Evinacumab for the treatment of homozygous familial hypercholesterolaemia: first patient
case report in Switzerland
DOI: https://doi.org/https://doi.org/10.57187/s.4024
Noé
Corpatauxa,
Fabienne Areggerb,
Konstantinos
C. Koskinasa,
Catherine
Gebharda
a Department
of Cardiology, Inselspital, Bern University Hospital and
University of Bern, Bern, Switzerland
b Department of Nephrology and Hypertension,
Inselspital, Bern University Hospital and University of Bern, Bern, Switzerland
Summary
We present the first case in Switzerland of a
patient with homozygous familial hypercholesterolaemia treated with evinacumab,
a new recombinant human monoclonal antibody currently approved in Europe and in
the USA but not yet in Switzerland. Homozygous familial hypercholesterolaemia
is a rare genetic disorder that causes severely elevated levels of low-density
lipoprotein (LDL) cholesterol and early atherosclerotic cardiovascular disease,
which, if left untreated, can lead to premature death. As a result of this newly
introduced treatment, the patient’s LDL cholesterol levels were reduced by more
than half, achieving recommended target values of secondary prevention for the
first time. This case underscores the efficacy of evinacumab in achieving LDL
cholesterol targets in homozygous familial hypercholesterolaemia patients and
highlights the importance of early identification and treatment initiation.
Introduction
Homozygous
familial hypercholesterolaemia (HoFH) is a very rare, autosomal, semi-dominant
disease caused mostly by two mutant alleles of the LDL receptor gene (LDLR) [1]. While the more prevalent heterozygous
familial hypercholesterolaemia affects about 1 in 250 individuals, it is
estimated that homozygous familial hypercholesterolaemia impacts approximately
1 in 300,000 individuals worldwide [2, 3].
Homozygous familial hypercholesterolaemia is characterised by substantially elevated
levels of low-density lipoprotein cholesterol (LDL-C) ranging from 10 mmol/l to
25 mmol/l from birth [4]. This leads to
accelerated development of early-onset atherosclerotic cardiovascular disease,
often resulting in premature mortality [2].
In addition
to lifestyle changes and patient education, early treatment with a potent
lipid-lowering therapy, typically consisting of a high-dose statin combined
with ezetimibe, is the cornerstone treatment for every homozygous familial
hypercholesterolaemia patient. In cases where the LDL cholesterol target (<1.8
mmol/l or <1.4 mmol/l for patients with additional atherosclerotic
cardiovascular disease risk factors or confirmed atherosclerotic cardiovascular
disease) is not achieved [5], treatment targeted
at proprotein convertase subtilisin / kexin type 9 (PCSK9) is an additional
therapeutic option. However, the efficacy of treatment with a PCSK9 inhibitor
depends on the level of residual LDL receptor activity [2] with a mean LDL cholesterol
reduction of about 20% in the homozygous
familial hypercholesterolaemia population [6, 7].
In Switzerland, weekly lipoprotein apheresis, combined with comprehensive
lipid-lowering therapy, remains the most effective strategy for managing LDL
cholesterol levels in these patients.
Evinacumab,
a recombinant human monoclonal antibody administered intravenously on a monthly
basis, targets angiopoietin-like protein 3 (ANGPTL3), a liver-secreted protein
that increases plasma levels of triglycerides, LDL cholesterol and high-density
lipoprotein (HDL). By inhibiting ANGPTL3, evinacumab significantly lowers LDL
cholesterol levels, independently of LDL receptor functionality [8]. On the basis
of the results of the phase 3
trial ELIPSE HoFH (Evinacumab Lipid Studies in Patients with Homozygous
Familial Hypercholesterolemia) [9], it was
approved both in the USA and in Europe in 2021 for the treatment of patients
with homozygous familial hypercholesterolaemia. ELIPSE HoFH demonstrated a
47.1% reduction in LDL cholesterol levels in patients with homozygous familial
hypercholesterolaemia who received evinacumab, corresponding to an absolute
decrease of approximately 3.5 mmol/l, irrespective of their LDL receptor
function. Additionally, the study by Rosenson et al. reported a sustained
reduction in LDL cholesterol levels over 72 weeks, with no safety concerns
observed [10]. Currently, evinacumab is
not approved in Switzerland and is only available under very strict and
controlled conditions through article 71c of the Ordinance on Health Insurance (KVV).
This article allows for the reimbursement of the cost of medicinal
products in individual cases, even if they are not included in the list of
pharmaceutical specialties [11].
In this
case report, we present the clinical data of the first patient in Switzerland
treated with evinacumab.
Case description
A 42-year-old
man with homozygous familial hypercholesterolaemia and a confirmed homozygous LDLR mutation experienced
significant progression of his atherosclerotic cardiovascular disease over the
years, despite receiving maximum lipid-lowering therapy. At the age of 11 years,
he underwent his first percutaneous coronary intervention (PCI) for a high-grade
stenosis of the left main coronary artery. Since then, he has been treated with
high-intensity statins, ezetimibe and weekly apheresis. In 2021, aged 39, he
underwent triple coronary bypass surgery and replacement of the ascending aorta
because of progressive coronary artery disease (figure 1) and a highly
calcified aortic root. Two years later, in 2023, he experienced a myocardial
infarction caused by an acute occlusion of the right coronary artery (saphenous
vein graft chronically occluded). Simultaneously, a computed tomography
angiography scan demonstrated diffuse peripheral arterial occlusive disease
with atheromatous infiltration in the ilio-femoral region (figure 2) as well as
bilateral atheromatous infiltration of the carotids, indicating systemic polyvascular
atherosclerotic infiltration.
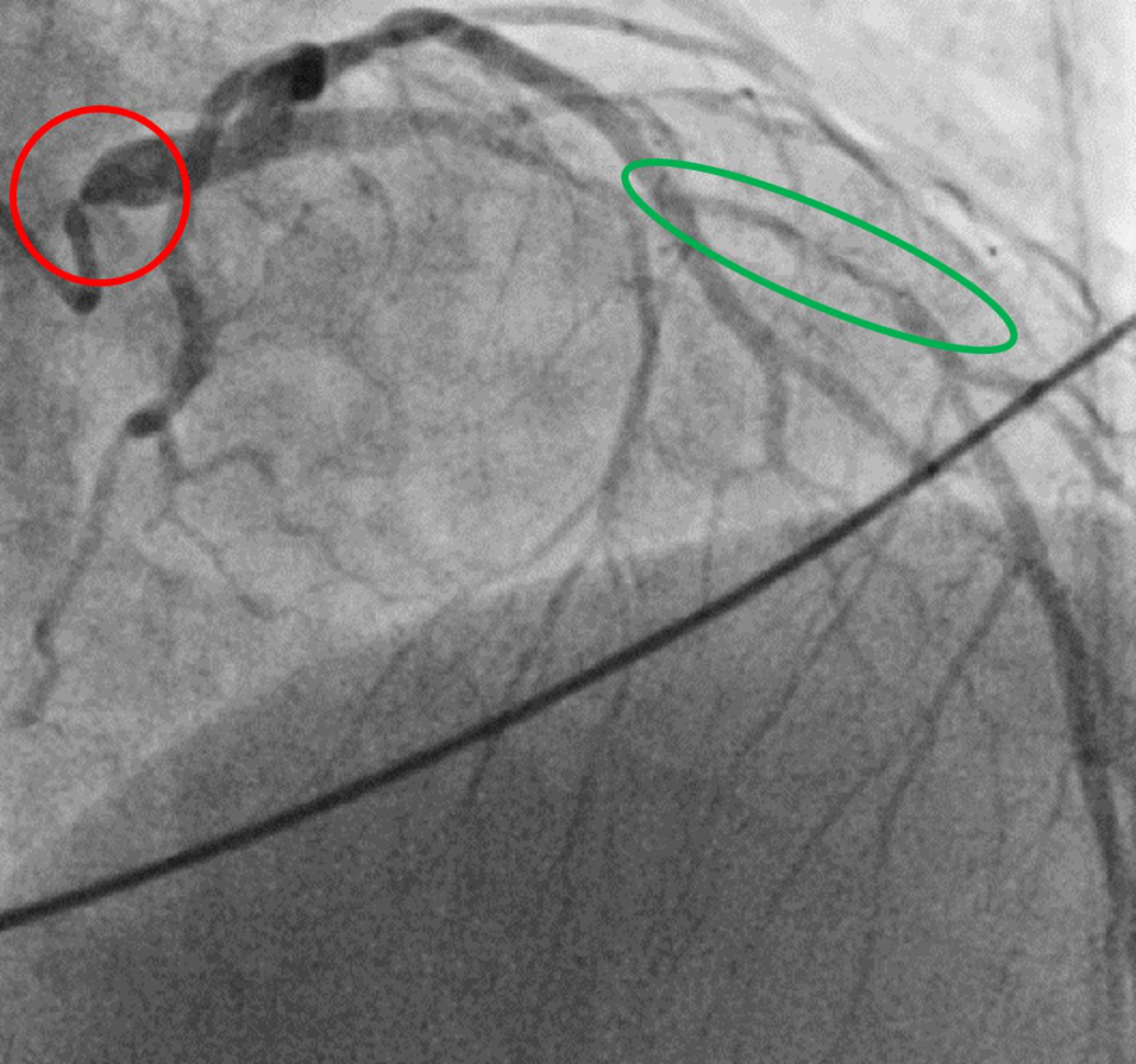
Figure 1Coronary angiogram. Diagnostic coronary angiogram showing an ostial subtotal
left main stenosis (red circle) as well as significant stenosis in the mid left
anterior descending artery (green circle) before coronary artery bypass surgery
in 2021 (age 39 years).
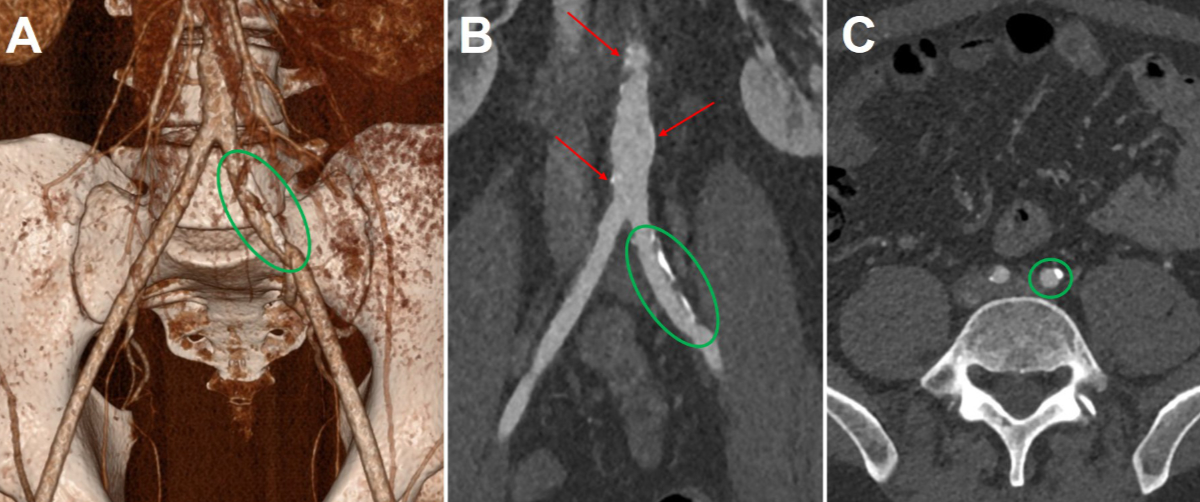
Figure 2Computed
tomography angiography. 3D reconstruction (A), coronal view (B) and axial view
(C) demonstrating atheromatous infiltration in the descending aorta (red
arrows) and in the two common iliac arteries (green circles) in 2023 (age 40 years).
Despite
receiving maximal lipid-lowering therapy, including the addition of a PCSK9
inhibitor by the end of 2023 which resulted in an additional ~10% reduction in
LDL cholesterol, mean LDL cholesterol levels ranged from 6.0 to 8.0 mmol/l before
weekly apheresis and 3.0 to 4.5 mmol/l post-apheresis.
Following
approval from the health insurance provider for a 6-month trial period, the
patient received the initial dose of evinacumab in April 2024, with the next dose
administered one month later. Both doses were delivered at the standard regimen
of 15 mg/kg. One week after the first dose, the LDL cholesterol value before
apheresis was 3.84 mmol/l decreasing to 0.76 mmol/l after apheresis. For the first
time in the patient’s medical history, an LDL cholesterol value within the
target range of <1.4 mmol/l was observed. After the second injection, which
is when the maximum effect is expected and a therapeutic plateau is reached, the
mean LDL cholesterol value before apheresis was 2.8 mmol/l and 0.6 mmol/l after
apheresis (−53% and −72%, respectively). A temporal plot of LDL values is
provided in figure 3. No significant changes in triglyceride or lipoprotein(a)
levels were observed after treatment initiation, with both values remaining
within the normal range prior to starting therapy. Consistent with the findings
in the ELIPSE HoFH trial, the treatment has been well tolerated by the patient,
with no reported side effects or safety concerns. Blood tests conducted
regularly during the weekly apheresis have shown no unusual findings since the
start of the treatment.
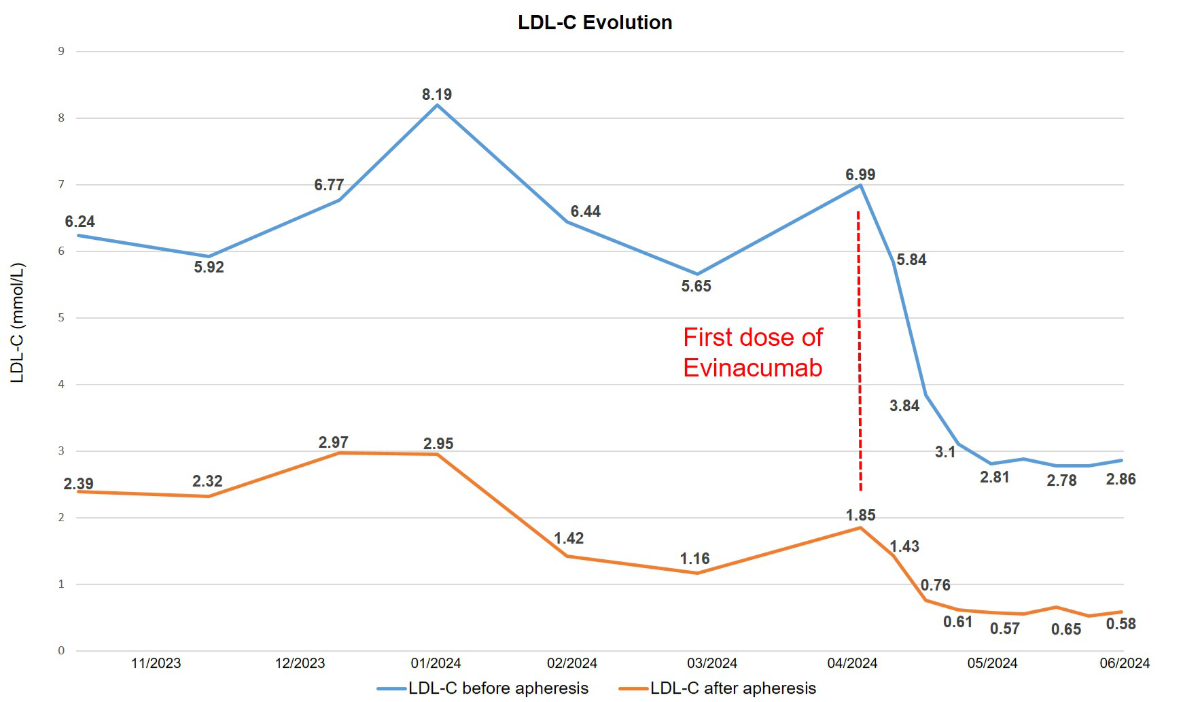
Figure 3Temporal evolution of low-density lipoprotein cholesterol (LDL-C) level (in mmol/l)
before and after initiation of evinacumab treatment during weekly apheresis.
Discussion
From the patient’s
perspective, despite the significant efficacy of the new treatment, the lowest
mean LDL cholesterol level achieved, calculated using the Kroon method [12], was 2.03
mmol/l, which remains
substantially above the target of <1.4 mmol/l. This makes it impractical to
discontinue or extend the interval between weekly apheresis sessions. A longer
treatment duration and extended follow-up are essential to evaluate the
long-term clinical effects and safety of the treatment.
Homozygous
familial hypercholesterolaemia is a rare condition associated with premature
cardiovascular death and represents a major challenge for physicians. Despite
current therapeutic interventions with high-intensity statins, ezetimibe, a PCSK9
inhibitor and lipid apheresis, patients often struggle to achieve LDL
cholesterol goals and prevent progression of atherosclerotic cardiovascular
disease. Evinacumab is a highly effective new treatment that should be added to
the therapeutic arsenal for managing these patients. The synergy between these
different treatments is crucial in optimising LDL cholesterol control. This highlights
the potential for achieving the ambitious guideline-recommended target LDL
cholesterol values despite the extremely elevated baseline levels in this small
patient population, thus avoiding the need for liver transplantation, which may
be considered a last-resort strategy for patients with progressive disease
despite maximal lipid-lowering therapy [13].
Furthermore,
this case highlights the importance of early identification of patients with homozygous
familial hypercholesterolaemia and early initiation of intensive lipid-lowering
therapy in mitigating disease progression. Similar to diabetes or smoking, the
number of years of exposure to high LDL cholesterol levels significantly
impacts the life expectancy of these patients. Early initiation of treatment in
childhood can prevent or delay the onset of atherosclerosis and its clinical manifestations
[13]. From a national perspective, establishing
a national registry in Switzerland could facilitate improved management
strategies and provide valuable insights into the utilisation of novel
therapies.
Informed consent
Written informed consent was obtained from the patient for the publication of this
article.
Dr. med. Noé Corpataux
Department of Cardiology
Bern University Hospital
University of Bern
CH-3010 Bern
noe.corpataux[at]insel.ch
References
1. Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al.; European
Atherosclerosis Society Consensus Panel on Familial Hypercholesterolaemia. Homozygous
familial hypercholesterolaemia: new insights and guidance for clinicians to improve
detection and clinical management. A position paper from the Consensus Panel on Familial
Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014 Aug;35(32):2146–57.
doi: https://doi.org/10.1093/eurheartj/ehu274
2. Stock J. EAS Consensus Panel statement on homozygous FH. Atherosclerosis. 2015 Sep;242(1):323–6.
doi: https://doi.org/10.1016/j.atherosclerosis.2015.06.050
3. Akioyamen LE, Genest J, Shan SD, Reel RL, Albaum JM, Chu A, et al. Estimating the
prevalence of heterozygous familial hypercholesterolaemia: a systematic review and
meta-analysis. BMJ Open. 2017 Sep;7(9):e016461. doi: https://doi.org/10.1136/bmjopen-2017-016461
4. France M, Rees A, Datta D, Thompson G, Capps N, Ferns G, et al.; for HEART UK Medical
Scientific and Research Committee. HEART UK statement on the management of homozygous
familial hypercholesterolaemia in the United Kingdom. Atherosclerosis. 2016 Dec;255:128–39.
doi: https://doi.org/10.1016/j.atherosclerosis.2016.10.017
5. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al.; ESC Scientific
Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid
modification to reduce cardiovascular risk. Eur Heart J. 2020 Jan;41(1):111–88. doi: https://doi.org/10.1093/eurheartj/ehz455
6. Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, et al.; TESLA Investigators.
Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA
Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015 Jan;385(9965):341–50.
doi: https://doi.org/10.1016/S0140-6736(14)61374-X
7. Santos RD, Stein EA, Hovingh GK, Blom DJ, Soran H, Watts GF, et al. Long-Term Evolocumab
in Patients With Familial Hypercholesterolemia. J Am Coll Cardiol. 2020 Feb;75(6):565–74.
doi: https://doi.org/10.1016/j.jacc.2019.12.020
8. Gaudet D, Gipe DA, Pordy R, Ahmad Z, Cuchel M, Shah PK, et al. ANGPTL3 Inhibition
in Homozygous Familial Hypercholesterolemia. N Engl J Med. 2017 Jul;377(3):296–7.
doi: https://doi.org/10.1056/NEJMc1705994
9. Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJ, Rubba P, et al.; ELIPSE
HoFH Investigators. Evinacumab for Homozygous Familial Hypercholesterolemia. N Engl
J Med. 2020 Aug;383(8):711–20. doi: https://doi.org/10.1056/NEJMoa2004215
10. Rosenson RS, Burgess LJ, Ebenbichler CF, Baum SJ, Stroes ES, Ali S, et al. Longer-Term
Efficacy and Safety of Evinacumab in Patients With Refractory Hypercholesterolemia.
JAMA Cardiol. 2023 Nov;8(11):1070–6. doi: https://doi.org/10.1001/jamacardio.2023.2921
11. Reimbursement for the cost of medical products in individual cases. Federal Office
of Public Health (BAG). 12.6.2024. Available from: https://www.bag.admin.ch/bag/en/home/versicherungen/krankenversicherung/krankenversicherung-leistungen-tarife/Arzneimittel/verguetung-arzneimittel-im-einzelfall.html
12. Kroon AA, van’t Hof MA, Demacker PN, Stalenhoef AF. The rebound of lipoproteins after
LDL-apheresis. Kinetics and estimation of mean lipoprotein levels. Atherosclerosis.
2000 Oct;152(2):519–26. doi: https://doi.org/10.1016/S0021-9150(00)00371-3
13. Cuchel M, Raal FJ, Hegele RA, Al-Rasadi K, Arca M, Averna M, et al. 2023 Update on
European Atherosclerosis Society Consensus Statement on Homozygous Familial Hypercholesterolaemia:
new treatments and clinical guidance. Eur Heart J. 2023 Jul;44(25):2277–91. doi: https://doi.org/10.1093/eurheartj/ehad197


