: an observational study. Lancet. 2018 May;391(10132):1775–82.
doi: https://doi.org/10.1016/S0140-6736(18)30668-8
28. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)
statement: guidelines for reporting observational studies. Lancet. 2007 Oct;370(9596):1453–7.
doi: https://doi.org/10.1016/S0140-6736(07)61602-X
29. Swiss DR. Regeln und Definitionen zur Fallabrechnung unter SwissDRG. 2016. Available
from : https://www.swissdrg.org/application/files/4714/8111/3146/160620_SwissDRG_Falldefinitionen_v5.pdf
30. Volpato S, Landi F, Incalzi RA. A Frail Health Care System for an Old Population:
lesson form the COVID-19 Outbreak in Italy. J Gerontol A Biol Sci Med Sci. 2020 Sep;75(9):e126–7.
doi: https://doi.org/10.1093/gerona/glaa087
31. Diebold M, Martinez AE, Adam KM, Bassetti S, Osthoff M, Kassi E, et al. Temporal trends
of COVID-19 related in-hospital mortality and demographics in Switzerland - a retrospective
single centre cohort study. Swiss Med Wkly. 2021 Jul;151(2930):w20572. doi: https://doi.org/10.4414/smw.2021.20572
32. Leidi F, Boari GE, Scarano O, Mangili B, Gorla G, Corbani A, et al. Comparison of
the characteristics, morbidity and mortality of COVID-19 between first and second/third
wave in a hospital setting in Lombardy: a retrospective cohort study. Intern Emerg
Med. 2022 Oct;17(7):1941–9. doi: https://doi.org/10.1007/s11739-022-03034-5
33. Gregoriano C, Koch D, Haubitz S, Conen A, Fux CA, Mueller B, et al. Characteristics,
predictors and outcomes among 99 patients hospitalised with COVID-19 in a tertiary
care centre in Switzerland: an observational analysis. Swiss Med Wkly. 2020 Jul;150(2930):w20316.
doi: https://doi.org/10.4414/smw.2020.20316
34. Wolfisberg S, Gregoriano C, Struja T, Kutz A, Koch D, Bernasconi L, et al. Comparison
of characteristics, predictors and outcomes between the first and second COVID-19
waves in a tertiary care centre in Switzerland: an observational analysis. Swiss Med
Wkly. 2021 Aug;151(3132):w20569. doi: https://doi.org/10.4414/smw.2021.20569
35. Thiabaud A, Iten A, Balmelli C, Senn L, Troillet N, Widmer A, et al. Cohort profile:
SARS-CoV-2/COVID-19 hospitalised patients in Switzerland. Swiss Med Wkly. 2021 Feb;151(708):w20475.
doi: https://doi.org/10.4414/smw.2021.20475
36. Fröhlich GM, De Kraker ME, Abbas M, Keiser O, Thiabaud A, Roelens M, et al. Hospital
outcomes of community-acquired COVID-19 versus influenza: insights from the Swiss
hospital-based surveillance of influenza and COVID-19. Euro Surveill. 2022 Jan;27(1):2001848.
doi: https://doi.org/10.2807/1560-7917.ES.2022.27.1.2001848
37. Hothorn T, Bopp M, Günthard H, Keiser O, Roelens M, Weibull CE, et al. Assessing relative
COVID-19 mortality: a Swiss population-based study. BMJ Open. 2021 Mar;11(3):e042387.
doi: https://doi.org/10.1136/bmjopen-2020-042387
38. Goel S, Jain T, Hooda A, Malhotra R, Johal G, Masoomi R, et al. Clinical characteristics
and in-hospital mortality for COVID-19 across the globe. Cardiol Ther. 2020 Dec;9(2):553–9.
doi: https://doi.org/10.1007/s40119-020-00189-0
39. Giacomelli A, Ridolfo AL, Pezzati L, Oreni L, Carrozzo G, Beltrami M, et al. Mortality
rates among COVID-19 patients hospitalised during the first three waves of the epidemic
in Milan, Italy: A prospective observational study. PLoS One. 2022 Apr;17(4):e0263548.
doi: https://doi.org/10.1371/journal.pone.0263548
40. Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et
al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19
admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020 Sep;8(9):853–62.
doi: https://doi.org/10.1016/S2213-2600(20)30316-7
41. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al.
Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized
With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: https://doi.org/10.1001/jama.2020.7681
42. Mallow PJ, Belk KW, Topmiller M, Hooker EA. Outcomes of hospitalized COVID-19 patients
by risk factors: results from a United States hospital claims database. J Health Econ
Outcomes Res. 2020 Sep;7(2):165–74. doi: https://doi.org/10.36469/jheor.2020.17331
43. Docherty AB, Mulholland RH, Lone NI, Cheyne CP, De Angelis D, Diaz-Ordaz K, et al.;
ISARIC4C Investigators. Changes in in-hospital mortality in the first wave of COVID-19:
a multicentre prospective observational cohort study using the WHO Clinical Characterisation
Protocol UK. Lancet Respir Med. 2021 Jul;9(7):773–85. doi: https://doi.org/10.1016/S2213-2600(21)00175-2
44. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al.; ISARIC4C
investigators. Features of 20 133 UK patients in hospital with covid-19 using the
ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study.
BMJ. 2020 May;369:m1985. doi: https://doi.org/10.1136/bmj.m1985
45. Wu P, Hao X, Lau EH, Wong JY, Leung KS, Wu JT, et al. Real-time tentative assessment
of the epidemiological characteristics of novel coronavirus infections in Wuhan, China,
as at 22 January 2020. Euro Surveill. 2020 Jan;25(3):2000044. doi: https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000044
46. Roso-Llorach A, Serra-Picamal X, Cos FX, Pallejà-Millán M, Mateu L, Rosell A, et al. Evolving
mortality and clinical outcomes of hospitalized subjects during successive COVID-19
waves in Catalonia, Spain. Glob Epidemiol. 2022 Dec;4:100071. doi: https://doi.org/10.1016/j.gloepi.2022.100071
47. Zuil M, Benítez ID, Cabo-Gambín R, Manzano Senra C, Moncusí-Moix A, Gort-Paniello C,
et al. Clinical management and outcome differences between first and second waves
among COVID-19 hospitalized patients: A regional prospective observational cohort.
PLoS One. 2021 Oct;16(10):e0258918. doi: https://doi.org/10.1371/journal.pone.0258918
48. Bechman K, Yates M, Mann K, Nagra D, Smith LJ, Rutherford AI, et al. Inpatient COVID-19
mortality has reduced over time: results from an observational cohort. PLoS One. 2022 Jan;17(1):e0261142.
doi: https://doi.org/10.1371/journal.pone.0261142
49. Oladunjoye O, Gallagher M, Wasser T, Oladunjoye A, Paladugu S, Donato A. Mortality
due to COVID-19 infection: A comparison of first and second waves. J Community Hosp
Intern Med Perspect. 2021 Nov;11(6):747–52. doi: https://doi.org/10.1080/20009666.2021.1978154
50. James N, Menzies M, Radchenko P. COVID-19 second wave mortality in Europe and the
United States. Chaos. 2021 Mar;31(3):031105. doi: https://doi.org/10.1063/5.0041569
51. Ludwig M, Jacob J, Basedow F, Andersohn F, Walker J. Clinical outcomes and characteristics
of patients hospitalized for Influenza or COVID-19 in Germany. Int J Infect Dis. 2021 Feb;103:316–22.
doi: https://doi.org/10.1016/j.ijid.2020.11.204
52. Rodriguez-Nunez N, Gude F, Lama A, Rábade C, Varela A, Abelleira R, et al. Health
indicators in hospitalized patients with SARS-CoV-2 pneumonia: a comparison between
the first and second wave. Arch Bronconeumol. 2021;57(11):717–719. doi: https://doi.org/10.1016/j.arbr.2021.03.019
53. Di Fusco M, Shea KM, Lin J, Nguyen JL, Angulo FJ, Benigno M, et al. Health outcomes
and economic burden of hospitalized COVID-19 patients in the United States. J Med
Econ. 2021;24(1):308–17. doi: https://doi.org/10.1080/13696998.2021.1886109
54. Zeleke AJ, Moscato S, Miglio R, Chiari L. Length of stay analysis of COVID-19 hospitalizations
using a count regression model and Quantile regression: a study in Bologna, Italy.
Int J Environ Res Public Health. 2022 Feb;19(4):2224. doi: https://doi.org/10.3390/ijerph19042224
55. Sabbatini AK, Robicsek A, Chiu ST, Gluckman TJ. Excess mortality among patients hospitalized
during the COVID‐19 pandemic. J Hosp Med. 2021 Oct;16(10):596–602. doi: https://doi.org/10.12788/jhm.3633
56. Dang A, Thakker R, Li S, Hommel E, Mehta HB, Goodwin JS. Hospitalizations and mortality
from non–SARS-CoV-2 causes among Medicare beneficiaries at US hospitals during the
SARS-CoV-2 pandemic. JAMA Netw Open. 2022 Mar;5(3):e221754. doi: https://doi.org/10.1001/jamanetworkopen.2022.1754
57. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19
pandemic on hospital admissions in the United States: study examines trends in US
hospital admissions during the COVID-19 pandemic. Health Aff (Millwood). 2020 Nov;39(11):2010–7.
doi: https://doi.org/10.1377/hlthaff.2020.00980
58. Bartolomeo N, Giotta M, Trerotoli P. In-hospital mortality in non-COVID-19-related
diseases before and during the pandemic: A regional retrospective study. Int J Environ
Res Public Health. 2021 Oct;18(20):10886. doi: https://doi.org/10.3390/ijerph182010886
59. Huggins A, Husaini M, Wang F, Waken RJ, Epstein AM, Orav EJ, et al. Care Disruption
During COVID-19: a National Survey of Hospital Leaders. J Gen Intern Med. 2023 Apr;38(5):1232–8.
doi: https://doi.org/10.1007/s11606-022-08002-5
60. Moon RC, Brown H, Rosenthal N. Healthcare resource utilization of patients with COVID-19
visiting US hospitals. Value Health. 2022 May;25(5):751–60. doi: https://doi.org/10.1016/j.jval.2021.12.005
61. Isath A, Malik AH, Goel A, Gupta R, Shrivastav R, Bandyopadhyay D. Nationwide analysis
of the outcomes and mortality of hospitalized COVID-19 patients. Curr Probl Cardiol.
2023 Feb;48(2):101440. doi: https://doi.org/10.1016/j.cpcardiol.2022.101440
62. Li X, Guan B, Su T, Liu W, Chen M, Bin Waleed K, et al. Impact of cardiovascular disease
and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic
review and meta-analysis. Heart. 2020 Aug;106(15):1142–7. doi: https://doi.org/10.1136/heartjnl-2020-317062
63. Mason KE, Maudsley G, McHale P, Pennington A, Day J, Barr B. Age-Adjusted Associations
Between Comorbidity and Outcomes of COVID-19: A Review of the Evidence From the Early
Stages of the Pandemic. Front Public Health. 2021 Aug;9:584182. doi: https://doi.org/10.3389/fpubh.2021.584182
64. Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms
contributing to the double burden of diabetes and intracellular bacterial infections.
Immunology. 2015 Feb;144(2):171–85. doi: https://doi.org/10.1111/imm.12394
65. Yang C, Jin Z. An acute respiratory infection runs into the most common noncommunicable
epidemic—COVID-19 and cardiovascular diseases. JAMA Cardiol. 2020 Jul;5(7):743–4.
doi: https://doi.org/10.1001/jamacardio.2020.0934
66. Luzi L, Radaelli MG. Influenza and obesity: its odd relationship and the lessons for
COVID-19 pandemic. Acta Diabetol. 2020 Jun;57(6):759–64. doi: https://doi.org/10.1007/s00592-020-01522-8
67. Kwok S, Adam S, Ho JH, Iqbal Z, Turkington P, Razvi S, et al. Obesity: A critical
risk factor in the COVID-19 pandemic. Clin Obes. 2020 Dec;10(6):e12403. doi: https://doi.org/10.1111/cob.12403
68. Nagy É, Cseh V, Barcs I, Ludwig E. The Impact of Comorbidities and Obesity on the
Severity and Outcome of COVID-19 in Hospitalized Patients-A Retrospective Study in
a Hungarian Hospital. Int J Environ Res Public Health. 2023 Jan;20(2):1372. doi: https://doi.org/10.3390/ijerph20021372
69. Sawadogo W, Tsegaye M, Gizaw A, Adera T. Overweight and obesity as risk factors for
COVID-19-associated hospitalisations and death: systematic review and meta-analysis.
BMJ Nutr Prev Health. 2022 Jan;5(1):10–8. doi: https://doi.org/10.1136/bmjnph-2021-000375
70. Lehmann M, Peeters S, Streuter M, Nawrocki M, Kösters K, Kröger K. COVID 19 – Hospitalisierung
in der ersten und zweiten Welle [COVID 19 – Hospital Admission in the First and Second
Wave in Germany]. Dtsch Med Wochenschr. 2023; 148(4):e14–e20. German. doi: https://doi.org/10.1055/a-1951-0629
Appendix
The appendix is available for download as a separate file at https://doi.org/10.57187/s.4109.

