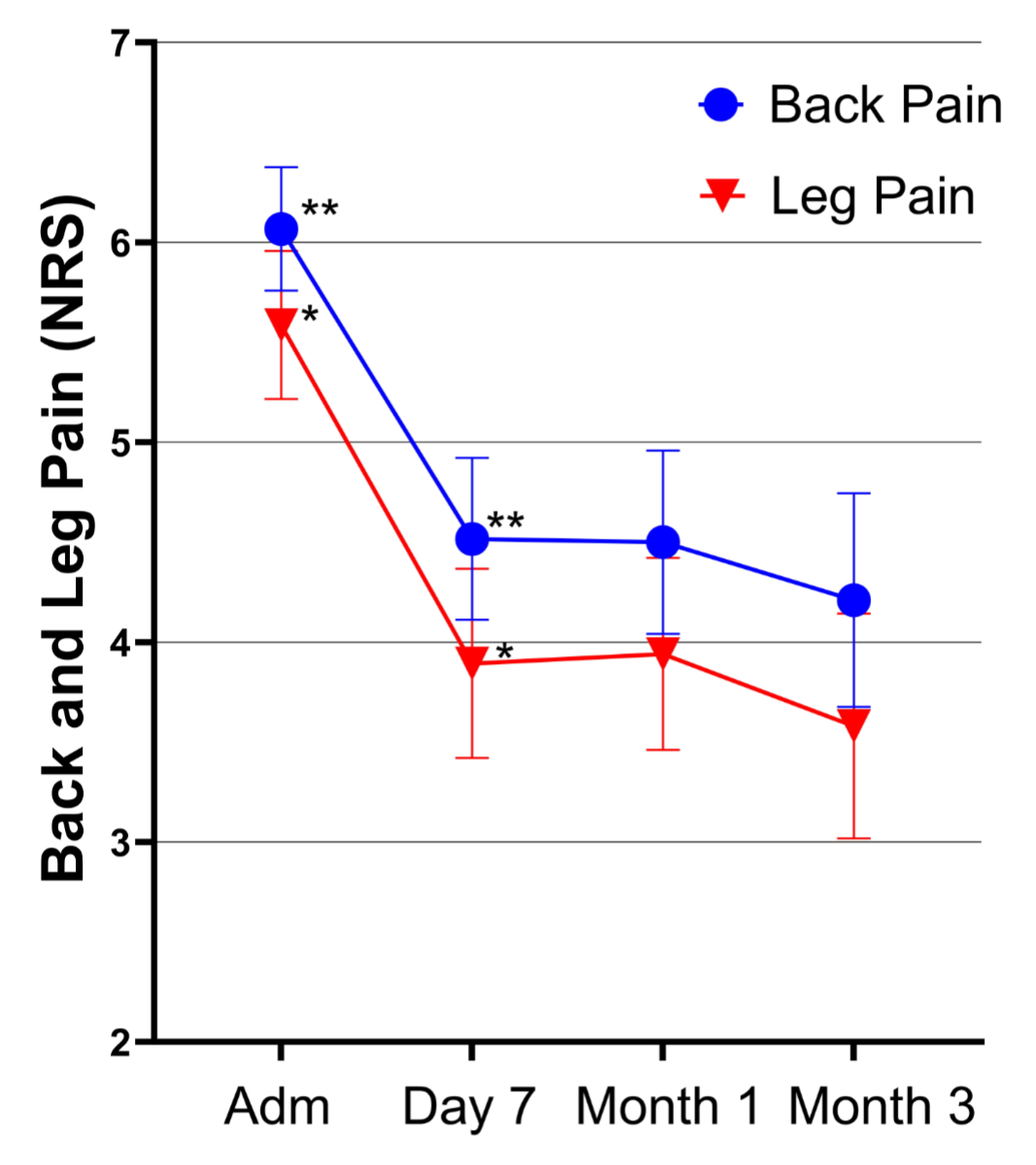
Figure 1Time course of back and leg pain. Adm: Admission; NRS: Numeric Rating Scale.
DOI: https://doi.org/https://doi.org/10.57187/s.4100
European Quality of Life 5 Dimensions
European Quality of Life 5 Dimensions Questionnaire
Numeric Pain Rating Scale
Numeric Rating Scale
Oswestry Disability Index
Pain and Enjoyment of Life and General Activity
In Switzerland in 2017, 35% of the adult population reported experiencing mild back pain, while 8% reported suffering from severe back or low back pain [1]. Chronic back pain and radicular pain resulting from central or neuroforaminal spinal stenosis represent significant issues requiring treatment [2]. In addition, radiculopathy in the lumbosacral region is a prevalent neurological syndrome, contributing significantly to disability and healthcare utilisation [3]. Between 2015 and 2019, Swiss healthcare costs attributable to back pain averaged CHF 518 million annually. Moreover, it was discovered that patients with low back pain incurred 72% higher overall costs compared to those without low back pain [4].
Non-surgical, conservative treatments provide viable options to address symptoms, particularly pain, especially for patients who decline surgery or for whom surgery is not feasible or advisable [5].
Epidural steroid infiltration has served as a symptomatic pain therapy for decades [6].
However, since the introduction of interlaminar and transforaminal epidural steroid infiltration, a debate has emerged regarding the advantages and disadvantages of particulate versus nonparticulate steroids [7, 8]. The depot effect of particulate steroids is believed to confer an advantage over non-particulate steroids [9, 10]; however, clinical studies have failed to demonstrate superior function-related and pain-reducing outcomes. In addition, case reports have warned of permanent neurological impairments following cervical transforaminal epidural steroid infiltration. Moreover, there is a risk of arterial occlusion of the perfusing vessels leading to embolic infarction if an intra-arterial injection of particular steroids is inadvertently performed [11].
In Switzerland, the debate over the use of particulate steroids for epidural infiltrations has become obsolete. While the US Food and Drug Administration (FDA) continues to permit and the World Institute of Pain Benelux Work Group still recommends the epidural use of particulate steroids [12], the Swiss regulatory authority Swissmedic banned their use in 2018 [13] due to the neurotoxicity of benzyl alcohol used in their production [14]. Consequently, only non-particulate steroids, primarily water-soluble dexamethasone – or off-label use of particulate steroids without benzyl alcohol (magistral formula) – are now available for epidural infiltration. Another available option is the lipid emulsion dexamethasone-21-palmitate (Lipotalon®) in which the steroid is emulsified with soybean oil, potentially prolonging the anti-inflammatory effect due to greater uptake by macrophages compared to water-soluble dexamethasone [15]. Furthermore, there are fewer systemic side effects due to slower absorption [16].
Due to the potential benefits of lipid-packed vs water-soluble dexamethasone, our pain clinic at University Hospital Basel decided to transition to off-label use of dexamethasone-21-palmitate after the ban on particulate steroids for epidural use. In parallel with this transition, we began implementing patient-reported outcome measures as a quality control for our interventional pain-management procedures. Patient-reported outcome measures are follow-up assessments that focus on patients’ perspectives on the impact of medical treatments [17]. They provide an opportunity to gather information about a patient’s discomfort, quality of life, daily functioning and various other aspects related to health and wellbeing [18]. As they directly reflect the impact on patients’ experiences, they serve as indicators of quality of care and treatment effectiveness [19], and complement objective clinical values with subjective outcome measures [20].
As pain cannot be objectively measured, patient-reported outcome measures are especially useful in pain medicine. The efficacy of pain therapy interventions can only be assessed through subjective outcomes.
To assess treatment response to epidural application of dexamethasone-21-palmitate, we analysed patient-reported outcome data collected from the first 212 patients receiving interlaminar or transforaminal epidural steroid infiltration with Lipotalon® for low back pain and/or leg pain due to central or neuroforaminal spinal stenosis in our clinic. Our main outcome of interest was the time course of pain measured by the 11-point Numeric Rating Scale (NRS). The time course of quality of life and function served as outcomes of secondary interest and were assessed using the following instruments: Numeric Pain Rating Scale (NPRS); Pain, Enjoyment of Life and General Activity (PEG) Scale; Oswestry Disability Index (ODI); European Quality of Life 5 Dimensions (EQ-5D); and patient satisfaction with treatment. All outcomes were assessed at predetermined time points: directly before intervention and at 7 days, 1 month and 3 months after intervention. This data analysis was conducted as a retrospective exploratory study to evaluate dexamethasone-21-palmitate as a treatment option for epidural steroid infiltrations in Switzerland.
This retrospective cohort study examining patient-reported outcome data was approved by the local ethics committee (BASEC ID 2023-00454). All patients undergoing epidural steroid infiltration were provided with written information about the survey and were invited to participate. Research consent was sought from all patients on the day they began treatment at University Hospital Basel. Patients who refused to consent were excluded from the analysis.
The analysis included patients treated between July 2019 and April 2022.
All patients who were deemed suitable for treatment with epidural steroid infiltration at the pain clinic of University Hospital Basel were considered potential candidates for the study.
Exclusion criteria were as follows: patients who refused to consent to study participation; patients unable to complete the questionnaire in either German or English; and/or patients incapable of participating due to their health status. To be eligible for analysis, patients were required to complete the baseline assessment and at least one of the three follow-up assessments.
The treatments evaluated in this study included X-ray image-guided interlaminar or transforaminal epidural steroid infiltrations at the level of the stenosis or impacted nerve root, performed in an outpatient setting. The interventions were conducted in accordance with the Practice Guidelines for Spinal Diagnostic and Treatment Procedures [21]. The procedures involved the following accesses: for interlaminar epidural steroid infiltration, a needle was inserted through an interlaminar space, allowing entry into the dorsal epidural space using the “loss of resistance” technique; for transforaminal epidural steroid infiltration, a needle was inserted into the intervertebral foramen to reach the affected nerve root and surrounding tissue [21].
The correct needle position was confirmed by administering 0.2–0.4 ml of radio-opaque substance (Iopamidol 300 mg/ml) and conducting X-ray control in at least two planes. For interlaminar epidural steroid infiltration, an 18-gauge Touhy cannula was used to inject a standard 12 mg dose of dexamethasone. For transforaminal epidural steroid infiltration, a 22-gauge spinal needle was used to inject a standard 4 mg (or in rare cases up to 6 mg) dose of dexamethasone. In the event of neurological symptoms due to compressive volume-effect during drug application, the dose could be reduced.
Outcome measures including pre-interventional health status and post-interventional follow-ups were assessed using patient-reported outcome measures. Data were collected via an electronic platform (Heartbeat ONE®, Heartbeat Medical Solutions GmbH, Berlin, Germany). Heartbeat ONE® stores data using 256-bit AES encryption and employs data centres located in Switzerland certified by ISO 5001 and ISO 27001 [22].
Data were collected at four predefined time points: directly before the intervention and 7 days, 1 month and 3 months after the intervention. The initial interview was conducted on-site using a tablet computer. Assistance from a healthcare worker was provided if patients encountered difficulties with electronic data collection. Follow-up assessments were completed online, with assistance provided via phone call from a healthcare worker if needed.
Health-related data and outcome measures collected in the patient-reported outcome surveys are detailed below. Baseline data and data regarding duration of back and leg pain were collected on admission. Patient satisfaction with the treatment procedure was assessed at the 7-day follow-up assessment. All other patient-reported outcome measures were assessed at all four assessment time points.
Baseline data included age, sex, educational level, work status, sick leave, comorbidities, structural pathologies and details concerning prescription and non-prescription medications. In addition, information regarding the intervention technique, intervention site, dosage of administered medication, reported side effects and any complications during the intervention were documented.
The duration of back and leg pain prior to the intervention was assessed. Response options were: no pain, pain lasting <3 months, 3–12 months, 1–2 years and >2 years.
Patients were asked to rate their satisfaction with the pain therapy. Questions were posed regarding whether patients felt they were taken seriously, whether they received new strategies for pain self-management, and whether the staff were friendly. Each category was rated on a scale from 0 to 10, with 0 being the worst and 10 being the best rating.
The Numeric Pain Raiting Scale (NPRS) is an 11-point scale for expressing pain intensity, with responses ranging from 0 (no pain) to 10 (worst pain imaginable). Back and leg pain were assessed separately using the NPRS.
The Pain, Enjoyment of Life and General Activity (PEG) scale is a validated multidimensional pain measurement method, which can be used to complement unidimensional pain documentation [23]. On the PEG scale, the average pain per week (Q1), the hindrance to a normal and satisfying life due to pain (Q2) and the influence of pain on general activities (Q3) are assessed and rated on scales between 0 (no impairment) and 10 (maximum imaginable negative interference). The PEG-Main score is determined by the mean of the three categories Q1, Q2 and Q3. The resulting graduated mean score is categorised as mild pain (<4), moderate pain (4–7) or severe pain (>7) [23].
Quality of life was assessed using the improved European Quality of Life 5 Dimensions (EQ-5D-5L) questionnaire [24], which is a descriptive system including five dimensions, each with five possible levels. The dimensions are “mobility”, “self-care”, “activity”, “pain” and “anxiety”. There is a choice of five ordinal answers (levels) per dimension [25].
The EQ-5D-5L can be summarised, resulting in the EQ-5D summary index. The EQ-5D summary index combines these dimensions to compare to a reference population, with 1.0 indicating the best possible health status. In our calculation, we used 100 instead of 1.0 as the best possible health status so that it can be regarded as a percentage of the best possible state of health.
To derive the summary index, a proprietary formula is used, with dimensions weighted based on population norms [24, 25].
The Oswestry Disability Index (ODI) is a validated questionnaire for disabilities related to low back pain [26]. It comprises categories including pain, personal care, lifting, walking, sitting, standing, sleeping, sexual life, social life and traveling. Responses range from 0 (no limitation) to 5 (maximum limitation due to pain), with the overall score interpreted as mild disability (0–20%), moderate disability (20–40%), severe disability (40–60%), disabling (60–80%), or bedridden or functionally impaired (80–100%) [27].
For statistical analysis, pseudonymised data were extracted from Heartbeat ONE® and patient identifiers were encrypted within the Heartbeat database. All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 28.0 (IBM Corp, Armonk, NY, USA). Graphical content was designed with GraphPad Prism, version 2020 (GraphPad Software, San Diego, CA, USA). The references were organised using EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA).
Quantitative variables were summarised using means, standard deviations (SD), as well as minimum and maximum values. Qualitative variables were presented as raw numbers with corresponding percentages.
Due to the exploratory noncompetitive nature of the study, no sample size calculation was performed. We set the minimum dataset size at 200 patients receiving Lipotalon® to perform the analysis, as a compromise between clinical feasibility and achieving a collective as meaningful as possible.
After ensuring homogeneity of variance (visually) and normality (visually and via the Shapiro-Wilk test; data not shown), differences between the various time points of outcome assessments were assessed by performing a Friedman’s two-way analysis of variance (ANOVA) on NPRS for back and leg pain, the PEG scale, EQ-5D and ODI. Pairwise comparisons of the results at different time points were conducted using post-hoc t-tests. A Bonferroni correction was applied to adjust for multiple testing. A significance level of 0.05 was chosen for all comparative analyses.
We analysed data from 212 patients (mean age 65 [SD 15.17] years, 55.2% female) who completed the patient-reported outcome questionnaire on admission to the study and subsequently underwent an epidural steroid infiltration. Approximately one-third of the patients were unable to work, with half of these cases attributed to back or leg pain (17.6%). At the baseline assessment, 19% of the patients were employed, while the majority (54.8%) were not employed due to other reasons such as studying, retirement or housework (table 1).
Table 1Baseline patient characteristics. Percentages were calculated in relation to the number of patients responding to the question.
| Age at admission, in years (mean [SD]) | 65 [SD 15.17] | n = 212 | |
| Sex, female (total [%]) | 117 [55.2%] | n = 212 | |
| Work status (total [%]) | n = 210 | ||
| Ready to work | 2 [1%] | – | |
| Employed full-time | 23 [11%] | – | |
| Employed part-time | 17 [8.1%] | ||
| Not able to work because of back/leg pain | 37 [17.6%] | ||
| Not working (student, retired, homework) | 115 [54.8%] | – | |
| Not able to work, other reason | 16 [7.6%] | – | |
Most patients had a history of long-lasting pain in the treatment target area. Specifically, 55% of patients reported experiencing back pain and 41.5% reported leg pain persisting for at least one year. The most frequently reported symptoms at baseline were prolonged dominant back pain (26.4%), prolonged dominant leg pain (19%) and prolonged combined back and leg pain (16.5%). Acute back and leg pain, along with spinal claudication, were less common. The prevalence of acute symptoms was 28%, whereas prolonged symptoms were observed in 61.9% of the cases. Tables 1 and 2 provide a detailed overview of baseline patient characteristics and pathology.
Table 2Baseline pathology. Percentages were calculated in relation to the number of patients providing values in the category.
| Duration of back pain prior to admission (total [%]) | n = 209 | ||
| No pain | 14 [6.7%] | – | |
| <3 months | 31 [14.8%] | – | |
| 3–12 months | 49 [23.4%] | – | |
| 1–2 years | 27 [12.9%] | – | |
| >2 years | 88 [42.1%] | – | |
| Duration of leg pain prior to admission (total [%]) | n = 209 | ||
| No pain | 20 [9.6%] | – | |
| <3 months | 47 [22.5%] | – | |
| 3–12 months | 56 [26.8%] | – | |
| 1–2 years | 28 [13.4%] | – | |
| >2 years | 58 [27.8%] | – | |
| Symptoms at baseline (total [%]) | n = 121 | ||
| Leg pain dominant, acute | 14 [11.6%] | – | |
| Leg pain dominant, chronic | 23 [19%] | – | |
| Back pain dominant, acute | 7 [5.8%] | ||
| Back pain dominant, chronic | 32 [26.4%] | – | |
| Back pain and leg pain, acute | 13 [10.7%] | – | |
| Back pain and leg pain, chronic | 20 [16.5%] | – | |
| Claudicatio spinalis | 12 [9.9%] | – | |
| Structural pathology at baseline (total [%]) | n = 120 | ||
| Appropriate to age | 11 [9.2%] | – | |
| Altered disc with normal intervertebral space | 18 [15%] | – | |
| Laterally altered facet joint | 4 [3.3%] | – | |
| Collapse of the intervertebral space not defined | 18 [15.0%] | – | |
| Spondylolisthesis/spondylolysis | 3 [2.5%] | – | |
| Compressive pathology at baseline (total [%]) | n = 122 | ||
| No | 3 [2.5%] | – | |
| Lateral | 15 [12.3%] | – | |
| Central | 43 [35.2%] | – | |
| Combined | 21 [17.2%] | – | |
| Not defined | 40 [32.8%] | – |
The vast majority of epidural steroid infiltrations (90.5%) were performed at the lumbar level. Of these, the interlaminar approach was used in 67.9% and the transforaminal approach in 32.1% of cases. For lumbar interlaminar treatments, the most commonly used dosage was 12 mg Lipotalon® (67.2%). In less frequent cases, dosages were reduced to 8 mg (13.9%) or 4 mg (18.9%), primarily due to neurological symptoms during the procedure (e.g. radiating pain) caused by volume effects on constricted spinal nerves. For the transforaminal approach, the preferred dose was 4 mg; 8 mg was used occasionally.
During four interventions, complications including accidental vascular perforation, severe pain, and presyncope post-intervention were recorded. None of these complications resulted in permanent damage or limitations. Detailed information on infiltration is provided in table 3.
Table 3Therapeutic variables. Percentages were calculated in relation to the number of patients providing values in this category.
| Access (total [%]) | n = 134 | ||
| Transforaminal | 43 [32.1%] | – | |
| Interlaminar | 91 [67.9%] | – | |
| Dose of injected Lipotalon® (lumbar) (total [%]) | n = 122 | ||
| 12 mg | 82 [67.2%] | – | |
| 8 mg | 17 [13.9%] | – | |
| 4 mg | 23 [18.9%] | – | |
| Procedure location (total [%]) | n = 137 | ||
| Cervical | 4 [2.9%] | – | |
| Thoracic | 1 [0.7%] | – | |
| Lumbar | 124 [90.5%] | – | |
| Sacral | 8 [5.8%] | – | |
| Level of injection (total [%]) | n = 113 | ||
| L1/2 | 2 [1.8%] | – | |
| L2/3 | 6 [5.3%] | – | |
| L3/4 | 17 [15.0%] | – | |
| L4/5 | 34 [30.1%] | – | |
| L5/S1 | 54 [47.8%] | – | |
For back and leg pain assessments, 210 responses were available at admission, 122 at the second assessment (7 days post-intervention), 118 at the third assessment (1 month post-intervention) and 100 at the fourth assessment (3 months post-intervention).
Baseline back pain was recorded as 6.07 (SD 2.27) on the NPRS. After the intervention, pain significantly decreased compared to baseline levels (p <0.001): 7 days post-intervention, the mean pain score was 4.52 (SD 2.26); after 1 month, it was 4.50 (SD 2.51); and after 3 months, it was 4.21 (SD 2.69). There was no statistically significant difference in back pain between the follow-up assessments.
Baseline leg pain was 5.59 (SD 2.72) on the NPRS. Similar to back pain, leg pain significantly decreased during the follow-ups (p <0.001). Mean pain scores were 3.89 (SD 2.64) at day 7, 3.94 (SD 2.64) after 1 month and 3.58 (SD 2.84) after 3 months. There was no statistically significant difference in leg pain between the follow-up assessments.
Significant differences between the sexes were found for both back and leg pain at 1 month (back pain: p = 0.032, leg pain: p = 0.029) and at 3 months (back pain: p = 0.002, leg pain: p = 0.001). On average, women reported more back and leg pain than men at all assessment points. Further details and the time course of back and leg pain can be reviewed in table 4 and figure 1, respectively.
Table 4Back and leg pain, by sex. This table presents means and standard deviations for average back and leg pain across all participants, with additional insights into central tendency and variability provided by sex-specific means.
| Back pain | Leg pain | |||||
| Sex | n | Mean | SD | Mean | SD | |
| Pain at admission | Female | 116 | 6.33 | 2.14 | 6.08 | 2.37 |
| Male | 94 | 5.74 | 2.4 | 4.98 | 3.00 | |
| Overall | 210 | 6.07 | 2.27 | 5.59 | 2.72 | |
| Pain at day 7 | Female | 65 | 4.66 | 2.24 | 4.22 | 2.79 |
| Male | 57 | 4.35 | 2.29 | 3.53 | 2.42 | |
| Overall | 122 | 4.52* | 2.26 | 3.89* | 2.64 | |
| Pain at 1 month | Female | 66 | 4.94 | 2.4 | 4.41 | 2.74 |
| Male | 52 | 3.94 | 2.56 | 3.35 | 2.39 | |
| Overall | 118 | 4.50* | 2.51 | 3.94* | 2.64 | |
| Pain at 3 months | Female | 57 | 4.91 | 2.44 | 4.35 | 2.74 |
| Male | 43 | 3.28 | 2.75 | 2.56 | 2.67 | |
| Overall | 100 | 4.21* | 2.69 | 3.58* | 2.84 | |
* Indicates overall values that differ significantly from the baseline.

Figure 1Time course of back and leg pain. Adm: Admission; NRS: Numeric Rating Scale.
The PEG, ODI and EQ-5D scores provide insights into the patients’ quality of life (see Methods section). Unless otherwise noted, total scores were compared over time. An equal number of responses was available for each of the scores: 210 patients responded to the first, 122 to the second, 118 to the third and 100 to the fourth assessment. All values are presented in table 5, and the time course of the scores is visualised in figure 2.
Table 5PEG, ODI and EQ-5D scores, by sex. This table displays means and standard deviations for the PEG, ODI and EQ-5D score, both overall and by sex.
| PEG | ODI | EQ-5D | ||||||
| Sex | n | Mean | SD | Mean | SD | Mean | SD | |
| Admission | Female | 116 | 6.42 | 1.79 | 37.91 | 16.13 | 55.7 | 19.98 |
| Male | 94 | 6.25 | 1.9 | 32.9 | 14.89 | 60.47 | 18.71 | |
| Overall | 210 | 6.34 | 1.84 | 35.67 | 15.75 | 57.83 | 19.52 | |
| 7 days | Female | 65 | 4.81 | 2.13 | 31.54 | 16.19 | 56.82 | 20.16 |
| Male | 57 | 4.18 | 2.13 | 24.47 | 15.3 | 64.67 | 18.14 | |
| Overall | 122* | 4.52 | 2.15* | 28.24 | 16.11 | 60.48 | 19.56 | |
| 1 month | Female | 66 | 4.84 | 2.39 | 28.97 | 13.88 | 61.42 | 19.68 |
| Male | 52 | 4.1 | 2.44 | 25.94 | 14.85 | 62.46 | 19.1 | |
| Overall | 118* | 4.49 | 2.43* | 27.64 | 14.33 | 61.88 | 19.35 | |
| 3 months | Female | 57 | 4.62 | 2.43 | 29.47 | 15.01 | 57.16 | 20.49 |
| Male | 43 | 3 | 2.56 | 19.47 | 16.16 | 71.6 | 19.72 | |
| Overall | 100* | 3.93 | 2.6* | 25.17 | 16.22 | 63.37 | 21.31 | |
EQ-5D: European Quality of Life 5 Dimensions; ODI: Oswestry Disability Index; PEG: Pain, Enjoyment of Life and General Activity Scale.
* Highlights overall values that differ significantly from the baseline (at admission).
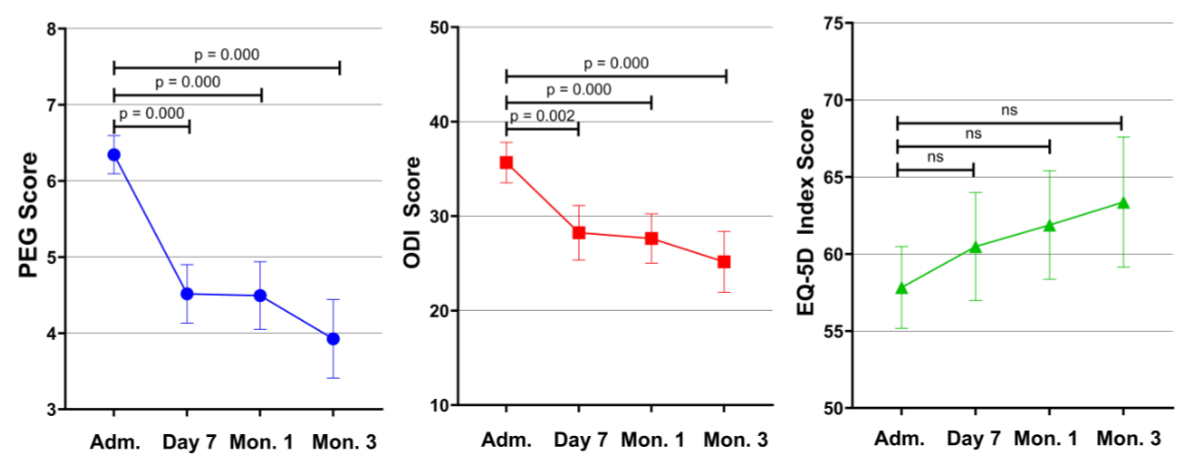
Figure 2Visual display illustrating the mean progression of the PEG Scale, ODI and EQ-5D across the evaluated assessments. Adm: Admission; EQ-5D: European Quality of Life 5 Dimensions; ODI: Oswestry Disability Index; PEG: Pain, Enjoyment of Life and General Activity Scale.
Mean PEG scores were 6.34 (SD 1.84) before the intervention, 4.52 (SD 2.15) after 7 days, 4.49 (SD 2.43) after 1 month and 3.93 (SD 2.60) after 3 months. The difference in PEG scores between admission and follow-up assessments was statistically significant (all p-values <0.001). However, there was no difference between the follow-up assessments. Similar to pain ratings, there was a statistically significant difference between men and women. At 3 months post-intervention, the average PEG score was 4.62 (SD 2.43) for women and 3.00 (SD 2.56) for men (p = 0.002).
The mean ODI score was 35.67 (SD 15.75) at admission, 28.24 (SD 16.11) after 7 days, 27.64 (SD 14.33) after 1 month and 25.17 (SD 16.22) after 3 months. The difference in ODI scores between admission and follow-up assessments was statistically significant (all p-values <0.002). However, the difference between the follow-up assessments was not significant. ODI scores between male and female patients differed significantly at admission (p = 0.02), 7 days post-intervention (p = 0.015) and 3 months post-intervention (p = 0.002). On average, women showed higher mean values at all measurement points than men (table 5). The time course of the individual ODI items is graphically presented in figure 3.
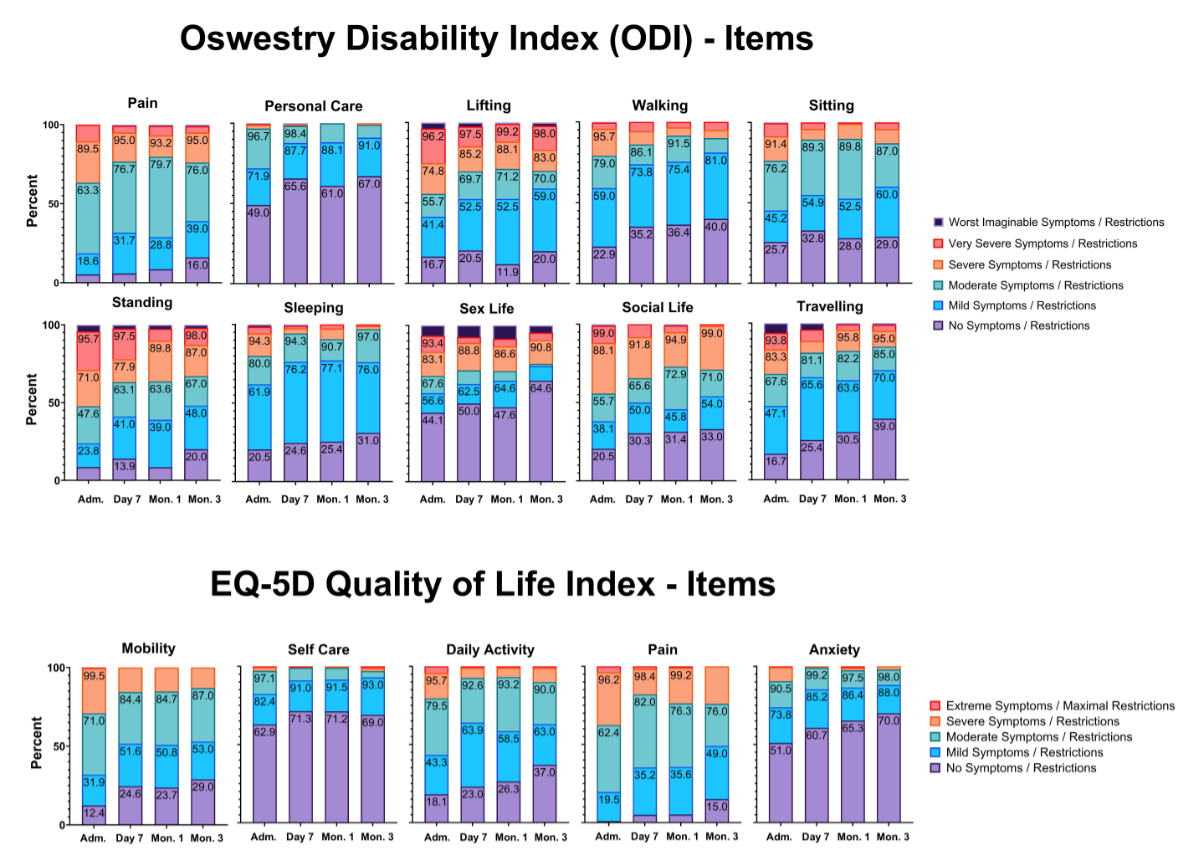
Figure 3Individual items of the Oswestry Disability Index (ODI) and European Quality of Life 5 Dimensions (EQ-5D).
The mean EQ-5D score was 57.83 (SD 19.52) before the intervention, 60.48 (SD 19.56) after 7 days, 61.88 (SD 19.35) after 1 month and 63.37 (SD 21.31) after 3 months. While the mean EQ-5D score increased overall, this was only statistically significant when comparing the 1-month and 3-month post-intervention assessments (p = 0.031). There was no statistically significant difference in EQ-5D scores between admission and follow-up assessments. Significant differences in sex were found at 7 days post-intervention (p = 0.03) and at 3 months post-intervention (p <0.001), with women having lower EQ-5D scores than men (table 5). The time course of the individual EQ-5D items is displayed in figure 3. Table 6 provides an overview of all outcome measures.
Table 6Mean differences. This table provides a summary of the mean values of various scores, including the back and leg pain, EQ-5D, ODI and PEG scales. Additionally, the table presents the corresponding p-values and confidence intervals for each score.
| Mean at admission (SD) | Mean at 3 months (SD) | Mean difference (95% CI) | p-Value | |
| Back pain | 6.07 (2.27) | 4.21 (2.69) | 1.86 (1.24–2.48) | <0.001 |
| Leg pain | 5.59 (2.72) | 3.58 (2.84) | 2.01 (1.34–2.68) | <0.001 |
| PEG | 6.34 (1.84) | 3.93 (2.6) | 2.41 (1.84–2.98) | <0.001 |
| ODI | 37.91 (16.13) | 25.17 (16.22) | 12.74 (8.86–16.62) | <0.001 |
| EQ-5D | 55.7 (19.98) | 63.37 (21.31) | −7.67 (−12.65–−2.69) | n.s. |
EQ-5D: European Quality of Life 5 Dimensions; n.s.: not significant; ODI: Oswestry Disability Index; PEG: Pain, Enjoyment of Life and General Activity Scale.
By analysing patient-reported outcome data regarding the effect of steroid infiltration with non-particulate steroids on back and leg pain due to central or neuroforaminal spinal stenosis, we observed a clinically and statistically significant decrease in pain intensity, improvement in quality of life and reduction in daily disability. A general pain reduction of over 30%, classified as moderate according to the IMMPACT recommendation, was observed, indicating a relevant pain reduction [28].
All three quality of life scores examined (PEG, ODI and EQ-5D) showed improved mean values after the intervention. Both the PEG and ODI scores demonstrated significant differences, with a change in classification from moderate to mild symptoms, indicating not only a statistical but also a clinically relevant reduction in disability due to pain.
Focusing on different categories (figure 3) also indicates that in the categories of pain, lifting, standing, sex life, social life and traveling, the proportions of patients reporting very severe and worst imaginable symptoms decreased. Conversely, the proportion of patients with mild to no symptoms increased.
Unexpectedly, no significant improvement was observed in the overall EQ-5D score. However, improvements were noted in the individual items “mobility”, “daily activity”, “pain” and “anxiety” (figure 3 and table 5). The item “self-care” was already quite high at admission, with more than half of the participants reporting no problems, making it difficult to show significant improvement in this area.
Although no significance was found for the change in overall EQ-5D score, the improved items align well with the ODI score, where corresponding items showed significant improvement (figure 3). The EQ-5D summary index can be compared with total population scores. For reference, the EQ-5D summary index reference value for the German general elderly population is 0.84 (SD 0.012, 95% CI: 0.814–0.864). No reference values have been published for Switzerland as of 9 June 2024. The observation of consistently lower EQ-5D summary index scores compared to the German index group indicates a lingering reduction in quality of life after treatment compared to the general population (table 5).
On average, women reported more pain and poorer outcomes across all assessments (tables 4 and 5). Men experienced an average pain reduction of approximately 43% for back pain and 48% for leg pain, while women recorded reductions of about 22% for back pain and 28% for leg pain. Beyond the known poorer outcomes for women compared to men in pain trials, we have no scientific explanation for the unfavourable outcomes in women in our trial [29].
All side effects observed during our study were transient and attributable to the infiltration itself rather than the off-label use of dexamethasone-21-palmitate. However, the small sample size does not allow us to conclusively state that the treatment is entirely safe and free of side effects. A larger patient population would be needed for a definitive conclusion.
The strengths of the study include the long observation period of three months, as well as the use of validated tools in the pain, quality of life and disability analyses combining pain outcomes with functional outcomes and patients’ subjective impressions, which generates a more precise picture of patients’ health status in response to treatment. In addition, a relatively large population was included to investigate the off-label use of dexamethasone-21-palmitate in the interventional treatment of pain due to spinal stenosis.
The lack of a control group in our study is a major limitation, and the observed changes in clinical outcome measures could be influenced by natural evolution rather than solely by the treatment. However, as 55% of patients had suffered from pain for more than one year, and the majority of these for more than two years, spontaneous reduction due to natural evolution is highly unlikely (table 2).
We cannot rule out a non-response bias, as the response rate to follow-up questionnaires decreased to 50% at the 3-month follow-up. However, our 50–60% response rate is in accordance with quality requirements in survey trials [30].
There could also be a recall bias, as study participants were asked to report on their past pain and quality of life, which could be distorted by their current experience. Since pain treatment focuses on improving subjective experience, this potential distortion is probably less relevant for assessing the study’s significance.
Various doses of epidural steroid infiltrations were used in our study (table 3), with the most prevalent doses being 12 mg for interlaminar and 4 mg for transforaminal injections. These doses were grouped together, and no individual evaluations were performed for the respective doses used.
In a 2011 study, Ahadian et al. reported that 4 mg dexamethasone was sufficient for transforaminal epidural steroid infiltration [31]. No significant improvements were observed when higher doses of 8 mg or 12 mg were used [31]. In agreement, a 2022 study by Park et al. comparing 4 mg and 8 mg doses dexamethasone for interlaminar epidural steroid infiltrations found no statistically significant differences in outcome measures (VAS and ODI) [32]. In addition, no differences were found in measured serum cortisol and glucose levels between enrolment and follow-up or between the two study groups (4 vs 8 mg). These findings suggest that increasing the dosage would not significantly change the outcome. The improved VAS and ODI scores and the absence of severe adverse outcomes observed in our study agree with results of both Ahadian et al. and Park et al. [31, 32]. However, in a 2023 publication, Shermon et al. describe the occurrence of flushing as an adverse event in about 5% of cases after lumbar transforaminal epidural steroid infiltrations with 4 mg dexamethasone [33]. Moreover, in 2019, Fischer et al. reported a case of induced psychosis after a single transforaminal epidural steroid injection with 10 mg dexamethasone and 0.5 ml lidocaine in a patient with a history of anxiety and adverse reactions to high-dose systemic corticosteroids [34]. Nonetheless, although none of our patients reported any adverse events, our sample size was too small to draw definitive conclusions about the side effect profile of dexamethasone-21-palmitate compared to other steroids used for epidural steroid infiltration.
The results suggest that epidural steroid infiltration with dexamethasone-21-palmitate is an effective and apparently safe treatment option for patients with chronic leg and back pain. Nonetheless, further studies are needed to assess safety and side effects, as well as to conduct head-to-head comparisons against other steroids or controlled trials to evaluate treatment efficacy. In addition, a study on the minimally effective dose of dexamethasone-21-palmitate could further optimise treatment.
Based on ODI, PEG and EQ-5D scores, patients with prolonged leg and/or back pain experienced significant reductions in pain and daily disability after epidural steroid infiltration with favourable changes observed for at least three months, suggesting potential benefits over this period. No significant side effects associated with the application were observed, suggesting that dexamethasone-21-palmitate may be a potential treatment alternative to traditional water-soluble steroids. However, further prospective controlled trials are necessary to confirm the efficacy and safety of this treatment.
The authors certify that this manuscript reports original clinical trial data. Deidentified individual-level data that underlie the results reported in this article (text, tables, figures and appendices) along with the study protocol will be available indefinitely to any researcher who wants access to them.
The authors would like to thank Allison Dwileski, MSc, Clinic For Anaesthesia, University Hospital Basel, for providing editorial assistance.
This work should be attributed to the Clinic for Anaesthesia, Intermediate Care, Prehospital Emergency Medicine and Pain Therapy, University Hospital Basel, Basel, Switzerland.
The study was funded solely by the Clinic for Anaesthesia, University Hospital Basel.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed. The financing party has not influenced the protocol, analysis or publication.
1. Bundesamt für Statistik (BFS). Gesundheitsstatistik 2019.34. Available from: https://www.bfs.admin.ch/asset/de/10227275
2. Patel EA, Perloff MD. Radicular Pain Syndromes: Cervical, Lumbar, and Spinal Stenosis. Semin Neurol. 2018;38(6):634-9. Epub 20181206. doi: .
3. Tarulli AW, Raynor EM. Lumbosacral radiculopathy. Neurol Clin. 2007 May;25(2):387–405.
4. Di Gangi S, Bagnoud C, Pichierri G, Rosemann T, Plate A. Characteristics and health care costs in patients with a diagnostic imaging for low back pain in Switzerland. Eur J Health Econ. 2022;23(5):823-35. Epub 20211030. doi:
5. Chou R, Loeser JD, Owens DK, Rosenquist RW, Atlas SJ, Baisden J, et al.; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009 May;34(10):1066–77.
6. Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988 Feb;318(5):291–300. doi: https://doi.org/10.1056/NEJM198802043180506
7. FDA Drug Safety Communication. FDA Drug Safety Communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain 2014 [1.05.2022]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-label-changes-warn-rare-serious-neurologic-problems-after
8. Dietrich TJ, Sutter R, Froehlich JM, Pfirrmann CW. Particulate versus non-particulate steroids for lumbar transforaminal or interlaminar epidural steroid injections: an update. Skeletal Radiol. 2015 Feb;44(2):149–55.
9. Abraham G, Demiraj F, Ungemach FR. Comparison of the hypothalamic-pituitary-adrenal axis susceptibility upon single-dose i.m. depot versus long-acting i.v. triamcinolone acetonide therapy: a direct pharmacokinetic correlation. J Endocrinol. 2006 Nov;191(2):491–6.
10. Cohen SP, Ross JD. Lumbar transforaminal epidural steroid injections with particulate vs. nonparticulate steroid: an evidence-informed review on shifting gear to a personalized medicine paradigm. Curr Opin Anaesthesiol. 2024;37(5):565-74. Epub 20240611. doi: doi: https://doi.org/10.1097/ACO.0000000000001402
11. House LM, Barrette K, Mattie R, McCormick ZL. Cervical Epidural Steroid Injection: techniques and Evidence. Phys Med Rehabil Clin N Am. 2018 Feb;29(1):1–17.
12. Van Boxem K, Rijsdijk M, Hans G, de Jong J, Kallewaard JW, Vissers K, et al. Safe Use of Epidural Corticosteroid Injections: Recommendations of the WIP Benelux Work Group. Pain Pract. 2019;19(1):61-92. Epub 20180702. doi:
13. Swissmedic. Swissmedicinfo: Official medicinal product information. Available from: www.swissmedicinfo.ch
14. Hodgson PS, Neal JM, Pollock JE, Liu SS. The neurotoxicity of drugs given intrathecally (spinal). Anesth Analg. 1999 Apr;88(4):797–809.
15. Wakiguchi H, Ohga S. [Clinical utility of the liposteroid therapy: potential effects on the macrophage activation]. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39(3):190–6.
16. Saha BK, Milman NT. Short Review of Liposteroid: A Novel Targeted Glucocorticoid Preparation for Treatment of Autoimmune and Inflammatory Diseases. Prague Med Rep. 2021;122(4):257–68.
17. Weldring T, Smith SM. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv Insights. 2013;6:61-8. Epub 20130804. doi: doi: https://doi.org/10.4137/HSI.S11093
18. Australian Commission on Safety and Quality in Health Care. About PROMs 2023. Available from: https://www.safetyandquality.gov.au/our-work/indicators-measurement-and-reporting/patient-reported-outcomes/about-proms
19. Australian Commission on Safety and Quality in Health Care. Patient-reported outcome measures 2022. Available from: https://www.safetyandquality.gov.au/our-work/indicators-measurement-and-reporting/patient-reported-outcome-measures
20. Hostettler S, Kraft E, Bosshard C, Patient-reported outcome measures: Die Patientensicht zählt. Schweizerische Ärztezeitung. 2018 Oct 3;99(40):1348. doi: https://doi.org/
21. International Spine Intervention Society (Bogduk N, editor). ISIS Practice Guidelines for Spinal Diagnostic and Treatment Procedures. 2nd ed. Hinsdale, Chicago: International Spine Intervention Society; 2014.
22. Heartbeat Medical. Outcome Measurement for Clinicians and Hospitals Heartbeat Medical. Available from: https://heartbeat-med.com/solutions/clinicians-hospitals/
23. Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009 Jun;24(6):733–8.
24. Heartbeat Medical. EQ-5D-5L Fragebogen 2023. Available from: https://heartbeat-med.com/de/resources/eq-5d-5l/
25. EuroQol Research Foundation. EQ-5D-5L User Guide 2019. Available from: https://euroqol.org/publications/user-guides
26. Mannion AF, Junge A, Fairbank JC, Dvorak J, Grob D. Development of a German version of the Oswestry Disability Index. Part 1: cross-cultural adaptation, reliability, and validity. Eur Spine J. 2006;15(1):55-65. Epub 20050426. doi:
27. Heartbeat Medical. Oswestry Disability Index (ODI). Available from: https://heartbeat-med.com/resources/oswestry-disability-index-odi/#anchor10
28. Smith SM, Dworkin RH, Turk DC, McDermott MP, Eccleston C, Farrar JT, et al. Interpretation of chronic pain clinical trial outcomes: IMMPACT recommended considerations. Pain. 2020 Nov;161(11):2446–61.
29. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013 Jul;111(1):52–8.
30. Meyer VM, Benjamens S, Moumni ME, Lange JF, Pol RA. Global Overview of Response Rates in Patient and Health Care Professional Surveys in Surgery: A Systematic Review. Ann Surg. 2022 Jan;275(1):e75–81. doi: https://doi.org/10.1097/SLA.0000000000004078
31. Ahadian FM, McGreevy K, Schulteis G. Lumbar transforaminal epidural dexamethasone: a prospective, randomized, double-blind, dose-response trial. Reg Anesth Pain Med. 2011;36(6):572–8.
32. Park CH, Jang YH, Lee SH. Comparison of Pain Reduction and Changes in Serum Cortisol and Glucose Levels to Different Doses of Lumbar Epidural Dexamethasone: A Prospective Study. Pain Physician. 2022 Oct;25(7):E1081–5.
33. Shermon S, Van Acker G, Suric V, Kim C, Abd-Elsayed A, Mata N. Flushing After Lumbar Epidural Steroid Injection with Dexamethasone. Curr Pain Headache Rep. 2023 Jun;27(6):143–8.
34. Fischer M, Kim PY. Corticosteroid-Induced Psychosis After a Single Transforaminal Epidural Steroid Injection. WMJ. 2019 Jul;118(2):91–4.