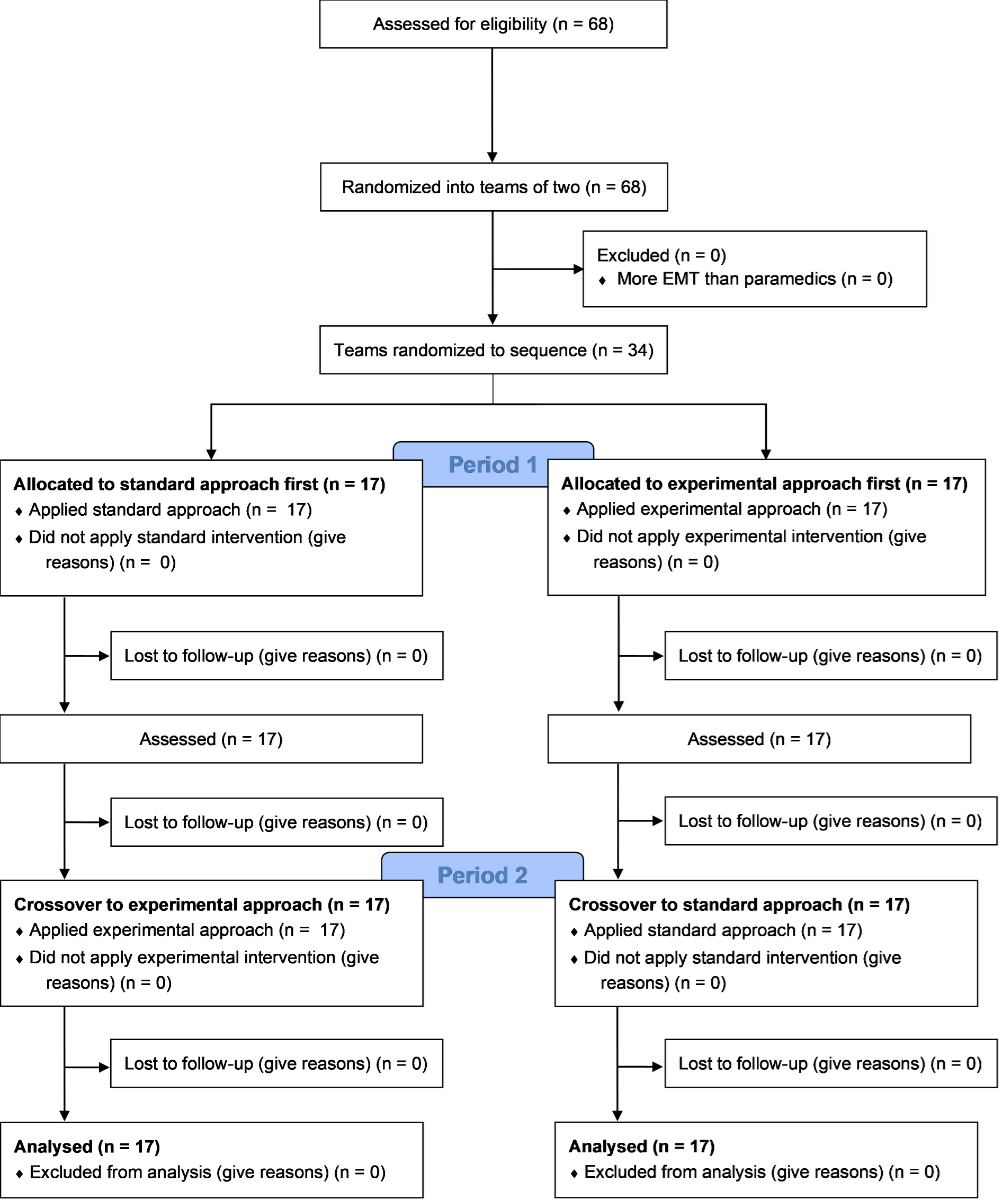
Figure 1Study flowchart. EMT: emergency medical technicians.
DOI: https://doi.org/https://doi.org/10.57187/s.4079
Despite significant advancements in resuscitation science, paediatric out-of-hospital cardiac arrest survival rates remain low (0.0% to 21.2%) [1–3]. These unsatisfactory rates, combined with the particularly high incidence of paediatric out-of-hospital cardiac arrest in infants less than a year old (20.9 to 23.42 per 100,000) [4, 5], underscore the need for continued efforts to mitigate risk factors and optimise survival. Causes of paediatric out-of-hospital cardiac arrest include hypoxia, heart diseases, trauma and sudden infant death syndrome, among others [4–8]. In older children, the incidence of paediatric out-of-hospital cardiac arrest is 3.7 per 100,000, with mostly noncardiac aetiologies (particularly respiratory events), leading to non-shockable rhythms [9]. Prompt restoration of oxygenation is crucial for achieving return of spontaneous circulation. Delays in airway management are associated with decreased survival rates [10]. However, there is limited data on the effects of different paediatric airway management strategies in paediatric out-of-hospital cardiac arrest [11, 12]. Simple and straightforward airway management procedures are often advocated since young age is associated with higher rates of adverse events when advanced airway management procedures are used in prehospital paediatric out-of-hospital cardiac arrest [13].
Emergency medical services typically use bag-valve-mask devices for oxygenation in these cases [14]. However, bag-valve-mask devices have several clinically significant limitations, including leakage and gastric insufflation [15–20], and negatively impact venous return [19, 21, 22]. Additionally, using bag-valve-mask devices requires interruption of chest compressions, which decreases blood flow and is associated with lower survival rates [20]. Advanced airway management procedures such as endotracheal intubation offer optimal airtightness but require advanced skills and may introduce delays [23–28].
Intermediate airway management using supraglottic airway devices such as the i-gel® could be a promising alternative. In adult patients, in addition to ease of insertion and high success rates [29–38], intermediate airway management allows for continuous chest compressions and generates improved ventilation parameters [39–43]. Compared to endotracheal intubation, intermediate airway management leads to faster airway placement and potentially more return of spontaneous circulation, though the impact on long-term survival and aspiration events remains uncertain [44]. Emerging evidence supports the use of intermediate airway management devices in paediatric patients, with higher success rates and similar outcomes compared to endotracheal intubation [45–48].
However, data on the impact of intermediate airway management on ventilation parameters during paediatric out-of-hospital cardiac arrest remains limited, as most studies, primarily registry-based, have focused on advanced airway management, including both supraglottic airways and tracheal intubation, whereas each technique should be evaluated separately. Therefore, the need to specifically assess intermediate airway management as a distinct category has been previously emphasised [49]. Our study hypothesis was that early insertion of an i-gel® device without prior bag-valve-mask ventilation may enhance ventilation parameters compared to the standard bag-valve-mask-only approach.
The primary objective of this study was to determine whether an intermediate airway management strategy, consisting of immediate i-gel® insertion followed by asynchronous ventilations, improved the minute alveolar ventilation in a simulated model of paediatric out-of-hospital cardiac arrest, compared to the standard approach according to the American Heart Association (AHA) guidelines [14].
The secondary objective was to compare the impact of these approaches on metrics for ventilation quality and timing, as well as chest compression quality (rate, depth, chest recoil and chest compression fraction), and on the ability to rapidly administer intravenous adrenaline.
This was a multicentre, superiority, randomised crossover study based on a simulated model of paediatric out-of-hospital cardiac arrest. The study protocol has been published [50]. This manuscript complies with the extension for randomised crossover trials of the Consolidated Standards of Reporting Trials guidelines [51]. The trial was carried out according to the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. A waiver of consent was obtained from the local ethics committee (CCER – Req-2022-00859].
In Switzerland, the organisation of prehospital emergency medical services (EMS) varies considerably from canton to canton. Ambulances are mainly staffed by paramedics, who have completed a three-year curriculum and represent the highest level of non-medical prehospital care [52]. In several cantons, paramedics team up with emergency medical technicians (EMT), who are certified after one year of training. Depending on the cantonal organisation, nurses may be present in ambulances instead of EMTs or paramedics. In most cantons, when a life-threatening emergency is identified by dispatchers, medical reinforcement is provided by a light vehicle, the Service Mobile d'Urgence et de Réanimation, staffed by an emergency physician and a paramedic or specialised nurse. Emergency medical service helicopters, each staffed by a paramedic, an emergency physician and a pilot, are also available and dispatched according to specific criteria. Medical reinforcement can be sent simultaneously with the ambulance or requested by the paramedics after arriving at the scene. In certain regions, a paediatrician may be dispatched to manage specific paediatric cases.
Among the study centres, four were based in the Canton of Geneva (Genève TEAM Ambulances, SK Ambulances, ACE Genève Ambulances, SAG Secours Ambulances Genève), one in the Canton of Valais (Centre de Secours et d’Urgence de la Ville de Sion) and three in the Canton of Neuchâtel (Ambulances des Vallées Neuchâteloises, Service d’Incendie et de Secours [SIS] des Montagnes Neuchâteloises, Service de la protection et de la sécurité [SPS] Neuchâtel).
Paramedics and EMTs currently employed at any of the participating study centres were eligible for inclusion. All these centres use i-gel® devices as part of their standard clinical procedures. The sole exclusion criterion was being a member of the study team. A local study coordinator recruited the participants using a standardised email template that provided comprehensive information about the study, including data protection policies. Participants were blinded to specific study outcomes to prevent preparation bias, even though they were informed that the study was about out-of-hospital cardiac arrest management. Participation was voluntary, and participants were free to withdraw at any time without providing a reason. No incentives were provided. All participants of the study provided written informed consent. Prior to signing the informed consent form, participants were given ample time and opportunity to ask questions. All participants were presumed to possess comparable skills in bag-valve-mask ventilation and i-gel® use, consistent with their regular practice and training background.
Two levels of randomisation were applied. First, each trial centre served as a cluster, and teams underwent initial intra-cluster randomisation. This process employed an online balanced team generator [53], stratified by professional status to ensure that there was at least one paramedic per team. When mixed teams were created (i.e. with an EMT), the paramedic consistently assumed the role of “team leader”. Within paramedic-only teams, participants chose their roles freely, consistent with actual clinical practice. Throughout all scenarios, team leaders retained their positions.
After a self-directed training session supported by two demonstration videos, the second level of randomisation (team level) took place. The videos, each lasting just over a minute, served as a presentation of the approach to be adopted. For the standard approach, the video demonstrated the application of the AHA recommendations [14], which involved alternating cycles of 15 compressions and 2 ventilations, starting with compressions. For the experimental approach, the sequence also began with compressions, followed by the insertion of the i-gel without prior bag-valve-mask ventilation, after which asynchronous ventilations were delivered at a rate of 20–30 per minute (or one ventilation every 2–3 seconds). For both approaches, the videos stressed the importance of alternating roles every 2 minutes. No mention of the placement or use of defibrillation pads was provided in either video.
Teams were then randomly assigned to one of the two study paths by picking up an opaque, sealed envelope created using a block randomisation list (blocks of size 2 and 4) generated online, with a 1:1 ratio [54]. This randomisation was stratified by centre, primarily due to logistical considerations (different sessions) but also to accommodate differences among participants, such as initial airway management strategies, task allocation, local cardiopulmonary resuscitation (CPR) procedures, quality assurance processes or previous specialised training.
The allocation was only disclosed when the team leader opened the sealed envelope just before starting the first simulation, thereby minimising induced biases. Once allocation was known, no further contact between the investigators and the participating teams was allowed.
Consistent with previous findings showing no significant difference between the European Resuscitation Council approach and the AHA approach [55], the AHA guidelines were chosen as the standard [14]. Both guidelines recommend alternating 15 compressions and 2 ventilations. The AHA guidelines start with compressions, whereas the ERC guidelines begin with five initial rescue breaths.
The experimental approach, i.e. using intermediate airway management, involved the immediate insertion of an i-gel® device without prior bag-valve-mask ventilation. Continuous chest compressions were to be initiated upon identification of cardiac arrest. Once the i-gel® device was inserted, ventilations were administered asynchronously at a rate of 20–30 per minute, in accordance with AHA recommendations [14].
Following the crossover design, teams applied both approaches in a random order. Half of the teams performed the first paediatric out-of-hospital cardiac arrest simulation using the standard approach, followed by a second simulation using the experimental approach. The other half did the reverse.
Throughout the study, the same high-fidelity Wi-Fi manikin and dedicated multiparametric monitor/defibrillator (Laerdal SimBaby, Laerdal Medical, Stavanger, Norway) were used. All study outcomes, including chest compression rate, depth, recoil, ventilation volume and rate, were automatically recorded by the manikin, except for the timing of the adrenaline injection, which was manually tagged. Representing a 9-month-old infant with a height of 71 cm, the SimBaby is marketed as a realistic manikin but actually weighs 4.9 kg. To maintain consistency with age, the simulated infant’s weight was communicated to participants to be 9 kg based on the appropriate Best Guess formula: (0.5 × age in months) + 4.5 [56].
Teams were allowed to use their full resuscitation equipment, such as oropharyngeal cannulas, allowing them the flexibility to choose their preferred tools during the simulation. The decision regarding the use of any specific item remained solely at the discretion of the team members, mirroring real-life resuscitation scenarios. A back compensation, using a folded blanket, was already set up. Only appropriately-sized airway management devices (bag-valve-mask device and i-gel®, size 1.5 Intersurgical Ltd, Wokingham, UK) were available.
The scenario was standardised and precisely detailed in the study protocol [50]. Participating teams engaged in two consecutive, identical 10-minute realistic paediatric out-of-hospital cardiac arrest scenarios. The start (T0) was defined as the first compression or the first ventilation, whichever occurred first. To enhance fidelity, two stressors were used: a simulated parent, portrayed by the same female investigator, whose role was to enquire about the situation at scripted intervals, and simulated traffic noises. The scenario began with a clinical statement acknowledging the life-threatening condition of the patient, followed by the team leader restating for confirmation. The simulated child was apnoeic and pulseless, displaying asystole upon electrode placement. CPR waves were automatically displayed during compressions, with subsequent rhythm analyses consistently showing refractory asystole. An intravenous/intraosseous access could be obtained successfully on the first attempt. After the first simulation, the equipment was restored. Then, the exact same scenario was repeated with the alternative airway management strategy, without additional interaction with the study team.
The primary outcome was the minute alveolar ventilation, calculated by subtracting the dead space volume from each ventilation, multiplying by the number of ventilations, and dividing by the time elapsed.
The secondary outcomes were:
Although not included in the protocol, it was decided before data collection to also assess overall ventilation volume and the total number of ventilations.
The protocol’s sample size calculation was incorrect in estimating the number of ventilations with the bag-valve-mask. The protocol anticipated ventilations being provided for only 8 minutes (given the time necessary to prepare ventilation devices). In an adult scenario lasting 10 minutes, there were 39 ventilations in the intermediate airway management approach (with a target rate of 10/minute) and 19 in the bag-valve-mask approach (ratio of 2 ventilations per 30 compressions) [41]. In this study, targeting a ventilation rate of 20–30/minute, around 100 ventilations were expected with the intermediate airway management approach, while the protocol erroneously expected 40 ventilations with the bag-valve-mask approach. The correct expectation should have been around 80 ventilations, given that a ratio of 2 ventilations per 15 compressions yields 10 ventilations/minute, totaling 80 ventilations over 8 minutes. The tidal volume was estimated to be similar with both devices [55], approximately 52 ml, equating to 25 ml of alveolar ventilation. The expected difference in alveolar minute ventilation between bag-valve-mask and intermediate airway management devices was planned to be 185 ml (125 ml versus 310 ml). Data variability was estimated with a standard deviation of 140 ml. A correlation of 0 was used in the calculation to ensure a larger and more cautious sample size, though this assumption disregards the expected positive correlation in a crossover design. With a Type 1 error set at 5% and a power of 90%, the erroneous requirement was 15 teams (30 simulations). However, with the corrected expectation of 80 ventilations (alveolar minute ventilation of 250 ml, with a difference of 60 ml), the actual required sample size should be 117 teams. Consequently, this study is underpowered, with an intraclass correlation coefficient of 0.0065 and a post hoc power estimate of 16.7%.
Data was automatically collected by the manikin’s sensors and exported to a CSV file to prevent assessment bias. A custom PHP script generated variables of interest [57]. Demographics were collected directly through a web-based platform to which participants logged in using the coded identifier of their team, which was written on the outside of the sealed envelope. The curated databases were sent in DTA format to the blinded data analyst. All investigators had access to the data file.
The statistical analysis plan was described in the published protocol [50]. Data distribution was assessed graphically and using the Shapiro-Wilk test in case of doubt. Variables were described accordingly using either median [Q1–Q3] or mean (standard deviation [SD] and/or 95% confidence interval [CI]) depending on their distribution. Due to the crossover design, variable dependency was considered by using paired tests (paired Student’s t-test or Wilcoxon matched-pairs signed-rank test depending on the assumptions) for continuous variables, and McNemar’s test for paired nominal data. Analysis was performed on an intention-to-treat basis. A sensitivity analysis, not anticipated in the protocol but decided prior to data collection, was performed by analysing the primary outcome with ventilation capped at 70 ml to ensure a potential difference was not related to hyperventilations. After a first team-based analysis (with means as unity of analysis), a more in-depth analysis was carried out by graphically assessing the ventilation volume at a ventilation-based level (with ventilation as the unit of analysis). All statistical tests were two-sided, with statistical significance set at 5%. Missing data was treated as such. Data analysis was carried out using Stata V15.1 (StataCorp LLC, College Station, TX, USA).
There was no major deviation from the planned protocol. However, minor protocol deviations occurred and were noted to ensure transparency and accuracy in reporting the study findings.
The trial received a declaration of no objection by the Geneva Cantonal Research Ethics Commission on 19/07/2022 (Req-2022-00859). The trial was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
Sixty-eight participants were recruited from 8 different emergency medical services and distributed into 34 teams (figure 1). Their characteristics are presented in table 1. There were no missing data.

Figure 1Study flowchart. EMT: emergency medical technicians.
Table 1Participants’ characteristics. Totals may not equal 100 due to rounding.
| Characteristic | Participants (n = 68) | |
| Sex, n (%) | Male | 38 (55.9%) |
| Female | 29 (42.7%) | |
| Other | 1 (1.5%) | |
| Profession, n (%) | Paramedic | 57 (83.8%) |
| Emergency medical technician | 9 (13.2%) | |
| Nurse | 2 (2.9%) | |
| Age, median [Q1–Q3] | 33 [28; 38] | |
| Years since diploma, median [Q1–Q3] | 5 [2; 11] | |
| Prehospital work experience in years, median [Q1–Q3] | 8 [4; 15] | |
| Emergency medical service, n (%) | Genève TEAM Ambulances | 16 (23.5%) |
| Centre de Secours et d’Urgence de la Ville de Sion | 6 (23.5%) | |
| SK Ambulances | 12 (17.7%) | |
| Ambulances des Vallées Neuchâteloises | 6 (8.8%) | |
| SIS des Montagnes Neuchâteloises | 6 (8.8%) | |
| ACE Genève Ambulances | 4 (5.9%) | |
| SAG Secours Ambulances Genève | 4 (5.9%) | |
| SPS Neuchâtel | 4 (5.9%) | |
| Actual number of paediatric out-of-hospital cardiac arrests responded to, median [Q1–Q3]. | 0 [0; 1] | |
| Estimated elapsed time since last paediatric out-of-hospital cardiac arrest in the field, n (%) | No prior paediatric resuscitation in the field | 35 (51.5%) |
| <6 months | 2 (2.9%) | |
| 6–12 months | 1 (1.5%) | |
| 12–24 months | 10 (14.7%) | |
| >24 months | 20 (29.4%) | |
| Estimated elapsed time since last simulated paediatric out-of-hospital cardiac arrest, n (%) | No prior simulated paediatric resuscitation | 5 (7.4%) |
| <6 months | 12 (17.7%) | |
| 6–12 months | 13 (19.1%) | |
| 12–24 months | 13 (19.1%) | |
| >24 months | 25 (36.8%) | |
| Specific post-graduate course in paediatric out-of-hospital cardiac arrest, n (%) | 38 (55.9%) | |
SIS: Service d’Incendie et de Secours: SPS: Service de la protection et de la sécurité
Minute alveolar ventilation was not significantly different when using the intermediate airway management strategy compared to bag-valve-mask (258 ml [95% CI 190 to 326] versus 222 ml [95% CI 194 to 250]; difference of 36 ml [95% CI −28 to 99]) (figure 2). When capping each ventilation at 70 ml according to the sensitivity analysis, similar results were found (197 ml [95% CI 155 to 238] with intermediate airway management compared to 185 ml [95% CI 164 to 206] with bag-valve-mask; difference of 11 ml [95% CI −29 to 51]).
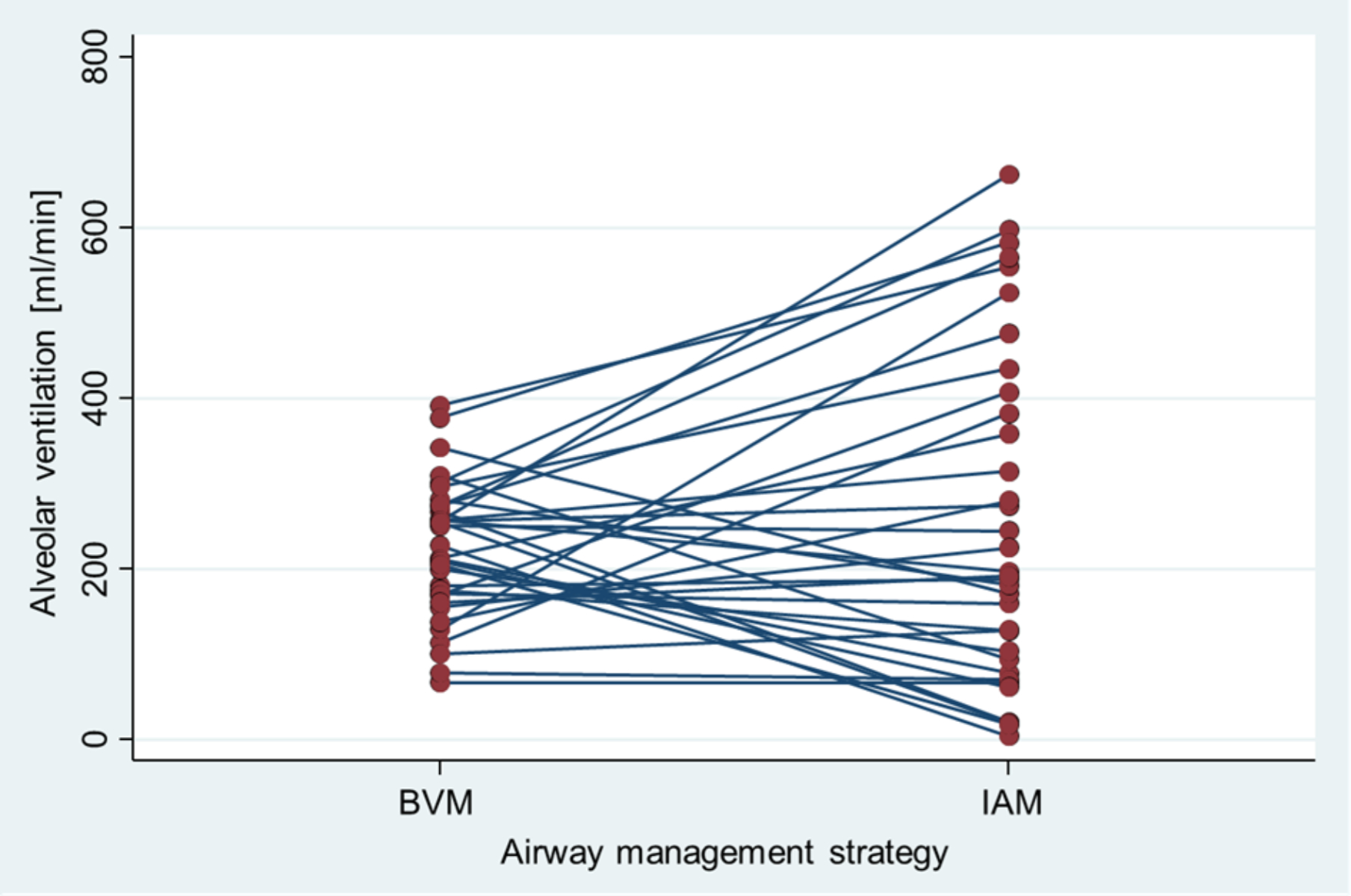
Figure 2Comparison of alveolar ventilation (ml/min) between Bag-Valve-Mask (BVM) and Intermediate Airway Management (IAM) strategies. Each line connects the data points for individual teams, highlighting changes in alveolar ventilation from BVM to IAM.
More ventilations were delivered when using intermediate airway management, with a consistent mean volume regardless of the airway management strategy. The use of bag-valve-mask enabled quicker initiation of ventilation and provided more ventilations within the target volume (table 2).
Table 2Ventilation outcomes. Totals may not equal 100 due to rounding.
| Bag-valve-mask | Intermediate airway management | Difference | ||
| Number of ventilations, mean (95% CI) | 79 (74 to 84) | 96 (89 to 103) | −17 (−23 to −10) | |
| Ventilation volume in ml, mean (95% CI) | 54 (51 to 58) | 53 (45 to 61) | 1 (−7 to 10) | |
| Time to first ventilation in s, median [Q1–Q3] | 49 [36; 65] | 72 [53; 98] | −27 [−48; −1] | |
| % of ventilations below/within/over target volume, mean (95% CI) | Below (<45 ml) | 39 (32 to 45) | 47 (35 to 59) | −8 (−20 to 3) |
| Within (45–72 ml) | 38 (34 to 42) | 28 (22 to 35) | 9 (2 to 16) | |
| Over (>72 ml) | 23 (19 to 28) | 24 (14 to 35) | −1 (−11 to 9) | |
All teams started with chest compressions, resulting in a duration of 0 seconds to the first compression. The chest compression fraction was significantly higher when using intermediate airway management compared to bag-valve-mask. In contrast, chest recoil was better with the bag-valve-mask strategy (table 3). All other compression outcomes were similar.
Table 3Chest compression outcomes. Totals may not equal 100 due to rounding.
| Bag-valve-mask | Intermediate airway management | Difference | ||
| Chest compression fraction in %, mean (95% CI) | 58 (56 to 60) | 70 (68 to 73) | −12 (−15 to −11) | |
| Compression rate in cpm, mean (95% CI) | 124 (121 to 127) | 123 (121 to 126) | 0 (−1 to 2) | |
| % of compressions below/within/over target rate, mean (95% CI) | Below (<100 cpm) | 1.0 (0.6 to 1.4) | 1.0 (0.4 to 1.5) | 0 (−0.5 to 0.5) |
| Within (100–120 cpm) | 38 (27 to 49) | 37 (26 to 48) | 1 (−4 to 7) | |
| Over (>120 cpm) | 61 (49 to 72) | 62 (51 to 73) | −1 (−7 to 4) | |
| Compression depth in cm, median [Q1–Q3] | 3.0 [2.9; 3.2] | 2.9 [2.8; 3.1] | 0 [−0.8; 2.0] | |
| % of compressions below/within guideline’s depth target, median [Q1–Q3] | Below (<4.3 cm) | 100 [100; 100] | 100 [100; 100] | 0 [0; 0] |
| Within (≥4.3 cm) | 0 [0; 0] | 0 [0; 0] | 0 [0; 0] | |
| % of compressions below/within/over manufacturer’s depth target, median [Q1–Q3] | Below (<4 cm) | 100 [98; 100] | 100 [97; 100] | 0 [0; 1] |
| Within (4–5 cm) | 0 [0; 2] | 0 [0; 3] | 0 [0; 0] | |
| Over (>5 cm) | 0 [0; 0] | 0 [0; 0] | 0 [0; 0] | |
| Complete chest recoil, %, median [Q1–Q3] | 58 [45; 74] | 46 [30; 56] | 13 [3; 26] | |
cpm: compressions per minute.
The proportion of scenarios in which adrenaline was administered was similar with both strategies (table 4), as was the proportion in which it was administered within 5 minutes (table 5).
Table 4Timing of adrenaline administration.
| Intermediate airway management | ||||
| Not administered | Administered | Total | ||
| Bag-valve-mask | Not administered | 1 | 2 | 3 |
| Administered | 5 | 26 | 31 | |
| Total | 6 | 28 | 34 | |
Table 5Timing of adrenaline administration within 5 minutes.
| Intermediate airway management | ||||
| After 5 minutes | Within 5 minutes | Total | ||
| Bag-valve-mask | After 5 minutes | 31 | 2 | 33 |
| Within 5 minutes | 1 | 0 | 1 | |
| Total | 32 | 2 | 34 | |
The time to the first adrenaline injection was similar with both strategies, with a median [Q1–Q3] time of 420 seconds [365; 494] when using bag-valve-mask versus 420 seconds [359; 490] with intermediate airway management, yielding a difference of 14 seconds [−85; 67].
A total of 5944 ventilations was included in the database (table 6).
Table 6Number of ventilations below/within/over target volume, n (%). Totals may not equal 100 due to rounding.
| Bag-valve-mask | Intermediate airway management | |
| <45 ml | 1058 (39.8%) | 1573 (48.3%) |
| 45–72 ml | 1023 (35.0%) | 941 (28.9%) |
| >72 ml | 606 (22.6%) | 743 (22.8%) |
The variability in ventilation volume was higher when using the intermediate airway management strategy compared to the bag-valve-mask strategy (figure 3).
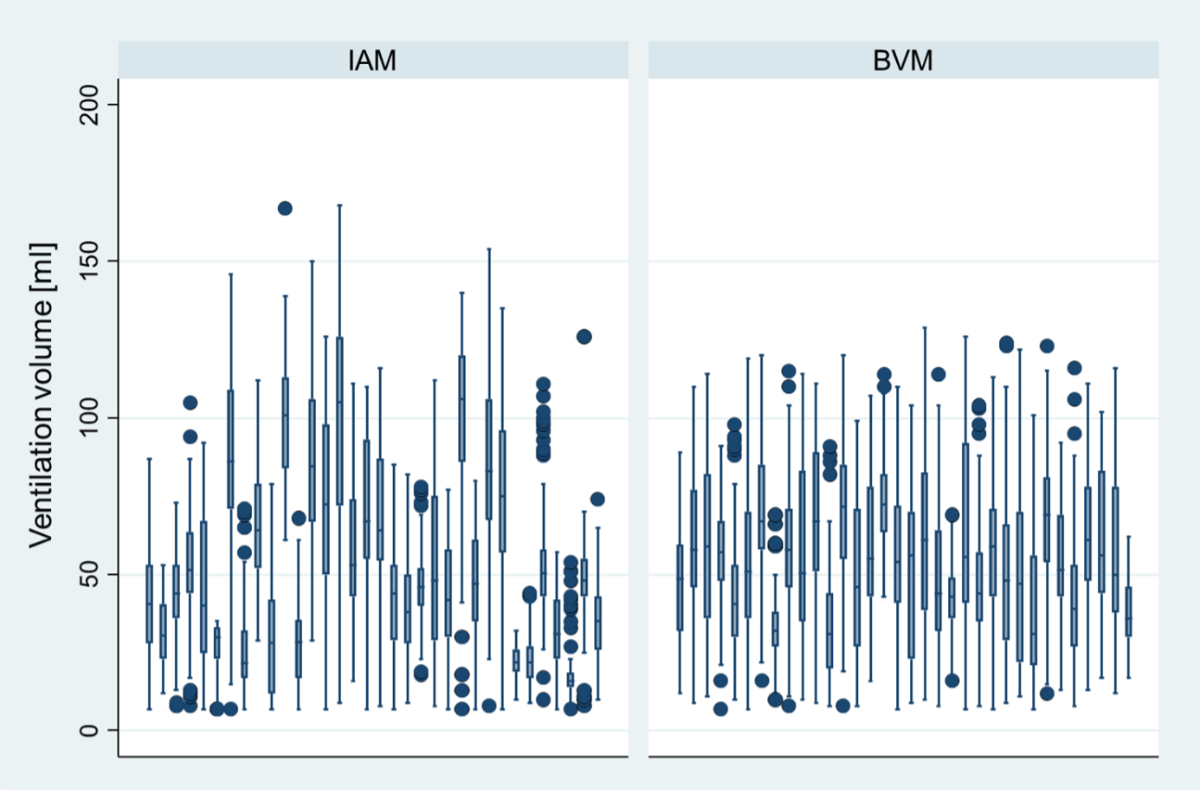
Figure 3Scaled analysis of ventilation volume per team (the same team corresponding to the same boxplot, e.g. the first boxplot in the Intermediate Airway Management [IAM] graph represents the data from the same team as the first boxplot in the Bag-Valve-Mask [BVM] graph). The box represents the interquartile range (IQR), which spans from the first quartile (Q1, 25th percentile) to the third quartile (Q3, 75th percentile). The line inside the box indicates the median (Q2, 50th percentile). The whiskers extend to the smallest and largest values within 1.5 times the IQR from Q1 and Q3, respectively. Data points outside this range are considered outliers and are represented as individual dots.
In this study comparing intermediate airway management and bag-valve-mask ventilation approaches, there was no clinically relevant difference in the primary outcome when an intermediate airway management strategy was used, with an increase of only 36 ml/min in alveolar ventilation. However, the wide 95% CI (−28 to 99 ml) surrounding this point estimate suggests considerable variability, and the clinical relevance may vary significantly depending on the actual value of the parameter. These findings underscore the need for a critical evaluation of the observed differences and their potential impact on clinical decisions and patient outcomes. Increasing the sample size to achieve adequate statistical power would not enhance the clinical impact of such a small difference in alveolar ventilation. This lack of difference aligns with the Amagasa et al. meta-analysis, which showed that prehospital advanced or intermediate airway management (including both endotracheal intubation and supraglottic airway) for paediatric out-of-hospital cardiac arrest did not improve outcomes compared to bag-valve-mask. Their ranking analysis indicated that bag-valve-mask was superior to supraglottic airway and endotracheal intubation for survival and favourable neurological outcomes, with a low to very low level of certainty [59].
All groups displayed a suboptimal approach to paediatric CPR by prioritising defibrillation pad placement over ventilation, following the adult approach. This was unexpected since prehospital providers are taught to tailor resuscitation to children’s specific needs by prioritising effective ventilation over pad placement. However, the time to first ventilation was relatively low (49 s for bag-valve-mask and 72 s for intermediate airway management) compared to simulated cases with larger CPR teams, where the time to first bag-valve-mask ventilation was 1.4 or 1.5 minutes depending on whether the cardiac rhythm was shockable [60]. It is important to note that the timing of different airway management interventions does not appear to affect patient outcomes [61]. On the other hand, a shorter time to first adrenaline dose was found in larger team responses [60], possibly due to different prioritisation strategies.
Another notable finding was the increased variability in ventilation volumes with the i-gel®, accompanied by a higher proportion of hyperinflation. Continuous chest compressions during intermediate airway management may have made self-assessment of ventilatory quality (by visualising chest rise) difficult compared to using a bag-valve-mask device. It is unclear to what extent the lack of sealing, linked to the fact that the use of a manikin prevents the gel cuff from reaching an adequate seal pressure, could impact this endpoint. Indeed, the seal pressure appears to improve over time in humans due to the thermoplastic properties [62]. Moreover, it is uncertain whether the measures of compression and ventilation parameters were reliable when performing continuous compressions with asynchronous ventilations using a non-tracheal device. The clinical impact of this variability should be questioned. Participant exposure to simulated or actual cases was very low. While 20 insertions are recommended for novices to develop skills in using the i-gel® [63], it remains unclear how to maintain the skill. More recent simulation, increased participation and simulation training during daytime hours may improve CPR performance [64]. The observed variability could likely be mitigated with continuous training and/or quality management. Hyperventilation occurs more often during CPR with a tracheal tube or a supraglottic airway in place than with a bag-valve-mask. Given the limited data on the impact of ventilation parameters on clinical outcomes in paediatric out-of-hospital cardiac arrest, future research should focus on respiratory physiology during paediatric CPR to determine the optimal ventilation rate [65]. Two potential solutions to mitigate higher variability in clinical settings include using ventilation feedback devices and/or using an i-gel® without continuous chest compressions, similar to bag-valve-mask with a 15:2 ratio, as this will still improve the chest compression fraction [41].
Prehospital providers should consider that impaired lung compliance can make bag-valve-mask use more difficult by increasing air leaks. Therefore, devices and strategies should be selected based on the clinical situation. The lack of clinically relevant differences between bag-valve-mask and intermediate airway management observed in the present study, and the higher odds ratios for survival associated with bag-valve-mask compared to intermediate airway management or advanced airway management in a registry-based American study [66], advocate for the use of bag-valve-mask as the first-line oxygenation tool in cases of paediatric out-of-hospital cardiac arrest. This is in line with the 2024 Internation Liaison Committee on Resuscitation Consensus on Science with Treatment Recommendations [67]. However, intermediate airway management could be useful when bag-valve-mask use is difficult and should not be entirely disregarded since ventilation was improved after supraglottic airway insertion in 96/135 (71%) of cases [68]. Moreover, a Japanese study reported no significant difference in one-month survival between prehospital endotracheal intubation and supraglottic airway insertion by emergency medical service personnel among paediatric out-of-hospital cardiac arrest patients [69], suggesting that emergency medical service personnel may rely on their familiar strategy when performing more advanced prehospital airway management during paediatric out-of-hospital cardiac arrest. These assertions should, to the extent possible, be confirmed by sufficiently powered randomised controlled trials.
The difference in chest compression fraction (favouring intermediate airway management) and chest recoil (favouring bag-valve-mask) suggests that neither approach was conclusively superior. Compressions were consistently too shallow in both groups. Regarding compression quality metrics, and especially compression depth, our results were similar to actual in-hospital cardiac arrests. Quality metrics often did not meet the guidelines, with the greatest difficulty in achieving the chest compression depth target in younger children (for children less than 1 year only, median [Q1–Q3] chest compression fraction was 88% [61; 98], rate was 119/min [110; 129] and depth 2.3 cm [1.9; 3.0]) [70]. The compression technique (thumbs/one hand/two hands) was not assessed in this study but could partly explain the results as different hand positions during CPR in young children have been shown to change compression depth [71]. Finally, the high cognitive load experienced by participants during paediatric out-of-hospital cardiac arrest [72] could explain a lower CPR quality in paediatric cases compared to adults [60].
This study has limitations. It was underpowered, due to an error in the sample size calculation, as detailed previously, which may have impacted the robustness of our findings and the generalisability of our conclusions. A possible bias was noted with an automatically displayed end-tidal CO2 value on the monitor even if ventilation was at 0 ml/min, which we found concerning for high-fidelity manikins. All scenarios were simulated and thus cannot be directly linked to clinical outcomes, potentially not accurately reflecting real-life resuscitation quality. The use of simulation was essential due to the infrequency of paediatric out-of-hospital cardiac arrest cases. Performance was likely superior to real-life scenarios due to the controlled environment and reduced stress, and a Hawthorne effect cannot be ruled out [73].
Despite these limitations, the study’s strong design i.e. the crossover design which controls for static confounders, the multicentric setting and the adherence to a published protocol represent clear strengths. The data generated provides valuable insights into the challenges and considerations surrounding paediatric ventilation in prehospital settings.
In a simulated paediatric out-of-hospital cardiac arrest model, a strategy of immediate intermediate airway management use without prior bag-valve-mask ventilations did not result in relevant differences. Thus, intermediate airway management devices cannot be recommended as first-line devices for paediatric out-of-hospital cardiac arrest but should be considered when bag-valve-mask oxygenation is challenging, particularly when lung compliance is altered. Overall, the focus should remain on providing high-quality ventilations regardless of the device used by emergency medical services providers.
The datasets supporting the results of this article are available on a Yareta repository (https://doi.org/10.26037/yareta:cyekjdvz75cdba4p3ifxjae6zm).
The authors sincerely thank the College of Higher Education in Ambulance Care in Geneva for the loan of the manikin and Sylvain Simonet for his assistance in organising the study sessions. They also express gratitude to all participating emergency medical service personnel, especially Basile Berger, Daisy La Monica, Marec Saillant, Olivia Mowat, Damien Hennet, Benjamin Ehresmann, Robin Vos, Alexi Borel, Julien Celi, Andy Willener, Jean-Daniel Zimmerli, Yves Challandes and Yves Vollenweider.
Author contributions: Conceptualisation: LSt, DT, JNS, LSu. Methodology: LSt, LJ, LSu, DT, JNS. Software: LJ, LSt, DT. Validation: LSt, LSu, JNS. Formal analysis: LSt. Investigation: LSt, DT, LJ, JMT, EM. Resources: LJ, LSt. Data curation: LJ, LSu, LSt. Writing – original draft preparation: LSt, LSu. Writing – review and editing: LSt, EM, DT, LJ, JNS, JMT, LSu. Visualisation: LSt. Supervision: LSt, LSu, JNS. Project administration: LSt. Funding acquisition: not applicable. All authors have read and agreed to the published version of the manuscript.
This study received no funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022 Feb;145(8):e153–639. doi: https://doi.org/10.1161/CIR.0000000000001052
2. Tham LP, Wah W, Phillips R, Shahidah N, Ng YY, Shin SD, et al. Epidemiology and outcome of paediatric out-of-hospital cardiac arrests: A paediatric sub-study of the Pan-Asian resuscitation outcomes study (PAROS). Resuscitation. 2018 Apr;125:111–7. doi: https://doi.org/10.1016/j.resuscitation.2018.01.040
3. Kim M, Yu J, Chang H, Heo S, Lee SU, Hwang SY, et al. National Surveillance of Pediatric Out-of-Hospital Cardiac Arrest in Korea: The 10-Year Trend From 2009 to 2018. J Korean Med Sci. 2022 Nov;37(44):e317. doi: https://doi.org/10.3346/jkms.2022.37.e317
4. Kelpanides IK, Katzenschlager S, Skogvoll E, Tjelmeland IB, Grindheim G, Alm-Kruse K, et al. Out-of-hospital cardiac arrest in children in Norway: A national cohort study, 2016-2021. Resusc Plus. 2024 May;18:100662. doi: https://doi.org/10.1016/j.resplu.2024.100662
5. Katzenschlager S, Kelpanides IK, Ristau P, Huck M, Seewald S, Brenner S, et al. Out-of-hospital cardiac arrest in children: an epidemiological study based on the German Resuscitation Registry identifying modifiable factors for return of spontaneous circulation. Crit Care. 2023 Sep;27(1):349. doi: https://doi.org/10.1186/s13054-023-04630-3
6. Holgersen MG, Jensen TW, Breindahl N, Kjerulff JL, Breindahl SH, Blomberg SN, et al.; Danish Cardiac Arrest Registry Group. Pediatric out-of-hospital cardiac arrest in Denmark. Scand J Trauma Resusc Emerg Med. 2022 Nov;30(1):58. doi: https://doi.org/10.1186/s13049-022-01045-x
7. Lee J, Yang WC, Lee EP, Huang JL, Hsiao HJ, Lin MJ, et al. Clinical Survey and Predictors of Outcomes of Pediatric Out-of-Hospital Cardiac Arrest Admitted to the Emergency Department. Sci Rep. 2019 May;9(1):7032. doi: https://doi.org/10.1038/s41598-019-43020-0
8. Fovaeus H, Holmen J, Mandalenakis Z, Herlitz J, Rawshani A, Castellheim AG. Out-of-hospital cardiac arrest: survival in children and young adults over 30 years, a nationwide registry-based cohort study. Resuscitation. 2024 Feb;195:110103. doi: https://doi.org/10.1016/j.resuscitation.2023.110103
9. Somma V, Pflaumer A, Connell V, Rowe S, Fahy L, Zentner D, et al. Epidemiology of pediatric out-of-hospital cardiac arrest compared with adults. Heart Rhythm. 2023 Nov;20(11):1525–31. doi: https://doi.org/10.1016/j.hrthm.2023.06.010
10. Ohashi-Fukuda N, Fukuda T, Doi K. Association between time to advanced airway management and survival during pediatric out-of-hospital cardiac arrest. Resusc Plus. 2022 Jun;11:100260. doi: https://doi.org/10.1016/j.resplu.2022.100260
11. Lavonas EJ, Ohshimo S, Nation K, Van de Voorde P, Nuthall G, Maconochie I, et al.; International Liaison Committee on Resuscitation (ILCOR) Pediatric Life Support Task Force. Advanced airway interventions for paediatric cardiac arrest: A systematic review and meta-analysis. Resuscitation. 2019 May;138:114–28. doi: https://doi.org/10.1016/j.resuscitation.2019.02.040
12. Weihing VK, Crowe EH, Wang HE, Ugalde IT. Prehospital airway management in the pediatric patient: A systematic review. Acad Emerg Med. 2022 Jun;29(6):765–71. doi: https://doi.org/10.1111/acem.14410
13. Eriksson CO, Bahr N, Meckler G, Hansen M, Walker-Stevenson G, Idris A, et al.; Child Safety Initiative–Emergency Medical Services for Children. Adverse Safety Events in Emergency Medical Services Care of Children With Out-of-Hospital Cardiac Arrest. JAMA Netw Open. 2024 Jan;7(1):e2351535. doi: https://doi.org/10.1001/jamanetworkopen.2023.51535
14. Topjian AA, Raymond TT, Atkins D, Chan M, Duff JP, Joyner BL Jr, et al.; Pediatric Basic and Advanced Life Support Collaborators. Part 4: Pediatric Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020 Oct;142(16_suppl_2 suppl_2):S469–523. doi: https://doi.org/10.1161/CIR.0000000000000901
15. Daigle CH, Fiadjoe JE, Laverriere EK, Bruins BB, Lockman JL, Shults J, et al.; National Emergency Airway Registry for Children (NEAR4KIDS) Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI). Difficult Bag-Mask Ventilation in Critically Ill Children Is Independently Associated With Adverse Events. Crit Care Med. 2020 Sep;48(9):e744–52. doi: https://doi.org/10.1097/CCM.0000000000004425
16. Santos-Folgar M, Lafuente-Filgueira P, Otero-Agra M, Fernández-Méndez F, Barcala-Furelos R, Trastoy-Quintela J, et al. Quality of Ventilations during Infant Resuscitation: A Simulation Study Comparing Endotracheal Tube with Face Mask. Children (Basel). 2022 Nov;9(11):1757. doi: https://doi.org/10.3390/children9111757
17. Becker HJ, Langhan ML. Can Providers Use Clinical Skills to Assess the Adequacy of Ventilation in Children During Bag-Valve Mask Ventilation? Pediatr Emerg Care. 2020 Dec;36(12):e695–9. doi: https://doi.org/10.1097/PEC.0000000000001314
18. Fitz-Clarke JR. Fast or Slow Rescue Ventilations: A Predictive Model of Gastric Inflation. Respir Care. 2018 May;63(5):502–9. doi: https://doi.org/10.4187/respcare.05620
19. Wenzel V, Idris AH, Banner MJ, Kubilis PS, Band R, Williams JL, et al. Respiratory system compliance decreases after cardiopulmonary resuscitation and stomach inflation: impact of large and small tidal volumes on calculated peak airway pressure1Presented, in part, at the 71st Scientific Sessions of the American Heart Association, Dallas, TX, November, 1998.1. Resuscitation. 1998 Aug;38(2):113–8. doi: https://doi.org/10.1016/S0300-9572(98)00095-1
20. Aramendi E, Irusta U. To interrupt, or not to interrupt chest compressions for ventilation: that is the question! J Thorac Dis. 2016 Jan;8(1):E121–3.
21. Ruben H, Knudsen EJ, Carugati G. Gastric inflation in relation to airway pressure. Acta Anaesthesiol Scand. 1961;5(3):107–14. doi: https://doi.org/10.1111/j.1399-6576.1961.tb00089.x
22. Paal P, Neurauter A, Loedl M, Brandner J, Herff H, Knotzer H, et al. Effects of stomach inflation on haemodynamic and pulmonary function during spontaneous circulation in pigs. Resuscitation. 2009 Apr;80(4):470–7. doi: https://doi.org/10.1016/j.resuscitation.2009.01.005
23. Buis ML, Maissan IM, Hoeks SE, Klimek M, Stolker RJ. Defining the learning curve for endotracheal intubation using direct laryngoscopy: A systematic review. Resuscitation. 2016 Feb;99:63–71. doi: https://doi.org/10.1016/j.resuscitation.2015.11.005
24. Galinski M, Wrobel M, Boyer R, Reuter PG, Ruscev M, Debaty G, et al. Risk factors for failed first intubation attempt in an out-of-hospital setting: a multicenter prospective study. Intern Emerg Med. 2022 Oct;19:
25. Walas W, Aleksandrowicz D, Borszewska-Kornacka M, Gaszyński T, Helwich E, Migdał M, et al. Unanticipated difficult airway management in children - the consensus statement of the Paediatric Anaesthesiology and Intensive Care Section and the Airway Management Section of the Polish Society of Anaesthesiology and Intensive Therapy and the Polish So. Anaesthesiol Intensive Ther. 2017;49(5):336–49. doi: https://doi.org/10.5603/AIT.2017.0079
26. Hansen M, Lambert W, Guise JM, Warden CR, Mann NC, Wang H. Out-of-hospital pediatric airway management in the United States. Resuscitation. 2015 May;90:104–10. doi: https://doi.org/10.1016/j.resuscitation.2015.02.018
27. Pacheco GS, Patanwala AE, Leetch AN, Mendelson JS, Hurst NB, Sakles JC. Intubation During Pediatric Cardiac Arrest in the Emergency Department Is Associated With Reduced First-Pass Success. Pediatr Emerg Care. 2022 May;38(5):e1271–6. doi: https://doi.org/10.1097/PEC.0000000000002592
28. Funakoshi H, Kunitani Y, Goto T, Okamoto H, Hagiwara Y, Watase H, et al.; Japanese Emergency Medicine Network Investigators. Association Between Repeated Tracheal Intubation Attempts and Adverse Events in Children in the Emergency Department. Pediatr Emerg Care. 2022 Feb;38(2):e563–8. doi: https://doi.org/10.1097/PEC.0000000000002356
29. Leventis C, Chalkias A, Sampanis MA, Foulidou X, Xanthos T. Emergency airway management by paramedics: comparison between standard endotracheal intubation, laryngeal mask airway, and I-gel. Eur J Emerg Med. 2014 Oct;21(5):371–3. doi: https://doi.org/10.1097/MEJ.0000000000000101
30. Beylacq L, Bordes M, Semjen F, Cros AM. The I-gel, a single-use supraglottic airway device with a non-inflatable cuff and an esophageal vent: an observational study in children. Acta Anaesthesiol Scand. 2009 Mar;53(3):376–9. doi: https://doi.org/10.1111/j.1399-6576.2008.01869.x
31. Theiler L, Gutzmann M, Kleine-Brueggeney M, Urwyler N, Kaempfen B, Greif R. i-gel™ supraglottic airway in clinical practice: a prospective observational multicentre study. Br J Anaesth. 2012 Dec;109(6):990–5. doi: https://doi.org/10.1093/bja/aes309
32. Middleton PM, Simpson PM, Thomas RE, Bendall JC. Higher insertion success with the i-gel supraglottic airway in out-of-hospital cardiac arrest: a randomised controlled trial. Resuscitation. 2014 Jul;85(7):893–7. doi: https://doi.org/10.1016/j.resuscitation.2014.02.021
33. Duckett J, Fell P, Han K, Kimber C, Taylor C. Introduction of the I-gel supraglottic airway device for prehospital airway management in a UK ambulance service. Emerg Med J. 2014 Jun;31(6):505–7. doi: https://doi.org/10.1136/emermed-2012-202126
34. Ruetzler K, Roessler B, Potura L, Priemayr A, Robak O, Schuster E, et al. Performance and skill retention of intubation by paramedics using seven different airway devices—a manikin study. Resuscitation. 2011 May;82(5):593–7. doi: https://doi.org/10.1016/j.resuscitation.2011.01.008
35. Benger JR, Kirby K, Black S, Brett SJ, Clout M, Lazaroo MJ, et al. Supraglottic airway device versus tracheal intubation in the initial airway management of out-of-hospital cardiac arrest: the AIRWAYS-2 cluster RCT. Health Technol Assess. 2022 Apr;26(21):1–158. doi: https://doi.org/10.3310/VHOH9034
36. Stone BJ, Chantler PJ, Baskett PJ. The incidence of regurgitation during cardiopulmonary resuscitation: a comparison between the bag valve mask and laryngeal mask airway. Resuscitation. 1998 Jul;38(1):3–6. doi: https://doi.org/10.1016/S0300-9572(98)00068-9
37. Lønvik MP, Elden OE, Lunde MJ, Nordseth T, Bakkelund KE, Uleberg O. A prospective observational study comparing two supraglottic airway devices in out-of-hospital cardiac arrest. BMC Emerg Med. 2021 Apr;21(1):51. doi: https://doi.org/10.1186/s12873-021-00444-0
38. Smida T, Menegazzi J, Scheidler J, Martin PS, Salcido D, Bardes J; CARES Surveillance Group. A retrospective comparison of the King Laryngeal Tube and iGel supraglottic airway devices: A study for the CARES surveillance group. Resuscitation. 2023 Jul;188:109812. doi: https://doi.org/10.1016/j.resuscitation.2023.109812
39. Häske D, Schempf B, Gaier G, Niederberger C. Performance of the i-gel™ during pre-hospital cardiopulmonary resuscitation. Resuscitation. 2013 Sep;84(9):1229–32. doi: https://doi.org/10.1016/j.resuscitation.2013.04.025
40. Stuby L, Jampen L, Sierro J, Paus E, Spichiger T, Suppan L, et al. Effect on Chest Compression Fraction of Continuous Manual Compressions with Asynchronous Ventilations Using an i-gel® versus 30:2 Approach during Simulated Out-of-Hospital Cardiac Arrest: Protocol for a Manikin Multicenter Randomized Controlled Trial. Healthcare (Basel). 2021 Mar;9(3):354. doi: https://doi.org/10.3390/healthcare9030354
41. Stuby L, Jampen L, Sierro J, Bergeron M, Paus E, Spichiger T, et al. Effect of Early Supraglottic Airway Device Insertion on Chest Compression Fraction during Simulated Out-of-Hospital Cardiac Arrest: Randomised Controlled Trial. J Clin Med. 2021 Dec;11(1):217. doi: https://doi.org/10.3390/jcm11010217
42. Stuby L, Suppan L, Jampen L, Thurre D. Impact of the Over-the-Head Position with a Supraglottic Airway Device on Chest Compression Depth and Rate: A Post Hoc Analysis of a Randomized Controlled Trial. Healthcare (Basel). 2022 Apr;10(4):718. doi: https://doi.org/10.3390/healthcare10040718
43. Benger JR, Lazaroo MJ, Clout M, Voss S, Black S, Brett SJ, et al. Randomized trial of the i-gel supraglottic airway device versus tracheal intubation during out of hospital cardiac arrest (AIRWAYS-2): patient outcomes at three and six months. Resuscitation. 2020 Dec;157:74–82. doi: https://doi.org/10.1016/j.resuscitation.2020.09.026
44. Forestell B, Ramsden S, Sharif S, Centofanti J, Al Lawati K, Fernando SM, et al. Supraglottic Airway Versus Tracheal Intubation for Airway Management in Out-of-Hospital Cardiac Arrest: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis of Randomized Controlled Trials. Crit Care Med. 2024 Feb;52(2):e89–99. doi: https://doi.org/10.1097/CCM.0000000000006112
45. Hansen M, Wang H, Le N, Lin A, Idris A, Kornegay J, et al. Prospective evaluation of airway management in pediatric out-of-hospital cardiac arrest. Resuscitation. 2020 Nov;156:53–60. doi: https://doi.org/10.1016/j.resuscitation.2020.08.003
46. Hanlin ER, Chan HK, Hansen M, Wendelberger B, Shah MI, Bosson N, et al. Epidemiology of out-of-hospital pediatric airway management in the 2019 national emergency medical services information system data set. Resuscitation. 2022 Apr;173:124–33. doi: https://doi.org/10.1016/j.resuscitation.2022.01.008
47. Mani S, Gugino S, Helman J, Bawa M, Nair J, Chandrasekharan P, et al. Laryngeal mask ventilation with chest compression during neonatal resuscitation: randomized, non-inferiority trial in lambs. Pediatr Res. 2021;3(nov):1–7.
48. Le Bastard Q, Rouzioux J, Montassier E, Baert V, Recher M, Hubert H, et al.; GR-RéAC. Endotracheal intubation versus supraglottic procedure in paediatric out-of-hospital cardiac arrest: a registry-based study. Resuscitation. 2021 Nov;168:191–8. doi: https://doi.org/10.1016/j.resuscitation.2021.08.015
49. Suppan L, Fehlmann CA, Stuby L, Suppan M. The Importance of Acknowledging an Intermediate Category of Airway Management Devices in the Prehospital Setting. Healthcare (Basel). 2022 May;10(5):961. doi: https://doi.org/10.3390/healthcare10050961
50. Stuby L, Mühlemann E, Jampen L, Thurre D, Siebert JN, Suppan L. Effect of Intermediate Airway Management on Ventilation Parameters in Simulated Pediatric Out-of-Hospital Cardiac Arrest: Protocol for a Multicenter, Randomized, Crossover Trial. Children (Basel). 2023 Jan;10(1):148. doi: https://doi.org/10.3390/children10010148
51. Dwan K, Li T, Altman DG, Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019 Jul;366:l4378. doi: https://doi.org/10.1136/bmj.l4378
52. Schmutz T, Guechi Y, Denereaz S, Ozainne F, Nuoffer M, Exadaktylos A, et al. Paramedics in Switzerland: A Mature Profession. Int J Environ Res Public Health. 2022 Jul;19(14):8429. doi: https://doi.org/10.3390/ijerph19148429
53. Keamk - Create random and balanced teams. Available from: https://www.keamk.com/
54. Create a blocked randomisation list | Sealed Envelope. Available from: https://www.sealedenvelope.com/simple-randomiser/v1/lists
55. Suppan L, Jampen L, Siebert JN, Zünd S, Stuby L, Ozainne F. Impact of Two Resuscitation Sequences on Alveolar Ventilation during the First Minute of Simulated Pediatric Cardiac Arrest: Randomized Cross-Over Trial. Healthcare (Basel). 2022 Dec;10(12):2451. doi: https://doi.org/10.3390/healthcare10122451
56. Tinning K, Acworth J. Make your Best Guess: an updated method for paediatric weight estimation in emergencies. Emerg Med Australas. 2007 Dec;19(6):528–34. doi: https://doi.org/10.1111/j.1742-6723.2007.01026.x
57. Jampen L, Stuby L. CPR - Early Insertion Effect of a Supraglottic Airway Device on CCF in simulated OHCA - PHP code for data extraction from SimMan® 3G. 16 juill 2021;1. Available from: https://data.mendeley.com/datasets/s8d2gpfhyw/1
58. Suppan L, Jampen L, Siebert JN, Zünd S, Stuby L, Ozainne F. Correction: Suppan et al. Impact of Two Resuscitation Sequences on Alveolar Ventilation during the First Minute of Simulated Pediatric Cardiac Arrest: Randomized Cross-Over Trial. Healthcare 2022, 10, 2451. Healthcare (Basel). 2023 Jun;11(12):1799. doi: https://doi.org/10.3390/healthcare11121799
59. Amagasa S, Utsumi S, Moriwaki T, Yasuda H, Kashiura M, Uematsu S, et al. Advanced airway management for pediatric out-of-hospital cardiac arrest: A systematic review and network meta-analysis. Am J Emerg Med. 2023 Jun;68:161–9. doi: https://doi.org/10.1016/j.ajem.2023.03.049
60. Hansen M, Walker-Stevenson G, Bahr N, Harrod T, Meckler G, Eriksson C, et al. Comparison of Resuscitation Quality in Simulated Pediatric and Adult Out-of-Hospital Cardiac Arrest. JAMA Netw Open. 2023 May;6(5):e2313969. doi: https://doi.org/10.1001/jamanetworkopen.2023.13969
61. Amagasa S, Iwamoto S, Kashiura M, Yasuda H, Kishihara Y, Uematsu S, et al. Early versus late advanced airway management for adult patients with out-of-hospital cardiac arrest: A time-dependent propensity score-matched analysis. Acad Emerg Med. 2024 Aug;31(8):755–66.
62. Gabbott DA, Beringer R. The iGEL supraglottic airway: a potential role for resuscitation? Resuscitation. 2007 Apr;73(1):161–2. doi: https://doi.org/10.1016/j.resuscitation.2006.10.026
63. Nakanishi T, Sakamoto S, Yoshimura M, Fujiwara K, Toriumi T. Learning curve of i-gel insertion in novices using a cumulative sum analysis. Sci Rep. 2023 May;13(1):7121. doi: https://doi.org/10.1038/s41598-023-34152-5
64. Cashen K, Sutton RM, Reeder RW, Ahmed T, Bell MJ, Berg RA, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatrics Critical Care Research Network (CPCCRN); National Heart Lung and Blood Institute ICU-RESUScitation Project Investigators. Association of CPR simulation program characteristics with simulated and actual performance during paediatric in-hospital cardiac arrest. Resuscitation. 2023 Oct;191:109939. doi: https://doi.org/10.1016/j.resuscitation.2023.109939
65. O’Connell KJ, Dutta A, Myers S, Neubrand T, Sandler A, Keane R, et al. Association between the presence of an advanced airway and ventilation rate during pediatric CPR: A report from the Videography in Pediatric Resuscitation (VIPER) collaborative. Resuscitation. 2023 Oct;191:109923. doi: https://doi.org/10.1016/j.resuscitation.2023.109923
66. Hansen ML, Lin A, Eriksson C, Daya M, McNally B, Fu R, et al.; CARES surveillance group. A comparison of pediatric airway management techniques during out-of-hospital cardiac arrest using the CARES database. Resuscitation. 2017 Nov;120:51–6. doi: https://doi.org/10.1016/j.resuscitation.2017.08.015
67. Greif R, Bray JE, Djärv T, Drennan IR, Liley HG, Ng KC, et al. 2024 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces. Circulation. 2024 Dec;150(24):e580–687. doi: https://doi.org/10.1161/CIR.0000000000001288
68. Garcia-Marcinkiewicz AG, Lee LK, Haydar B, Fiadjoe JE, Matava CT, Kovatsis PG, et al.; PeDI Collaborative. Difficult or impossible facemask ventilation in children with difficult tracheal intubation: a retrospective analysis of the PeDI registry. Br J Anaesth. 2023 Jul;131(1):178–87. doi: https://doi.org/10.1016/j.bja.2023.02.035
69. Fukuda T, Sekiguchi H, Taira T, Hashizume N, Kitamura Y, Terada T, et al. Type of advanced airway and survival after pediatric out-of-hospital cardiac arrest. Resuscitation. 2020 May;150:145–53. doi: https://doi.org/10.1016/j.resuscitation.2020.02.005
70. Niles DE, Duval-Arnould J, Skellett S, Knight L, Su F, Raymond TT, et al.; pediatric Resuscitation Quality (pediRES-Q) Collaborative Investigators. Characterization of Pediatric In-Hospital Cardiopulmonary Resuscitation Quality Metrics Across an International Resuscitation Collaborative. Pediatr Crit Care Med. 2018 May;19(5):421–32. doi: https://doi.org/10.1097/PCC.0000000000001520
71. O’Connell KJ, Sandler A, Dutta A, Ahmed R, Neubrand T, Myers S, et al. The effect of hand position on chest compression quality during CPR in young children: Findings from the Videography in Pediatric Resuscitation (VIPER) collaborative. Resuscitation. 2023 Apr;185:109741. doi: https://doi.org/10.1016/j.resuscitation.2023.109741
72. Ivankovic J, Bahr N, Meckler GD, Hansen M, Eriksson C, Guise JM. Identifying high cognitive load activities during simulated pediatric cardiac arrest using functional near-infrared spectroscopy. Resusc Plus. 2023 Jun;14:100409. doi: https://doi.org/10.1016/j.resplu.2023.100409
73. Campbell JP, Maxey VA, Watson WA. Hawthorne effect: implications for prehospital research. Ann Emerg Med. 1995 Nov;26(5):590–4. doi: https://doi.org/10.1016/S0196-0644(95)70009-9