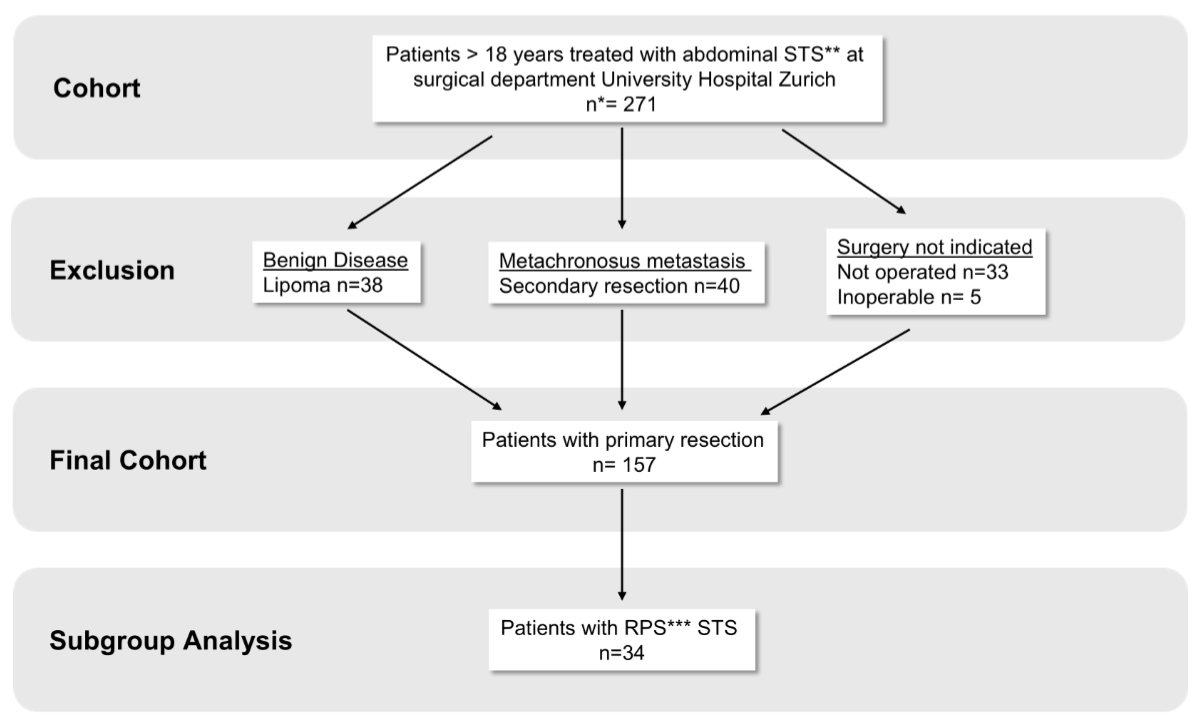
Figure 1STROBE flowchart. * n: number; ** STS: soft tissue sarcoma; *** RPS: retroperitoneal sarcoma.
DOI: https://doi.org/https://doi.org/10.57187/s.4091
Soft tissue sarcomas represent fewer than 1% of tumours in adulthood [1]. They are rare and frequently malignant, with more than 70 distinct histological subtypes occurring at any anatomical site [2]. In Switzerland, the annual incidence of sarcomas in general is 5.34 per 100,000 [3], similar to the rest of Europe, and the majority are soft tissue sarcomas (84%) [4]. Abdominal and retroperitoneal sarcomas represent only a minority of soft tissue sarcomas, comprising fewer than 20% in large surgical series [5]. Several multi-institutional reports suggest that improved patient outcomes (i.e. fewer reoperations, more tumour-free resection margins, significantly longer overall survival) are achieved in specialised centres [4, 6–8]. In Switzerland, limited data is available from two reports of patients with abdominal and retroperitoneal sarcomas. One is a registry-based study providing valuable information on sarcoma incidence and mortality based on the Swiss NICER database [3, 9]. The other report is derived from data from ICD codes from the Swiss Federal Statistical Office, and describes the trend towards higher rates of centralised, surgical treatment in Switzerland [9]. Despite the existence of multiple sarcoma centres in Switzerland, no patient-level data on surgical quality or outcomes has been published in this group of patients. The aim of the present study was to provide patient-level data for patients with abdominal and retroperitoneal soft tissue sarcoma in a Swiss tertiary centre. Overall survival was our primary endpoint. Secondary endpoints included disease-free survival, incidence of histological subtypes, completeness of surgical resection and tumour rupture.
The present study is a retrospective cohort study conducted at a single centre. Patients were treated according to standard care protocols at the clinic. The focus was on examining the number and characteristics of patients and their clinical outcomes.
The study included patients with abdominal soft tissue sarcoma treated between January 2012 and December 2022 at the Department of Abdominal Surgery, University Hospital Zurich, a Swiss tertiary certified referral centre for sarcoma treatment. This setting provided access to specialised care and a comprehensive database for retrospective data collection. Patients were managed by a specialised multidisciplinary team and presented at the Comprehensive Cancer Centre Zurich and the Balgrist Sarcoma Board before and after treatment. Pre-interventional core needle biopsies were indicated and performed whenever technically possible. The retroperitoneal surgery performed was compartmental resection by specialised sarcoma surgeons [6]. Data was retrospectively extracted from electronic medical records from April 2023 to September 2023. All analyses were performed from September 2023 to February 2024 using SPSS (IBM SPSS Statistics for Windows, Version 29.0. NY: IBM Corp). For the purpose of improving language clarity, ChatGPT (OpenAI, version May 2023) was used to assist with English editing of the manuscript. The scientific content and interpretation remain entirely the work of the authors. The study was approved by the cantonal ethics committee (BASEC 2023-00895).
We included patients aged >18 years with a diagnosis of abdominal and retroperitoneal soft tissue sarcoma who underwent surgical treatment during the study period. Exclusion criteria were: patients undergoing secondary surgery for local recurrence or metastasis; patients with benign lesions (e.g. lipomas); patients considered inoperable; and patients who refused surgery (figure 1).

Figure 1STROBE flowchart. * n: number; ** STS: soft tissue sarcoma; *** RPS: retroperitoneal sarcoma.
Histological subtypes were grouped as follows: gastrointestinal stroma tumour (GIST), liposarcoma (LPS), leiomyosarcoma (LMS), desmoid tumour (DT) and “Others” [10]. “Others” covers rarely occurring subtypes. Liposarcomas were divided into well-differentiated liposarcoma (WDLPS) and dedifferentiated liposarcoma (DDLPS). The FNCLCC (Fédération Nationale des Centres de Lutte Contre le Cancer) grading system was applied to liposarcomas [11]. Prognostic groups developed by Miettinen et al. were applied to GIST [12]. Demographic characteristics included patient age as a continuous variable, sex, height in metres and weight in kilograms. Clinical characteristics included the length of hospital and ICU stays in days, tumour location, tumour size in cm and multifocality, an important prognostic parameter in retroperitoneal soft tissue sarcoma [13]. Metastasis and performing a biopsy prior to treatment were also evaluated. Another clinical characteristic is tumour rupture, defined as any spontaneous or iatrogenic gross or microscopic breach of the tumour capsule into the peritoneal cavity or adjacent tissues. They occur particularly frequently in soft tissue sarcomas due to the large size of the tumour and are therefore an important prognostic factor [14]. The number of organs resected was recorded and is shown in tables 1 and 2. Surgical resection margins were classified as complete resection (R0), microscopic residual tumour (R1), macroscopic residual tumour (R2) or resection status unclear (RX). Postoperative complications were graded according to the Clavien-Dindo classification [15]. All grades (1–5) that occurred within 30 days after surgery, before or after discharge, were included. All deaths at 30 days after the definitive resection were recorded, regardless of whether they were related or not to complications of surgery.
Table 1Type and number of organs resected.
| n = 157 | %* | |
| Stomach | 56 | 35.7% |
| Small intestine | 30 | 19.1% |
| Kidney | 26 | 16.6% |
| Ureter | 24 | 15.3% |
| Adrenal gland | 22 | 14.0% |
| Left colon and rectum | 22 | 14.0% |
| Other | 19 | 12.1% |
| Gallbladder | 15 | 9.6% |
| Right colon | 13 | 8.3% |
| Appendix | 13 | 8.3% |
| Liver | 13 | 8.3% |
| Spleen | 11 | 7.0% |
| Pancreas | 11 | 7.0% |
| Cava | 9 | 5.7% |
| Duodenum | 8 | 5.1% |
| Abdominal wall | 7 | 4.5% |
| Adnexa/ovaries | 6 | 3.8% |
| Iliac vessels | 5 | 3.2% |
| Transverse colon | 4 | 2.5% |
| Diaphragm | 4 | 2.5% |
| Bladder | 4 | 2.5% |
| Testis / spermatic cord | 3 | 1.9% |
| Oesophagus | 2 | 1.3% |
* In several cases, >1 organ was resected such that the sum of cases is not 100%.
Table 2Type and number of organs resected in retroperitoneal sarcoma.
| n = 34 | %* | |
| Kidney | 21 | 61.8% |
| Ureter | 20 | 58.8% |
| Adrenal gland | 18 | 52.9% |
| Left colon and rectum | 12 | 35.3% |
| Right colon | 9 | 26.5% |
| Other | 8 | 23.5% |
| Appendix | 5 | 14.7% |
| Gallbladder | 5 | 14.7% |
| Spleen | 4 | 11.8% |
| Small intestine | 4 | 11.8% |
| Adnexa/ovaries | 4 | 11.8% |
| Iliac vessels | 3 | 8.8% |
| Diaphragm | 3 | 8.8% |
| Pancreas | 2 | 5.9% |
| Liver | 2 | 5.9% |
| Cava | 2 | 5.9% |
| Stomach | 1 | 2.9% |
| Duodenum | 1 | 2.9% |
| Bladder | 1 | 2.9% |
| Testis / spermatic cord | 1 | 2.9% |
* In several cases, >1 organ was resected such that the sum of cases is not 100%.
The primary outcome was overall survival, defined as the time from surgery to death from any cause; time was censored at the date of last follow-up for patients remaining alive. The secondary outcomes included disease-free survival, defined as the time from surgery to any kind of recurrence, either local recurrence or distant metastasis; time was censored at the date of last follow-up for patients without a recurrence event. Further secondary outcomes were incidence of histological subtypes, completeness of surgical resection and tumour rupture.
Data was extracted from the electronic medical records using KISIM, the internal software for patient data management. The data extraction was performed manually and transferred into SPSS Statistics for further analysis. Data was manually reviewed to ensure completeness and consistency. Each data point was checked for errors such as duplicate entries, invalid values or logical inconsistencies. Missing data was clearly marked in SPSS, and no imputation was performed as missing data was below 2.5% for all variables. Similarly no cases were excluded based on missing data. There was no cross-checking of data with external databases, as the study relied only on the KISIM dataset. Data was subsequently analysed using SPSS Statistics, applying appropriate statistical methods to assess the study outcomes. No formal study protocol was created prior to the data extraction. The study followed standard procedures for retrospective data analysis.
A total of 271 patients were included in the study. This number represents all eligible patients treated for abdominal and retroperitoneal soft tissue sarcoma at our centre during the study period. After applying the above-mentioned exclusion criteria, 157 patients remained for further analysis.
Adjuvant or/neoadjuvant treatment were indicated on a case-by-case basis following multidisciplinary discussion at the Comprehensive Cancer Centre Zurich and Balgrist Sarcoma Board. Patients underwent a regular follow-up between 2 and 6 weeks postoperatively at the surgical department by clinical examination. Further follow-up was orientated on the clinical guidelines of the Swiss Society of Medical Oncology (SSMO), genetic variety within the subtype and the Sarcoma Board’s recommendation. For the main subtypes analysed, the follow-up was carried out with CT or MRI every 3 months in the first two years, then every 6 months thereafter.
Continuous variables were described using mean, standard deviation and interquartile range (IQR) and categorical variables using counts and percentages. Overall and disease-free survival curves were estimated using the Kaplan-Meier method and statistically compared with the log-rank test. We considered a statistical test as significant when the corresponding p-value was below 0.05. Median follow-up was calculated using the reverse Kaplan-Meier method. Competing events were not taken into account in the statistical analysis, resulting in a conservative estimate of overall survival.
Overall, 271 patients were included in this study. Patients with recurrence, metachronous metastasis, irresectable tumours or benign disease were excluded. Finally, 157 patients with primary, resectable soft tissue sarcoma or sarcoma were included in the analysis. Median follow-up after surgery was 52.6 months (95% confidence interval [CI]: 42.24–62.95). Patient and tumour characteristics are summarised in table 3. The majority of patients (n = 82) presented with gastrointestinal stroma tumour; retroperitoneal sarcoma was diagnosed in 34 including well-differentiated liposarcoma, dedifferentiated liposarcoma and leiomyosarcoma. The median number of organs resected was one, while R0 and R1 resection rates were 80.3% and 11.5%, respectively. Postoperative complications were observed in 60 patients (38.2%), graded 3b in 5 (3.2%), 4a in 1 (0.6%) and 4b in 1 (0.6%). Reoperation was necessary in 7 (4.46%) patients. Three patients (1.91%) died after surgical intervention. Detailed surgical characteristics are shown in table 1.
Table 3Patient characteristics.
| n = 157 | Missing data (%) | ||
| Sex, n (%) | Female | 60 (38.2) | |
| Male | 97 (61.8) | ||
| Age in years, median (IQR) | 60 (48–70) | ||
| Tumour size in cm, median (IQR) | 8.50 (3.75–18.75) | 2.5 | |
| Tumour types, n (%) | Gastrointestinal stroma tumour | 82 (52.2) | |
| Liposarcoma | 36 (22.9) | ||
| Leiomyosarcoma | 13 (8.3) | ||
| Desmoid tumour | 8 (5.1) | ||
| Others | 18 (11.5) | ||
| Completeness of surgical resection | 1.3 | ||
| Completeness of surgical resection, n (%) | R0 | 126 (80.3) | |
| R1 | 18 (11.5) | ||
| R2 | 8 (5.1) | ||
| RX | 3 (1.9) | ||
| Number of resected organs, median (IQR) | 1 (1–3) | ||
| Number of resected organs, n (%) | 0 | 7 (4.5) | |
| 1 | 74 (47.1) | ||
| >1 | 76 (48.4) | ||
| Tumour rupture, n (%) | No | 151 (96.2) | |
| Yes | 6 (3.8) | ||
| Multifocality, n (%) | No | 144 (91.7) | |
| Yes | 13 (8.3) | ||
| Preoperative chemotherapy, n (%) | Done | 19 (12.1) | |
| Not done | 138 (87.9) | ||
| Postoperative chemotherapy, n (%) | Done | 45 (28.7) | |
| Not done | 112 (71.3) | ||
| Preoperative radiotherapy, n (%) | Done | 8 (5.1) | |
| Not done | 149 (94.9) | ||
| Postoperative radiotherapy, n (%) | Done | 9 (5.7) | |
| Not done | 148 (94.3) | ||
IQR: interquartile range.
Overall survival at 5, 8 and 10 years was 67.3%, 49.1% and 37.5%, respectively. Median overall survival was 87.9 months (95% CI: 54.95–120.74). Median overall survival according to subgroups was 117.5 months for gastrointestinal stroma tumours (95% CI: 76.96–158.00), 61.8 months for liposarcomas (95% CI: 40.16–83.36), 117.8 months for leiomyosarcomas (95% CI not achieved) and 100.2 months for Other (rare) subtypes (95% CI: 59.65–111.30). Median survival was not reached in the group of desmoid tumours. Forty-four (28%) patients developed tumour recurrence, which was localised in 18 (40.9%) and a distant metastasis in 26 (59.0%) patients. Disease-free survival at 3, 5 and 10 years was 71.3%, 61.5% and 52.7%, respectively. Median disease-free survival was not reached. Disease-free survival in subgroups was reached for liposarcomas with 33.1 months (95% CI: 21.78–44.45) and for leiomyosarcomas with 16.6 months (95% CI: 7.03–26.08). Median disease-free survival was not reached in the group of gastrointestinal stroma tumours, desmoid tumours and Other (rare) subtypes. Specific outcomes for well-differentiated liposarcomas, dedifferentiated liposarcomas and leiomyosarcomas are further shown in figure 2.
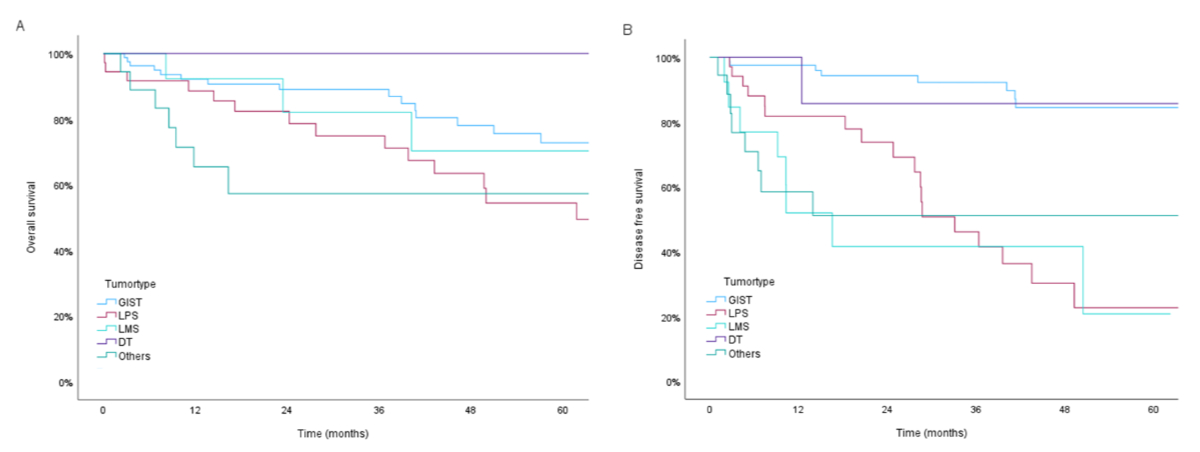
Figure 2Kaplan-Meier plots of (A) Overall survival and (B) Disease-free survival of 157 patients with abdominal soft tissue tumours according to tumour type. Five-year overall survival was 72.8% for gastrointestinal stroma tumour (GIST), 54.4% for liposarcoma (LPS), 70.3% for leiomyosarcoma (LMS), 100% for desmoid tumours (DT) and 57.3% for Other (rare) subtypes.
We analysed the subgroup of patients with retroperitoneal sarcoma (n = 34). Median follow-up after surgery was 60.6 months (95% CI: 37.94–83.28). Most patients in this group presented with liposarcoma (n = 27 or 79.4%), which was often dedifferentiated (77.8%). A median number of 4 organs was resected during compartmental surgery, enabling a combined R0/R1 rate of 90% (R0: 58.8%; R1: 32.4%) (tables 2 and 4). Postoperative complications were observed in 24 (70.6%) patients, and graded 3b in 2 (5.9%). One patient (2.9%) died, and two (5.9%) were reoperated. Overall survival at 5, 8 and 10 years was 53.1%, 23.9% and 23.9%, respectively. Median overall survival was 66.1 months (95% CI: 39.27–92.99). Regarding subgroups, median overall survival was 49.9 months (95% CI: 0.00–122.13) for well-differentiated liposarcomas, 66.1 months (95% CI: 24.71–107.55) for dedifferentiated liposarcomas and 9.5 months (95% CI: 0.00–22.02) for Other subtypes. Median overall survival was not reached for leiomyosarcomas. Nineteen (55.9%) patients developed recurrence, which was local in 11 (57.9%) and distant in 8 (42.1%) patients. Median disease-free survival was 28.7 months (95% CI: 22.54–34.88). Median disease-free survival of the subgroups was 33.1 months for well-differentiated liposarcomas (95% CI not achieved), 28.5 months for dedifferentiated liposarcomas (95% CI: 22.24–34.72), 10.3 months for leiomyosarcomas (95% CI: 8.50–12.07) and 6.5 months for Other subtypes (95% CI not achieved). Specific outcomes for well-differentiated liposarcomas, dedifferentiated liposarcomas, leiomyosarcomas and Other subtypes are shown in figure 3.
Table 4Patient characteristics of retroperitoneal sarcoma. No missing data.
| n = 34 | ||
| Sex, n (%) | Female | 15 (44.1) |
| Male | 19 (55.9) | |
| Patient age in years, median (IQR) | 62 (49–73) | |
| Tumour size in cm median (IQR) | 23.5 (15.87–32.65) | |
| Tumour types, n (%) | Liposarcoma | 27 (79.4) |
| Leiomyosarcoma | 3 (8.8) | |
| Other | 4 (11.8) | |
| Liposarcoma histological subtype, n (%) | Well-differentiated liposarcoma | 5 (18.5) |
| Dedifferentiated liposarcoma | 21 (77.8) | |
| Myxoid liposarcoma | 1 (3.7) | |
| Completeness of surgical resection, n (%) | R0 | 20 (58.8) |
| R1 | 11 (32.4) | |
| R2 | 3 (8.8) | |
| Number of resected organs, median (IQR) | 4 (2–5) | |
| Number of resected organs, n (%) | 0 | 1 (2.9) |
| 1 | 5 (14.7) | |
| >1 | 28 (82.4) | |
| Tumour rupture, n (%) | No | 33 (97.1) |
| Yes | 1 (2.9) | |
| Multifocality, n (%) | No | 29 (85.3) |
| Yes | 5 (14.7) | |
| Preoperative chemotherapy, n (%) | Done | 4 (11.8) |
| Not done | 30 (88.2) | |
| Postoperative chemotherapy, n (%) | Done | 5 (14.7) |
| Not done | 29 (85.3) | |
| Preoperative radiotherapy, n (%) | Done | 2 (5.9) |
| Not done | 32 (94.1) | |
| Postoperative radiotherapy, n (%) | Done | 4 (11.8) |
| Not done | 30 (88.2) | |
IQR: interquartile range.
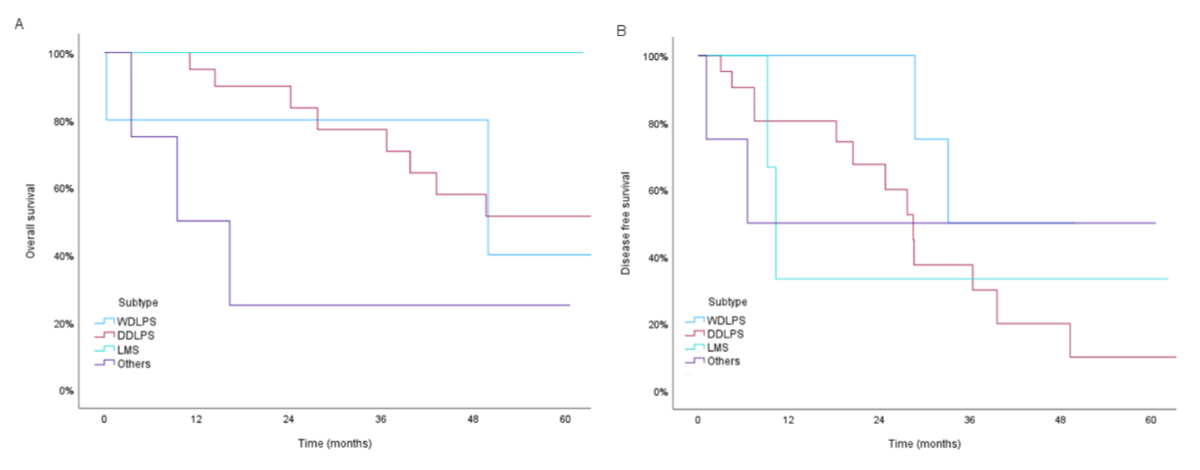
Figure 3Kaplan-Meier plots of (A) Overall survival and (B) Disease-free survival of 34 patients with retroperitoneal soft tissue tumours according to tumour types. WDLPS: well-differentiated liposarcoma; DDLPS: dedifferentiated liposarcoma; LMS: leiomyosarcoma; Other: rare retroperitoneal subtypes.
This study provides patient-level evidence from a specialised single centre in Switzerland regarding surgical quality and postoperative outcomes in addition to long-term survival in patients with abdominal and retroperitoneal soft tissue sarcoma. Despite a low case load in comparison to large European sarcoma centres, the overall quality parameters are comparable, indicating that multidisciplinary teams in Swiss sarcoma centres can provide adequate patient care. Sarcoma in the abdomen and retroperitoneum is a rare diagnosis. While the overall incidence in Switzerland is comparable to that of other countries, absolute numbers remain low, e.g. in comparison with some high-volume centres in France [9, 16]. Among the included 271 patients, treated between January 2012 and December 2022, a significant number of them were only referred for recurrent or highly advanced disease. We therefore analysed only the remaining 157 patients with primary abdominal and retroperitoneal sarcoma undergoing surgery. On average, we performed surgery for 15 primary sarcomas per year. The median age of 60 years is comparable to the results of the SEER database, a source of cancer statistics in the US, in addition to other studies [7, 17]. The observed male predominance is most likely due to the inclusion of gastrointestinal stroma tumours which are known for this gender disparity [18]. Overall, the median length of hospital stay was 11 days which compares well with previous Swiss data [9].
Looking at the occurrence of different histological subtypes in our cohort, gastrointestinal stroma tumours are the most prevalent type of sarcoma in the abdomen, also reflected by our study with n = 82 (52.2%) [19, 20]. Patient outcomes depend on prognostic grading and molecular profiles, which define susceptibility to targeted therapy [18]. There are two Swiss retrospective studies available which assessed surgical outcomes and prognostic factors [21, 22]. The observed 5-year overall survival of 72.8% for gastrointestinal stroma tumour is around 10% higher compared to the published literature [23]. Desmoid tumours, on the other hand, are rare tumours. They are associated with high recurrence rates despite a benign character on histology, due to their locally aggressive biology. Active surveillance, medical treatment or radiation therapy have increasingly replaced surgical resection as primary treatment over the last decade [24].
Our main focus was on patients with retroperitoneal sarcoma. The relatively low total number of 34 patients in 10 years compares to the situation in France, where a typical NetSarc (French clinical reference network for soft tissue and visceral sarcomas including 28 centres) centre treats a median of 23 patients in seven years [7]. Obviously, these numbers are not comparable to the exceptional high-volume centres known from European multicentre studies [4, 6, 7, 17, 25]. As in our cohort, liposarcoma was the predominant subtype in a French multicentre study [7] and dedifferentiated liposarcoma was the predominant histological subtype, also observed by other large cohorts [4, 6]. A critical parameter for surgical quality is the completeness of resection which is surprisingly defined as R0/R1 – and not R0 alone – in the international literature mentioned above. This is likely due to the high proportion of R1 resections, even in the context of a truly compartmental resection (figure 4) [6]. Usually, resection is considered complete if pathology shows R0/R1 in the context of a macroscopically complete and truly compartmental resection, and no signs of dedifferentiated liposarcoma tumour cells in the surgical margins, which was also used as a definition in this study. Our data in retroperitoneal sarcoma (R0: 58.8%, R1: 32.4%) compares well to large international series from Paris, France (R0: 41.9%, R1: 33.9%) [7] or Milan, Italy (R0/R1: 93.8%) [25]. Intraoperative tumour rupture is another surgical quality parameter observed in 2.9% in our series, which compares well to other reports [26]. The median number of four resected organs in patients with retroperitoneal sarcoma indicates the compartmental type of surgery. A significant number of patients in the present series was included after publication of the STRASS study, which concluded that preoperative radiation therapy should not be considered standard of care treatment for patients with retroperitoneal sarcoma [27]. Compared to older cohorts, our group of patients with preoperative RT is therefore significantly lower [4].
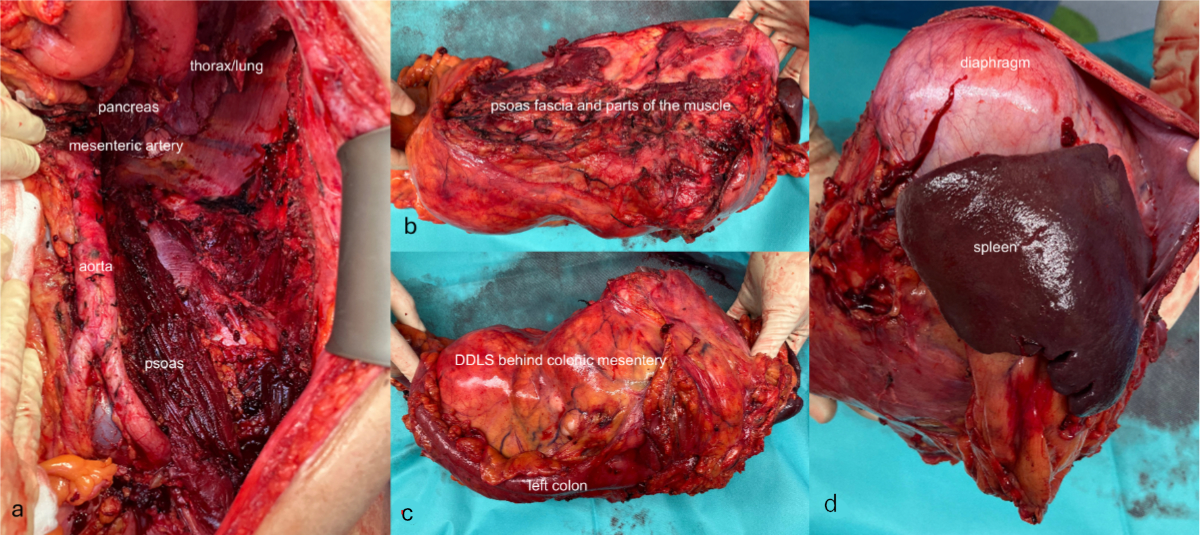
Figure 4Compartmental retroperitoneal resection (specimen and postoperative situs): left retroperitoneum after compartmental resection of a retroperitoneal dedifferentiated liposarcoma (DDLS) (A), including the adrenal gland, kidney and pancreatic tail, the psoas fascia (B), the left colon (C), and the spleen and diaphragm (D).
The critical outcome in sarcoma patients is cancer-related survival. In our series of retroperitoneal sarcoma, 5-year overall survival was 53.1%. The two large cancer centres in Paris, France and Milan, Italy published higher rates, e.g. a 5-year overall survival of 67% [4, 7]. Clearly, our small sample size and the high proportion of dedifferentiated liposarcoma limit the interpretation of this data as does the short follow-up period, and the difference might not be statistically significant. However, we believe that this finding calls for caution and further long-term follow-up.
We would like to acknowledge the limitations of this analysis. The first are limitations inherent to the retrospective design and the single-centre setting. In accordance with the disease rarity, we looked at a limited number of patients overall and this precluded a thorough analysis of specific subgroups – so far only possible in large, multi-institutional, international datasets. The relatively small number of patients does not allow for further analysis of confounding factors, e.g. multivariable Cox regression.
Due to the data collected from a specialised sarcoma centre, there were many cases with advanced disease and complicated course in the cohort, which are not representative of the overall population. To minimise this selection bias, we excluded patients with secondary treatment and inoperable patients. A time-related bias is likely due to the long study period of 10 years; however, treatment strategies of most subgroups has not changed significantly. Due to the retrospective nature of the study, an information bias due to incomplete data documentation with missing data is possible. To manage this, missing data was recorded.
An important confounding factor that was not taken into account in the classic Kaplan-Meier analysis is the presence of concomitant diseases and other factors, such as age, which influence overall survival and outcome. Due to the small sample size, multivariable Cox regression was not performed.
The effect modification is large in the cohort as it includes several subgroups that have different treatment regimens. At this point, a further subgroup analysis of the subtypes would be expedient, as we did with retroperitoneal sarcoma. However, a restriction then occurs due to the small sample size.
The transferability of this study to other clinics is difficult to assess due to the single-centre study design. Our results are, however, comparable to those of international sarcoma centres.
The clear strength of this study was to overcome the lack of surgical outcomes and overall survival of Swiss sarcoma patients. Despite the mentioned limitations, the results demonstrated comparability with international centres. However, there is a certain need to continuously collect and analyse this data on a national basis to ensure treatment quality and subsequently outcomes of Swiss sarcoma centres [4, 28].
To our knowledge, this single-centre retrospective analysis represents the first publication of data on surgical quality and survival of abdominal and retroperitoneal sarcoma patients in Switzerland. Observed surgical quality parameters compare well to large international centres. However, sarcoma-related survival deserves further follow-up and requires the establishment of a national database for this rare and complex disease. This would assist in improving patient management in Switzerland through benchmarking of surgical and other treatment outcomes.
Due to the nature of the data being patient-related and subject to data protection and privacy regulations, the datasets generated and analysed during the current study are not publicly available. Reasonable requests for deidentified data may be considered by the corresponding author in accordance with institutional and ethical guidelines.
This study received no funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006 Dec;119(12):2922–30.
2. Gounder MM, Agaram NP, Trabucco SE, Robinson V, Ferraro RA, Millis SZ, et al. Clinical genomic profiling in the management of patients with soft tissue and bone sarcoma. Nat Commun. 2022 Jun;13(1):3406.
3. Kollár A, Rothermundt C, Klenke F, Bode B, Baumhoer D, Arndt V, et al.; NICER Working Group. Incidence, mortality, and survival trends of soft tissue and bone sarcoma in Switzerland between 1996 and 2015. Cancer Epidemiol. 2019 Dec;63:101596.
4. Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, et al.; ESMO Guidelines Committee, EURACAN and GENTURIS. Electronic address: clinicalguidelines@esmo.org. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2021 Nov;32(11):1348–65.
5. Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014 Sep;260(3):416–21.
6. Swallow CJ, Strauss DC, Bonvalot S, Rutkowski P, Desai A, Gladdy RA, et al.; Transatlantic Australasian RPS Working Group (TARPSWG). Management of Primary Retroperitoneal Sarcoma (RPS) in the Adult: An Updated Consensus Approach from the Transatlantic Australasian RPS Working Group. Ann Surg Oncol. 2021 Nov;28(12):7873–88.
7. Bonvalot S, Gaignard E, Stoeckle E, Meeus P, Decanter G, Carrere S, et al. Survival Benefit of the Surgical Management of Retroperitoneal Sarcoma in a Reference Center: A Nationwide Study of the French Sarcoma Group from the NetSarc Database. Ann Surg Oncol. 2019 Jul;26(7):2286–93.
8. Berger NG, Silva JP, Mogal H, Clarke CN, Bedi M, Charlson J, et al. Overall survival after resection of retroperitoneal sarcoma at academic cancer centers versus community cancer centers: An analysis of the National Cancer Data Base. Surgery. 2018 Feb;163(2):318–23.
9. Willburger JC, von Strauss M, Peterson CJ, Glass TR, Kettelhack C. Incidence, Treatment and Outcome of Patients with Retroperitoneal Soft-Tissue Sarcoma in Switzerland 2005-2015: A Population-Based Analysis. World J Surg. 2022 Feb;46(2):461–8.
10.Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica. 2021 Apr;113(2):70–84.
11. Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. 2006 Oct;130(10):1448–53.
12. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006 May;23(2):70–83.
13. Anaya DA, Lahat G, Liu J, Xing Y, Cormier JN, Pisters PW, et al. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decision-making. Ann Surg. 2009 Jan;249(1):137–42.
14. Chen Y, Hao J, Yang Y, Yang J, Hao X. Tumor rupture predicts early metastasis and poor prognosis in stage III soft tissue sarcomas. World J Surg. 2011 May;35(5):1002–9.
15. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004 Aug;240(2):205–13.
16. Honoré C, Méeus P, Stoeckle E, Bonvalot S. Soft tissue sarcoma in France in 2015: Epidemiology, classification and organization of clinical care. J Visc Surg. 2015 Sep;152(4):223–30.
17. Dashti NK, Cates JM. Risk Assessment of Visceral Sarcomas: A Comparative Study of 2698 Cases from the SEER Database. Ann Surg Oncol. 2021 Oct;28(11):6852–60.
18. Group EE; ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014 Sep;25 Suppl 3:iii21–6.
19. Ducimetière F, Lurkin A, Ranchère-Vince D, Decouvelaere AV, Péoc’h M, Istier L, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6(8):e20294.
20. Dirnhofer S, Leyvraz S. Current standards and progress in understanding and treatment of GIST. Swiss Med Wkly. 2009 Feb;139(7-8):90–102.
21. Bucher P, Egger JF, Gervaz P, Ris F, Weintraub D, Villiger P, et al. An audit of surgical management of gastrointestinal stromal tumours (GIST). Eur J Surg Oncol. 2006 Apr;32(3):310–4.
22. Krajinovic K, Germer CT, Agaimy A, Wünsch PH, Isbert C. Outcome after resection of one hundred gastrointestinal stromal tumors. Dig Surg. 2010;27(4):313–9.
23. Bucher P, Villiger P, Egger JF, Buhler LH, Morel P. Management of gastrointestinal stromal tumors: from diagnosis to treatment. Swiss Med Wkly. 2004 Mar;134(11-12):145–53.
24. Bonvalot S, Cozic N, Le Cesne A, Blay JY, Penel N, Fau M, et al. ASO Visual Abstract: Initial Active Surveillance Strategy for Patients with Peripheral Sporadic Primary Desmoid-Type Fibromatosis: A Multicentric Phase II Observational Trial. Ann Surg Oncol. 2023;30(13):8671–2.
25. Gronchi A, Miceli R, Shurell E, Eilber FC, Eilber FR, Anaya DA, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013 May;31(13):1649–55.
26. Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS): A Report on 1007 Patients From the Multi-institutional Collaborative RPS Working Group. Ann Surg. 2016 May;263(5):1002–9.
27. Bonvalot S, Gronchi A, Le Péchoux C, Swallow CJ, Strauss D, Meeus P, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020 Oct;21(10):1366–77.
28. Derbel O, Heudel PE, Cropet C, Meeus P, Vaz G, Biron P, et al. Survival impact of centralization and clinical guidelines for soft tissue sarcoma (A prospective and exhaustive population-based cohort). PLoS One. 2017 Feb;12(2):e0158406.