Conference report: Trends, new technologies and implications for dementia diagnostics,
treatment and care in Switzerland
DOI: https://doi.org/https://doi.org/10.57187/s.4017
Julius Poppab,
Reto W. Kressigcd,
Mélanie
Bieler-Aeschlimanne,
Miriam Rabla,
Marcello
Iencafg,
Andreas U. Monschh,
Hans Pihani,
Stefan Klöppelj,
Tatjana Meyer-Heimk,
Stefanie
Beckerl
a Department of Adult Psychiatry and
Psychotherapy, Psychiatric University Hospital Zurich and University of Zurich,
Zurich, Switzerland
b Department of Old Age Psychiatry, University
Hospital of Lausanne, Lausanne, Switzerland
c University Department of Geriatric
Medicine Felix Platter, Basel, Switzerland
d University of Basel, Basel,
Switzerland
e Leenaards Memory Centre, Department
of Clinical Neurosciences, and Infections Disease Service, University Hospital
of Lausanne (CHUV), Lausanne, Switzerland
f Institute for History and Ethics of
Medicine, School of Medicine and Health, Technical University of Munich,
Munich, Germany
g College of Humanities, Swiss Federal
Institute of Technology in Lausanne, Lausanne, Switzerland
h Faculty of Psychology, University of
Basel, Basel, Switzerland
i Neurology Clinic and Memory Clinic,
Biel Hospital Centre, Biel, Switzerland
j University Hospital of Old Age Psychiatry and
Psychotherapy, University of Bern, Bern, Switzerland
k Zurichs Municipal Hospital, Waid, University
Geriatric Clinic, Zurich, Switzerland
l Alzheimer's Switzerland, Yverdon-les-Bains, Switzerland
Summary
Dementia
diseases represent a major burden for the directly affected people, their
relatives and modern society. Despite considerable efforts in recent years,
early and accurate disease diagnosis and monitoring is still a challenge while
no cure is available in most cases. New drugs, in particular disease-modifying
therapies, and recent technological advancements offer promising perspectives.
The integration of novel biomarkers, artificial intelligence and digital health
tools has the potential to transform dementia care, making it more personalised,
efficient and adapted to the living conditions and needs of older people. In
November 2023, the 7th Dementia Summit convened a panel of experts from
geriatrics, neurology, neuropsychology, psychiatry, ethics as well as general
medicine to discuss interdisciplinary challenges, advancements and their
implications for the future of dementia care in Switzerland. The conference
underscored the importance of a multidisciplinary approach to successfully
integrate new technologies in both clinical-translational research and dementia
prevention, diagnosis and care. While recent innovations represent major steps
forward, their implementation also comes with important challenges including
questions on healthcare system preparedness and adaptation, ethical aspects,
technology literacy, acceptance and appropriate use.
Introduction
Dementia
presents significant challenges to both the directly affected people, their
relatives and the healthcare system. Currently, around 156,900 people live with
dementia in Switzerland, with 33,800 new cases annually [1]. However, early and accurate
diagnosis is still a challenge,
and a sizeable proportion of people affected by dementia remains undiagnosed. Relevant
aspects such as neuropsychiatric symptom detection, monitoring and specific
treatment are often not addressed. Innovative approaches to diagnostics, prevention,
treatment and care are urgently needed. New drugs, in particular disease-modifying
therapies, and recent technological advancements offer promising perspectives.
The integration of novel biomarkers, artificial intelligence and digital health
tools has the potential to transform dementia care, making it more personalised,
efficient and adapted to the living conditions and needs of older people.
Implementing new technologies and multidomain prevention and treatment interventions
is essential for reducing the symptom burden and improving function – ultimately
enhancing the quality of life of dementia patients – and for supporting their
caregivers. However, important questions related to validation, costs,
acceptance and technology literacy need to be addressed. The 2023 Dementia
Summit brought together a panel of experts to discuss these advancements and
their implications for the future of dementia care in Switzerland. The two-day conference
addressed recent progress
and innovation in different fields with high clinical relevance including
nutrition and multidomain non-pharmacological interventions for the prevention
and early treatment of cognitive disorders, but also the development of
biomarkers for the early prediction and treatment of persisting
neuropsychiatric symptoms (day 1). A further important topic was the ethics of use
of innovative approaches and new technologies. Day 2 first focused on progress in
neuropsychological and biomarker diagnosis, then addressed the opportunities
and challenges related to the upcoming anti-amyloid drugs, and digital and
robotic technologies in the hospital and at the patient’s home. Finally, the
importance of literacy and attitudes of patients and caregivers regarding innovations
and new technologies was highlighted.
Role of nutrition
in prevention and treatment of cognitive decline in older people
Reto W. Kressig
A healthy
diet has potential in preserving brain and cognitive health. Depending on
adherence, the Mediterranean-DASH Diet Intervention for Neurodegenerative
Delay (MIND diet) has been shown to be possibly associated with improved
cognitive function in older adults [2]. The
MIND diet recommends nuts, leafy green and other vegetables, berries, beans,
whole grains, fish and poultry, olive oil and wine as cognitively healthy
components, whereas red meat, butter, pastries and sweets, fast food and fatty
cheese are considered unhealthy. As a possible mechanistic model for the MIND
diet-cognitive relationship, the healthy diet components are supposed to
decrease brain inflammation and brain oxidative stress by their
anti-inflammatory and anti-oxidant properties. In the FINGER study [3], in which general
lifestyle changes in
regard to physical and cognitive activity also included the MIND diet as a specific
brain-protective nutritional intervention, participants showed improvements in
several cognitive domains after two years, whereas the most recent MIND diet
single intervention trial for prevention in cognitive decline among older
persons did not reveal any cognitive benefits after three years [4]. Not surprisingly,
the MIND diet as a single
intervention for preservation of cognitive health seems significantly empowered
when combined with other healthy interventions such as exercise, cognitive
training and strict monitoring of vascular risk factors.
Another, more recent strategy to prevent and manage neurodegenerative
disease is to modify the diet and microbiota [5].
Published evidence indicates that fermented foods, including kefir, and foods
that are high in bioactive polyphenols and complex carbohydrates, such as
grapes, pomegranates and seaweed, may be effective at reducing
neuroinflammation, oxidative stress, neurotransmitter dysfunction, and neuronal
death associated with Alzheimer’s and Parkinson’s diseases. A phase 3 clinical
trial in China with a drug derived from marine brown algae, a seaweed that is supposed
to recondition dysbiosis of gut microbiota showed significant
cognitive benefits in patients with mild-to-moderate Alzheimer’s dementia after
36 weeks [6]. The same drug is currently being
tested for motor improvements in phase 2 studies among patients with
Parkinson’s disease.
Recently,
ketogenic therapies have been tested in randomised controlled trials, focusing
on delaying disease progression and ameliorating cognitive function [7]. It is hypothesised
that the brain energy
gap, created by worsened glucose metabolism in early cognitive decline, is covered
by the only alternative brain energy source: keto bodies. In general, the
ketogenic diet (sometimes shortened to “keto diet”) is a low-carbohydrate,
high-fat diet that focuses on reducing carbs and increasing fats to encourage
the body to enter a state of ketosis. In ketosis, the body uses fat as its
primary energy source instead of carbohydrates. Study interventions were heterogeneous,
acute or long-term (45–180 d),
including adherence to a ketogenic diet, intake of ready-to-consume drinks,
medium-chain triglyceride powder for drinks preparation, yoghurt enriched with medium-chain
triglycerides, medium-chain triglyceride capsules and ketogenic formulas/meals.
The use of isolated ketoneurotherapeutics in combination with continued
habitual eating patterns proved effective in improving general cognition using
the Alzheimer’s Disease Assessment Scale-Cognitive, in interventions of either
duration. In a six-month randomised controlled trial of ketogenic medium-chain
triglyceride versus placebo in patients with mild cognitive impairment, the ketogenic
medium-chain triglyceride drink improved three cognitive domains – executive
function, memory and language [8]. Although research on the subject is still in the
early stages and highly
heterogeneous in terms of study design, interventions and outcome measures,
ketogenic therapy appears promising in improving both acute and long-term
cognition among patients with Alzheimer’s disease and mild cognitive
impairment.
For clinicians, it is a most common phenomenon that accelerated weight
loss may precede diagnosis in Alzheimer’s disease [9]. This weight loss is mainly
due to a decrease in lean body mass
possibly leading to sarcopenia. The most recent data confirm that intramuscular adipose
tissue [3] – often seen in sarcopenia – may predict cognitive decline over the
next six years, independent of overall adiposity or muscle health [10]. This disease-related
muscle loss
underlines the increased protein need among patients with dementia [11], which seems
to be best prevented early on
by leucine-enriched whey protein supplementation [12].
Dementia
prevention with multidomain non-pharmacological interventions
Mélanie
Bieler-Aeschlimann
The
demographic evolution of Switzerland and the number of elderly people affected
by neurodegenerative disease are quite alarming and require the implementation
of risk-reduction strategies before reaching the stage of dementia [13]. Among people
referred to memory clinics, about one
third report subjective cognitive complaints, i.e. complaints without objective
cognitive impairment in neuropsychological tests. These people have an
increased risk of developing a neurodegenerative disease and there is growing evidence
that targeted interventions can have a positive influence on the ageing
cognitive functions of these individuals [3].
Relying on modifiable lifestyle factors [14]
currently appears to be an excellent and largely available opportunity for
seniors and especially for those with subjective cognitive complaints. Non-pharmacological
interventions aim to strengthen brain and cognitive reserves, i.e. optimise
structural and functional brain networks by training and/or adopting virtuous behaviours.
Multidomain interventions seem promising: they act on several modifiable risk
factors at once and optimise brain protection [15,
16]. While everyone can be recommended to follow a Mediterranean diet
and stay active, the type of cognitive training recommended depends on the
individual patient’s cognitive profile.
Primary
prevention targets older people in good cognitive health and proposes a programme
designed to keep them cognitively fit. For those with subjective cognitive
complaints or mild cognitive impairment, we rely on symptoms and apply the
principles of secondary prevention, proposing two types of approach: a
restorative approach to train attention and executive functions (such as
inhibition), and a compensatory approach based on strategies to improve (memory)
performances. Improving metacognition seems particularly appropriate for patients
with subjective cognitive complaints [17].
New technologies can assist in training cognition, but their efficiency depends
on whether they rely on both neuroscience models and therapeutic knowledge [18]. The
people who stand to benefit most from
non-pharmacological interventions are the frail or pre-frail older adults. At
the Leenaards Memory Centre, CHUV, Lausanne (CLM) we have set up three specific
projects to support these populations.
Firstly, in
a randomised controlled trial, we showed that a multidomain intervention
containing a digitised programme of cognitive and physical activities, enhanced
by motivational factors, and reinforcing social cohesion between users,
improved overall cognition and information processing speed in pre-frail
participants [19, 20]. This intervention
was carried out at home and gave participants a certain degree of autonomy to
choose their training programme. This study therefore demonstrates both the
feasibility and the efficacy of a digitised home-based training programme,
based on sound neuropsychological concepts.
Secondly,
as part of CareMENS, a programme supported by the “Promotion Santé Suisse”
foundation, the Leenaards Memory Centre has been offering nosognosic memory
clinic patients the opportunity to undergo non-pharmacological interventions.
To maintain the intervention’s quality of life improvements [21], patients are then
accompanied by a
Care Manager to start a community leisure activity. Non-pharmacological
interventions (neuropsychological, speech and/or physiotherapeutic therapy) are
proposed to patients with subjective cognitive complaints or mild cognitive
impairment. Over 1000 patients from all over French-speaking Switzerland have
been referred to us so far, and more than half of them have joined the CareMENS
programme, which has been yet extended to most memory centres in French-speaking
cantons.
Thirdly,
numerous young patients with Long COVID symptoms referred to the Leenaards
Memory Centre first went through a rigorous multidisciplinary assessment, then
were offered a 5-session holistic neuropsychological intervention. The symptoms
of Long COVID on three cognitive domains (memory, attention and executive
functions) were explained. Basic information on current research and the impact
of symptoms such as fatigue, stress and sleep disorders on daily, professional
and social life were also addressed in two sessions. As Long COVID patients in
most cases have subjective cognitive complaints, the intervention is based on
psychoeducation to reinforce patients’ metacognitive skills and provide them
with a large range of tools to help them better understand and overcome their
difficulties [22]. Further investigations
are needed to explore the subjective benefit of the intervention.
In a
nutshell, non-pharmacological interventions enable patients to become active agents
in their
own health and should be recommended in practice, and even reimbursed by our
healthcare system.
Multimodal
biomarkers of neuropsychiatric symptoms
Miriam Rabl
Neuropsychiatric
symptoms such as depression, apathy, agitation or hallucinations are very frequent
in older people, with a prevalence of up to 97% in people with dementia [23]. The
presence of neuropsychiatric symptoms
is the most common reason why patients with dementia are being admitted to
psychiatric hospitals. Also, having neuropsychiatric symptoms is associated
with worse outcomes such as more rapid cognitive decline and earlier death [24–26].
Little is
known about the neurobiology underlying neuropsychiatric symptoms yet, and no
biomarkers to detect the underlying pathology in neuropsychiatric symptoms are
available. Neuropsychiatric symptoms may evolve as part of the core Alzheimer’s
disease biology and/or may be caused by environmental stress factors, or by
other still-unknown pathologies. Different aetiologies may often be present at
the same time and contribute to the clinical symptoms to a different extent. An
aetiological differentiation based on the clinical manifestation is generally not
possible. Also, no biomarkers for the detection of the pathologies underlying neuropsychiatric
symptoms are available to date.
Some
studies investigated the association between neuropsychiatric symptoms and
biomarkers of the Alzheimer’s disease core pathology (cerebrospinal fluid
levels of total tau, phosphorylated tau and amyloid beta) with inconsistent
findings [27]. Also, neuropsychiatric
symptoms seem to not be robustly associated with markers of neurodegeneration
like neurogranin, neurofilament light chain and GAP-43 [28, 29].
Using a
data-driven untargeted proteomics approach in a memory clinic cohort, our group
at the University of Zurich recently identified a panel of 15 plasma and 27 cerebrospinal
fluid biomarker candidates for neuropsychiatric symptoms. These biomarkers also
predicted long-term outcomes such as persistence of neuropsychiatric symptoms
and associated cognitive decline [30, 31].
When combining findings from cerebrospinal fluid and plasma with structural MRI
data, we found region-specific pathophysiological changes related to neuropsychiatric
symptoms (figure 1). The most common enriched biological pathways related to neuropsychiatric
symptoms were immune reaction, protein metabolism and haemostasis (Rabl et al.,
in preparation). Using a more targeted approach and quantifying 38 markers of inflammation
and vascular injury in paired blood and cerebrospinal fluid samples, we confirmed
that neuroinflammatory processes play an important role in neuropsychiatric
symptoms [32]. Further, we partially replicated
the findings in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort.
Different biomarker candidates were identified as being associated with neuropsychiatric
symptoms and future neuropsychiatric symptoms up to two years later. Specific
involved biological pathways were identified for the most common single
symptoms of neuropsychiatric symptoms, whereas again the immune system was
among the enriched pathways related to overall neuropsychiatric symptoms [33]. Overall,
we found evidence suggesting
that there are pathological changes underlying neuropsychiatric symptoms that
are distinct from the core Alzheimer’s disease pathology and independently
contribute to more rapid cognitive decline [30].
Easily
available biomarkers to detect neuropsychiatric symptoms and its underlying
pathology would be helpful for the early detection of patients at risk of worse
longitudinal outcomes such as persistence of neuropsychiatric symptoms over
time and more rapid cognitive decline. This may be helpful in a memory clinic
setting to provide personalised treatment recommendations. Also, knowing more
about the involved pathophysiological mechanisms of neuropsychiatric symptoms opens
the perspective for the development of new treatment targets. Nevertheless,
research on this topic is still at the beginning and additional research is
required before the results might be available for clinical application. Further
and more targeted investigations and validation studies in independent and
larger cohorts are needed.
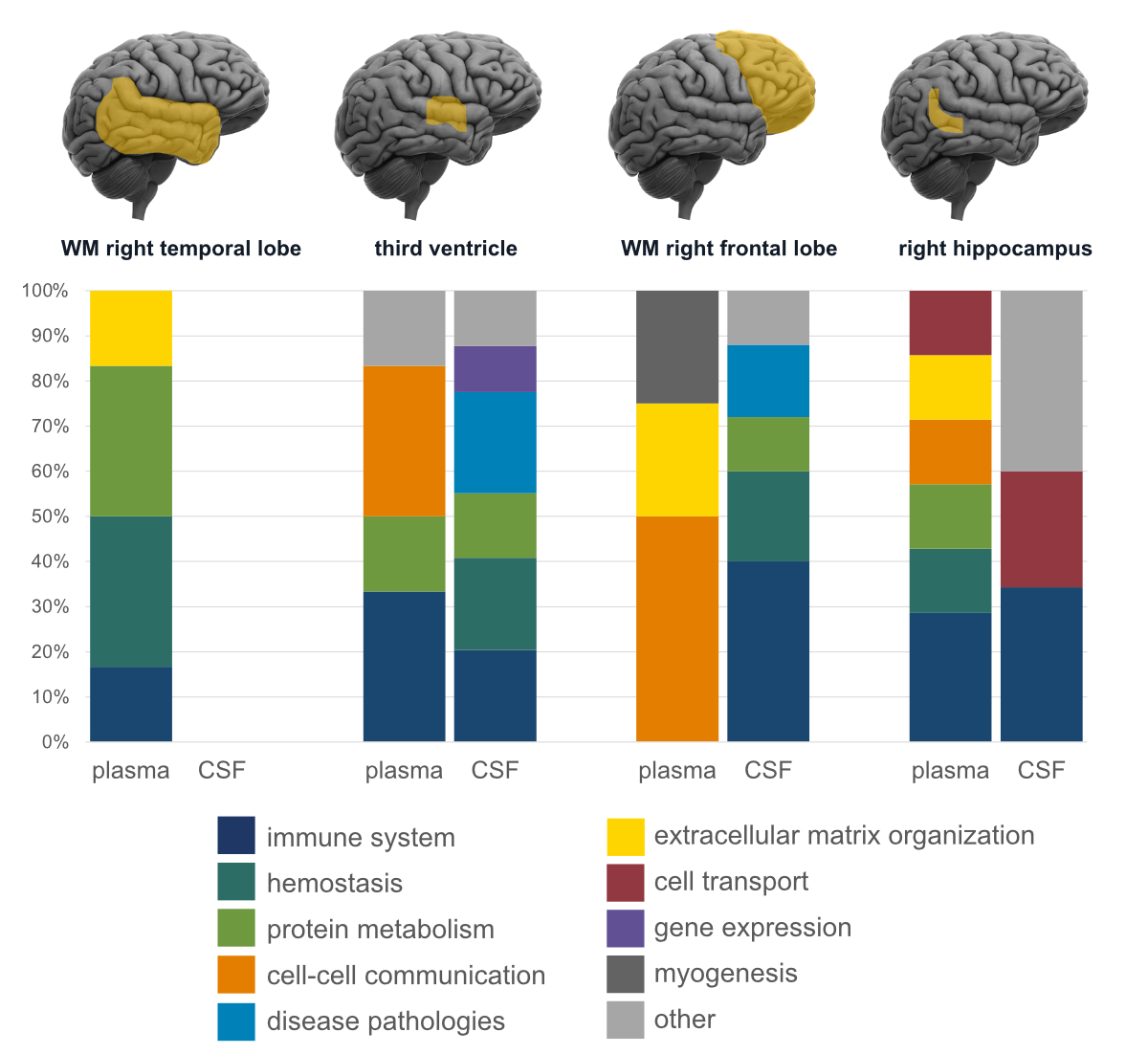
Figure 1Distribution
of enriched biological pathways (based on the Reactome database) of plasma and cerebrospinal
fluid (CSF) proteins (expressed as percentages) in four brain regions are
shown. The higher the percentage of enriched pathways of neuropsychiatric
symptoms (NPS), the stronger its association with atrophy in the respective
brain region. Regions and proteins were selected based on their associations
with NPS. WM: white matter.
Digital health and
artificial intelligence for dementia: Balancing potential with ethics
Marcello Ienca
As the
global population ages, dementia becomes increasingly prevalent, necessitating
innovative care approaches. Digital health, encompassing artificial
intelligence and Intelligent Assistive Technologies (IATs), offers
transformative potential [34, 35]. These
technologies can enhance the quality of life of dementia patients, reduce
healthcare costs and promote social inclusion [35,
36]. Artificial intelligence-driven tools like predictive analytics,
personalised care plans and automated monitoring systems exemplify how
technology can support dementia care.
The
integration of digital health technologies and artificial intelligence in
dementia care represents a transformative but ethically sensitive frontier.
Ethical considerations are especially pertinent as dementia patients are a
vulnerable group with unique needs. This section examines the ethical
dimensions of applying new technologies in dementia care, supported by concrete
examples to illustrate both the opportunities and the ethical challenges
involved. For instance, artificial intelligence-driven predictive analytics can
assist healthcare providers in forecasting disease progression, allowing for
timely and personalised interventions [37].
However, such predictive capabilities bring concerns about patient autonomy and
the potential for misinterpretation or misuse of predictive data [38]. Similarly,
wearable monitoring devices
can enhance patient safety by tracking movements and alerting caregivers to
falls or risky behaviours [39] yet these
devices may infringe upon privacy, raising questions about the balance between
patient safety and personal freedom [38].
Issues like
privacy, consent and data security are paramount, especially given the
vulnerable nature of dementia patients. Ethical design principles must guide
the development of these technologies, ensuring they respect human rights and
dignity [38]. Additionally, addressing
the digital divide is crucial to avoid exacerbating healthcare inequalities [40].
To ensure
ethical compliance and effectiveness, the development of digital health tools
must proactively involve stakeholders, particularly patients and caregivers [41, 42].
User-centred design principles can
help create solutions that are not only technically sound but also empathic and
practical [43]. Figure 2 provides a visual
overview of user-centred design for assistive digital systems for people with
dementia.
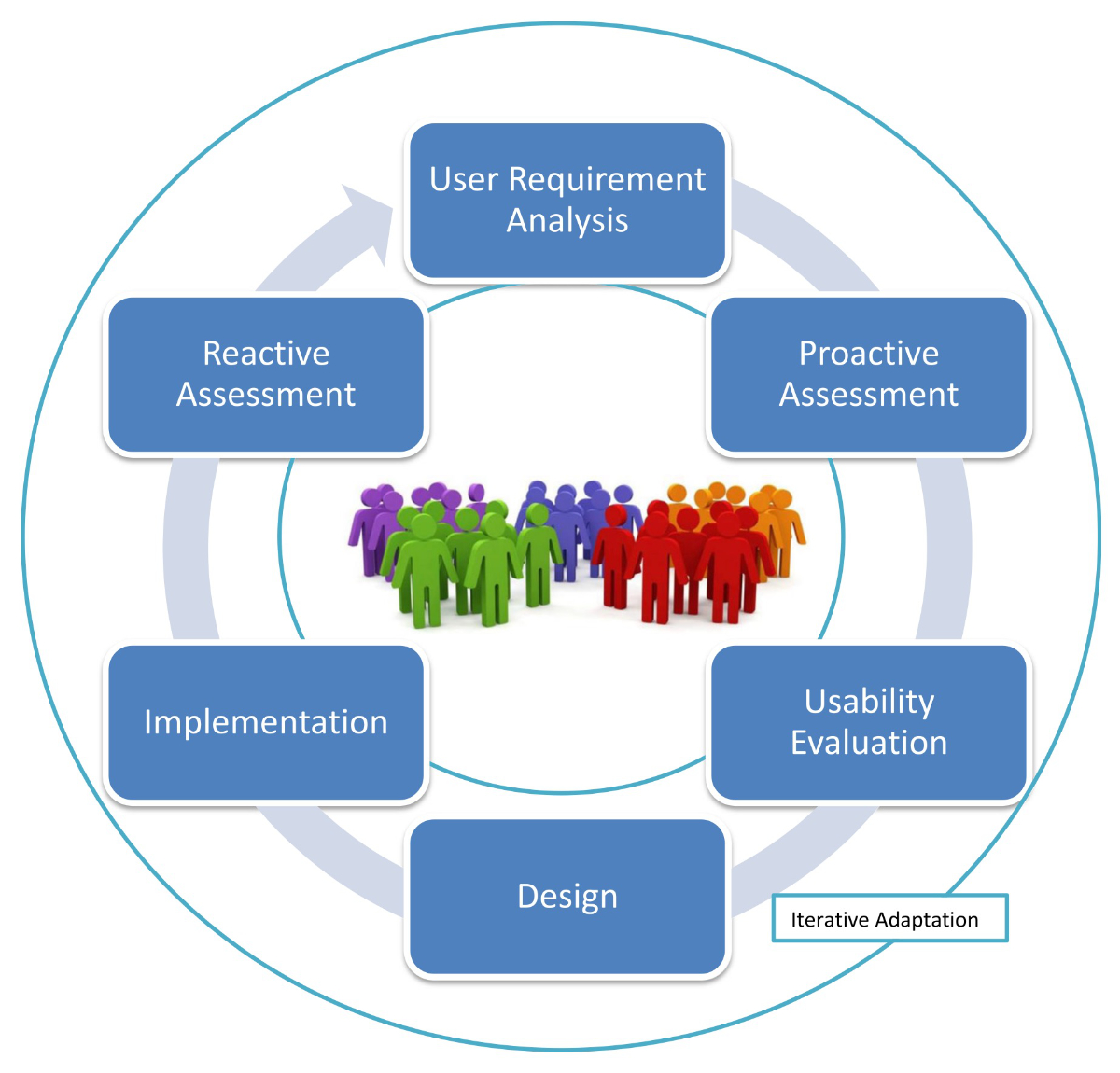
Figure 2Proactive user-centred design for assistive technologies. From: Ienca M, Kressig RW,
Jotterand F, Elger B. Proactive Ethical Design for Neuroengineering, Assistive and
Rehabilitation Technologies: the Cybathlon Lesson. J Neuroeng Rehabil. 2017 Nov;14(1):115
[84], https://doi.org/10.1186/s12984-017-0325-z, distributed
under the terms of the Creative Commons Attribution 4.0 International License
(http://creativecommons.org/licenses/by/4.0/).
Ethically aligned
innovation, which balances technological advancement with ethical
considerations, is critical in this domain. The integration of digital health
and artificial intelligence in dementia care holds immense promise for
improving patient outcomes and healthcare efficiency. However, this must be
navigated with a strong ethical compass. As we advance technologically, the
human aspect of care should remain at the forefront. Ethical considerations,
including privacy, consent and equity, must guide the development and
application of these technologies. By prioritising ethically aligned innovation
and user-centred design, we can harness the potential of artificial
intelligence and digital health to transform dementia care, making it more
effective, inclusive and humane.
Cognitive
assessment of neurocognitive disorders and new developments
Andreas U. Monsch
Current
neurocognitive assessment
The current
neuropsychological examination of brain disorders in older people focuses on
the criteria outlined in the Diagnostic and Statistical Manual of Mental
Disorders (DSM-5) [44] and requires the
assessment and classification of brain performance in the following six
domains: learning and memory, language, perceptual-motor function, executive
function, complex attention and social cognition. The examination often follows
a two-stage process of screening and actual, in-depth neuropsychological
examination. The brief screening examination (i.e. less than 15 minutes) allows
for detecting possible brain disorders, must be performed by trained healthcare
providers and does not provide a definite diagnosis. The assessment is based on
normative data (with correction for age, education and sex) and has an
established sensitivity and specificity. It provides guidance for the more
detailed neuropsychological assessment.
This
neuropsychological assessment evaluates all six cognitive domains and allows
for a specific diagnosis based on the cognitive deficit profile (figure 3, [45]).
However, it takes several hours and can
only be carried out competently by certified neuropsychologists. The results
are not only quantitative, but also qualitative in nature. The
neuropsychological examination makes an important contribution to finding the
cause of the brain disorder and is an important basis for treating patients and
providing counselling for their relatives.
Strengths and
weaknesses of the current neuropsychological assessment
The
strength of the current, paper-pencil-based, neuropsychological assessment
method encompasses the availability of extensive norms and knowledge of the
influence of demographic factors (age, education, sex). Weaknesses are that
single test scores rarely reflect a specific single brain activity; thus,
interpretation is demanding. Moreover, the field of neuropsychology is
methodologically rather weak with respect to meaningfully assessing change.
Most likely, the currently available diagnostic criteria (ICD-11, DSM-5, etc.)
reflect rather poorly the complexity of human brain function. Also, our brains
usually work in everyday life, not only in a well-controlled laboratory
setting.
Clearly,
new development to better understand, assess and diagnose human brain functions
is needed.
New developments in
the neuropsychology of dementia
The
development of an improved neuropsychological assessment must adopt findings
from neuroscience research and integrate them into its framework. Moreover,
neurocognitive processes must be understood within the everyday environment.
And, lastly, newly available tools and sophisticated analytical methods must
find their way into a new and improved methodology. New digital technologies
offer tremendous potential for shifting from traditional face-to-face
paper-pencil-based neuropsychological assessments to e.g. smartphone-based and
thus remote gathering of data in an everyday environment. Several new “toys”
have become available to serve this endeavour [46].
One such example is the remote digital memory composite (RDMC) score from an
unsupervised remote cognitive assessment battery focusing on episodic memory
and long-term recall [47] Participants
perform cognitive assessments in a fully remote and unsupervised setting via a
smartphone app. In addition to new tools to collect (big) data from study
participants and patients, new analytical methods with artificial intelligence
using e.g. machine learning are becoming more and more available.
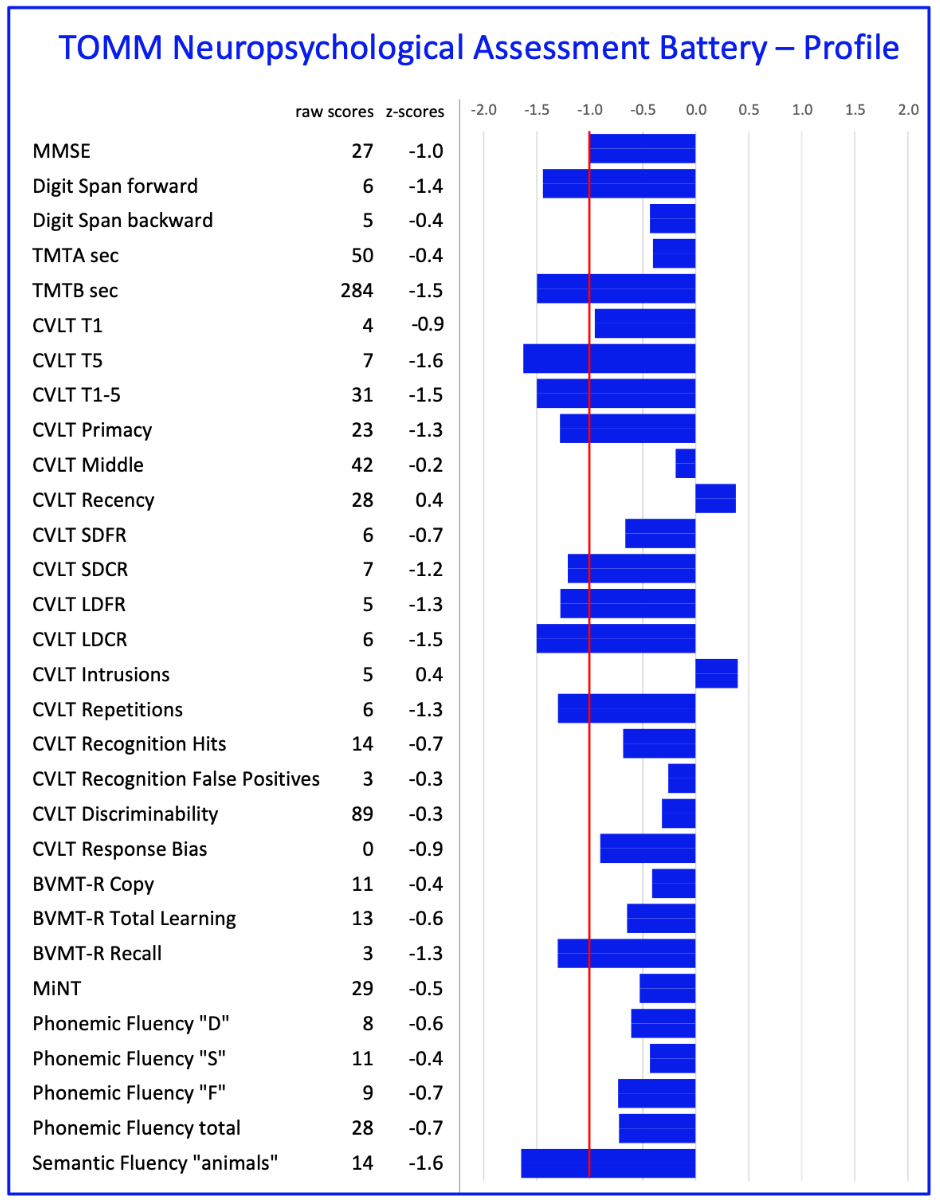
Figure 3Current
paper-pencil-based neuropsychological assessment profile. Example of a
comprehensive neuropsychological assessment using the TOMM-Neuropsychological
Assessment Battery [45]. Normative data
are based on 198 cognitively healthy adults. A cognitive profile is shown of a
patient with a mild neurocognitive disorder due to Alzheimer’s disease.
CVLT-II: California Verbal Learning Test; BVMT-R: Brief Visuospatial Memory
Test–Revised; TMT: Trail Making Test; MiNT: Multilingual Naming.
New biomarkers? – What
to expect
Julius Popp
Molecular
cerebrospinal fluid biomarkers in particular amyloid beta (Aβ)1–42, the Aβ1–42/Aβ1–40
ratio, tau and p-tau181 and amyloid-PET are
now established tools for the early and accurate detection of the specific
Alzheimer pathology. While they are both recommended by Swiss Memory Clinics and
covered by health insurance in Switzerland, these markers are still
restrictively used in clinical practice, mostly due to the relative
invasiveness of lumbar puncture and the costs and availability of amyloid-PET. The
use of biomarkers results in a change in the previous diagnosis and the management
plan in a large proportion of the investigated memory clinic patients [48, 49]. Accordingly,
these markers are now
increasingly often used as an additional investigation in the diagnostic work-up
of cognitive decline, including at the mild cognitive impairment stage [50, 51]. This
trend may be due to a growing
awareness about more personalised diagnosis and prognosis, and related
treatment and management options when first signs of cognitive decline occur. It
will likely be further accelerated by the upcoming anti-amyloid therapies which
require the clear detection of amyloid pathology along with clinical syndrome due
to cerebral Alzheimer’s disease pathology.
Current
research includes the identification of biomarker candidates for additional
clinically relevant aspects of the cerebral pathologies, such as
neuroinflammation and cholesterol metabolism [52,
53]. In addition, data-driven omics and integrative multi-omics are
deployed to both detect and better understand the involved biological pathways,
and to identify related biomarkers and intervention targets [54, 55].
Blood-based
biomarkers such as plasma p-tau indicating the presence of Alzheimer’s disease
pathology in the brain may be used to accurately identify those patients that
have a high or a low likelihood of having Alzheimer’s disease pathology in the
brain. Accordingly, only the remaining group of patients with inconclusive
results may require additional lumbar puncture for cerebrospinal fluid analysis
and/or PET to confirm or exclude Alzheimer’s disease [56]. If validated, this approach
will likely lead to a much
broader utilisation of biomarkers and make early and accurate diagnosis available
for a larger population while reducing costs for individual diagnostic workup.
This trend may also accelerate the development of effective and individually
tailored prevention and treatment interventions.
The use of
easily available, accurate and scalable biomarkers will also allow for repeated
measurements not only to confirm diagnosis but also to monitor the evolution of
relevant pathologies. This aspect may become particularly important when using disease-modifying
interventions including, but not limited to, the new anti-amyloid antibody
treatments [57]. Given the fact that the
development of brain pathologies leading to dementia, especially in Alzheimer’s
disease, start in most cases more than a decade before the onset of symptoms,
biomarkers may be deployed in the absence of any symptoms to identify
preclinical disease and assess the risk of cognitive decline [58]. So far, the use
of biomarkers is
currently not recommended neither in older people in the general population nor
in patients with subjective cognitive decline, i.e. cognitive complaints in the
absence of objective cognitive impairment according to standard cognitive
testing [50, 51]. However, we expect
blood-based biomarkers to be used in the near future as part of individual risk
profiling in people without any cognitive symptoms. Guidance and counselling
will be needed for the appropriate interpretation of the results.
New drug
approvals? What to expect
Hans Pihan
In July
2023, lecanemab received regular approval from the US Food and Drug
Administration and is expected to receive Swissmedic authorisation in 2024.
Following the controversial approval of aducanumab in 2021, there have been
many critical discussions in the specialist and lay press about the efficacy,
tolerability, side effects and costs of the new therapy. An exploratory prompt sent
to chatGPT on 9 November 2023 (“Negative expectations of
new Alzheimer’s therapies?”) showed that there are particular concerns about
long-term efficacy, side effects, patient safety, the cost-benefit ratio,
diagnostic status and a fundamentally poor understanding of the aetiological
factors.
The
long-awaited stabilisation of the disease with positive therapeutic effects on
the preservation of cognition and quality of life now appears to be within
reach. However, efficacy and safety still need to be substantiated with real-world
data.
Two
anti-amyloid beta antibodies will be available in the near future for the
treatment of Alzheimer’s disease. Both the Clarity study (lecanemab) and the
Trailblazer study (donanemab) showed positive results in terms of clinical
endpoints, with lecanemab reducing decline by 27% on the CDR-SB (Clinical Dementia
Rating - Sum of Boxes) and donanemab by 35% on the iADRS (integrated Alzheimer’s
Disease Rating Scale) [59, 60]. Limited
data from extension trials suggest that this effect may increase when treatment
is extended to two years or beyond [61].
One of the
important questions that needs to be answered is which patient characteristics
are predictive of a good response to anti-amyloid treatment. An extension study
of aducanumab, which is not approved in Europe, showed that patients who were “amyloid-negative”
on amyloid-PET after 18 months of treatment had a significantly slower decline on
the CDR-SB during the further treatment course [62].
Post-hoc
analyses from the Clarity (lecanemab) study showed that patients with low
baseline amyloid levels had a significantly slower decline in cognitive
parameters as compared to the whole group. The majority of patients with low
initial tau levels (Braak stage I and II) developed no decline in cognitive
performance over two years of treatment, and around half even showed an
improvement on the CDR-SB [61].
These
results must be interpreted with great caution given the post-hoc nature of the
analysis and the relatively small number of patients. For example, it is
unclear whether patients with low tau and low amyloid pathology represent an
earlier stage of the disease or whether unknown resilience factors slow the
progression of histopathology in this group. Despite the many uncertainties,
the latest data give hope that questions about treatment initiation, efficacy,
potential harm and treatment duration can be answered in the future.
Treatment
adverse events in the form of Amyloid-Related Imaging Abnormalities (ARIAs)
have occurred in all studies of anti-amyloid antibodies. Vasogenic oedema
(ARIA-E) and microbleeds (ARIA-H) can occur, particularly during the first 6–9
months of treatment. Their incidence depends on Apolipoprotein E (ApoE) status
and increases significantly with the number of ApoE4 alleles [63]. In the Trialblazer
study (donanemab),
they were detected in 36.8% of patients (24% ARIA-E; 31.4% ARIA-H). In the
Clarity study (lecanemab) in 21.5% (12.6% had ARIA-E and 17.3% had ARIA-H). ARIAs
are
visible on MRI in the FLAIR and SWI sequences and require treatment to be
interrupted or stopped once they reach a certain size or if symptoms occur. The
percentage of symptomatic ARIAs was 6.1% in the donanemab study and 2.8% in the
lecanemab trial. Typical symptoms were headache and confusion. In Clarity AD ARIA-E
generally occurred in the first 3 months, was mild and asymptomatic, did not lead
to discontinuation of lecanemab or placebo if mild, and resolved within 4 months.
In Trailblazer 57.9% of first ARIA-E occurred after receiving up to 3 donanemab infusions
[59, 60]. The first data on the
subcutaneous administration of lecanemab suggest a similar risk of
developing ARIA-E or ARIA-H [64].
Depending
on the Swissmedic decision, which is expected in early 2025, the first approved
anti-amyloid drug may be available in Switzerland in the near future. The new
treatments bring new hopes, but also new challenges. Some of these challenges
can be addressed as follows:
- Access:
An estimated maximum of 20% of patients assessed in memory clinics will meet
eligibility criteria or receive insurance coverage. However, 100% of people
with Alzheimer’s disease need high-quality care and the resources to provide
services at all stages of the disease.
- Cost:
Treatment costs are estimated at CHF 25,000 per year, an order of magnitude
higher than the drug costs of about CHF 1000 for currently available
anti-dementia pharmacological therapies. Do we need new standards for diagnosis
and treatment? What will be the role of primary care, specialists and memory
clinics? Do we need new reimbursement models?
- Efficacy
and safety: How does 27% disease
slowing (43% in men, 12% in women) with 18 months of treatment (lecanemab)
translate into quality of life (QoL)? Preliminary data from the Clarity study point
towards QoL benefits associated with treatment of lecanemab [59]. What is the significance
of the sex effect? Are
the therapeutic effects scalable to long-term therapy? Does low tau/low amyloid
indicate earlier disease stages or different disease pathogenesis (more
individual resilience)? What are the indicators of a good treatment response in
individual patients? What impact do ARIAs have on treatment outcome?
New technologies
for dementia treatment and care in the hospital setting
Stefan Klöppel
The
predicted shortage of staff in the Swiss healthcare system warrants new
approaches. Technology-based solutions hold promise with two developments in
particular.
Robots to
help lift patients or humanoids and pet robots to engage patients have entered
long-term care and hospitals, particularly in the dementia-care setting. Paro
is a baby seal robot and is often used to emotionally engage individuals
affected by dementia. When introduced more than ten years ago, Paro caused
ethical concerns as its appearance may deceive demented individuals in
perceiving Paro as a real pet. While early studies were positive, a more recent
meta-analysis found disappointing short- and long-term effects [65]. Best supported
by data is the role of
Paro in the treatment of agitation. While acceptance of robots is certainly
influenced by cultural background, reports on robots in care even from technology
savvy Japan recently sounded negative and listed time-consuming transfers as
well as rebooting and maintenance of robots. In addition, only a fraction of
patients consistently reacted positively to the robots [66].
Besides
robots, sensor technology is another new technology with the potential to
reduce the burden on professional care. In-bed sensors, for example, might
alert nurses to imminent falls when a frail patient attempts to stand up. When
the same sensors are set to a higher sensitivity, phases of restlessness can be
detected and again result in alerting staff. In these scenarios, alerting staff
serves to prevent more resource-intensive events (e.g. a fall) or enables them to
better structure their ward round (visiting an agitated patient when walking
past the room rather than reacting to the patient calling for help). While
studies consistently show the ability of sensors to monitor sleep and activity
of inpatients [67], there is a lack of
studies on the effectiveness of modern sensor technology to prevent falls [68].
In summary,
while solutions to battle staff shortage are urgently needed, robots and sensor
technology still have to prove that they may meaningfully contribute.
Clearly,
sensor technology can also aid patient monitoring outside hospitals, e.g. when
the aim is to monitor patients with dementia at an increased risk of developing
behavioural and psychological symptoms [69].
While reports of patients and relatives during a given consultation tend to
focus on symptoms or the recent past, sensor-based monitoring could provide a
more balanced view and thus support better treatment decisions. Lastly, sensors
can guide just-in-time adaptive interventions (JITAIs) and thus a dementia risk-reducing
lifestyle [70]. An example could be the
smartphone detecting elevator use resulting in a push message to encourage
taking the stairs.
Hospital at Home
for persons with cognitive impairment
Tatjana Meyer-Heim
The
emergence of hospitals in the early 19th century advanced medicine and to this
day hospitals are considered the standard of care for acute illness. However,
hospitals do not only have high maintenance costs, but can also, in particular
in older people, carry a high risk of developing a delirium or functional
decline.
In 1995,
the first Hospital at Home service was set up at Johns Hopkins hospital in
Baltimore – initiating a worldwide movement. Hospital at Home – also called home
treatment or virtual wards – provides hospital-level care in the patient’s home
environment, combining telemonitoring and in-person care.
In
many ways, the COVID-19 pandemic was a catalyst for an increase in demand for home-based
acute care. In the context of the pandemic, the use of telemedicine
technology has been the ideal solution in many countries, and patients realised
that hospitals are not
always the safest haven.
Telemedicine
combined with other new technologies can play an increasingly important role in
the diagnosis and treatment of acute illnesses such as heart failure,
pneumonia, complicated urinary tract infections, exacerbated chronic
obstructive pulmonary disease or COVID-19. In the
near future, the service could expand to support people with an even wider
range of conditions.
Wireless sensors are capable of monitoring heart rate, respiration rate,
heart rate variability, temperature, oxygen saturation and 1-lead ECG. In some brands,
an accelerometer is integrated into the sensor. Wireless blood pressure devices
are used for comprehensive monitoring.
Patients’
vital signs are continuously collected by sensors, then digitised and analysed.
Real-time patient data and early warning alerts are transmitted to central
monitoring stations and integrated with the provider’s system. Telemonitoring
is accompanied by in-person visits by nurses and physicians at the patient’s
home. Face-to-face visits are crucial.
Especially
persons living with dementia tend to do poorly in a hospital setting. Hospital
admissions harbour the risk of under- and overstimulation, sleep disruption,
immobility, malnutrition, loneliness and functional decline [71]among other negative
outcomes. The
risk of developing a delirium in the hospital is particularly high for patients
with preexisting neurocognitive disorders.
Person-centred
care in the patient’s home does improve patient satisfaction without jeopardising
safety.
Both a
recently published cohort study with more than 11,000 patients [72] and a Cochrane
review [73] confirmed the results of other studies showing
that mortality in a Hospital at Home setting was not higher than in patients
treated in a conventional hospital setting. Besides admission avoidance, a Hospital
at Home service
can decrease admissions to long-term residential care at 6 months [74].
In late
2019, Spital Zollikerberg in Zurich launched the first Swiss Hospital at Home
service for patients with acute internal medical diseases, called “Visit –
Spital Zollikerberg Zuhause”. Patients aged 19 to 99 years were included.
In agreement
with already published studies [75], there
were no safety issues and high patient and caregiver satisfaction were
demonstrated (5.8. and 5.7, respectively, on a scale from 1 to 6). These are
preliminary results of an ongoing pilot study.
Due to the
small number of cases (n = 78), no subgroup of patients with cognitive
impairment could be formed. However, individuals with dementia and their
relatives reported a positive experience. Further studies are needed.
Larger
randomised controlled trials which address the feasibility of Hospital at Home treatment
for patients with cognitive impairments are needed. NICE 2017 guidelines recommend
taking into account keeping a person living with dementia in a familiar
environment before a hospital admission. In the UK, so far over 240,000 people
have been treated in a Hospital at Home setting since April 2022 [76].
In addition
to finding ways of reimbursement and addressing other topics [77], healthcare systems
have to ensure that
nobody is left behind because of digital illiteracy while the use of digital
tools is increasing [78].
Technology literacy in old age – a neglected prevention strategy in
dementia care
Stefanie Becker
So-called gerontechnologies are increasingly penetrating the everyday
lives of dementia patients [79–81]. An
ever-wider range of technological solutions – from electronic bracelets with
geolocalisation to intelligent rollators, communication supporting devices or
smart homes – have emerged as pivotal allies. They offer innovative solutions
and help to promote values such as freedom of choice and autonomy, thus aiming
to reduce the impact of dementia-related impairments.
But they can only fully unfold their positive and supporting effects if
they are tailored and suited for the use of the individual and work for the
person. Unfortunately, despite the possible advantages for independent living, the
reality is that use of technology in dementia has been poor. A main concern
in connecting patients with dementia with gerontechnologies is the digital
divide that excludes individuals from benefiting from these resources. The
elderly population exhibits apprehension or difficulty in adopting new
technologies, often stemming from a lack of digital literacy. So, despite the
many potentially positive effects in empowering those affected by dementia and
supporting the family carers, (new) technologies are not yet widely used. Today,
among older adults, it is mainly those with a high interest in technology who use
digital services. Research shows that especially expectations like perceived ease
of use are related to age and interest in technology predict their use. Only a
few seniors have an overall positive attitude towards these digital services [82].
Technology acceptance and use – attitude as a crucial factor
Nevertheless, potential for greater use is evident. To foster the use of
better technology, acceptance, access, explanation, guidance and support to learn
the helpful use of new technologies in everyday life are crucial. It is
expected that the next generations of elderly people will be much more used to
the daily adaptation of technologies. But even with that future perspective,
technology acceptance or use are not just an “organic” development but require
awareness-raising and motivational linkage to the individuals’ preferences and
needs. Challenging current models of technology acceptance, [83] found that attitude
was the most important
factor which is influenced by facilitating conditions and social influence.
This had a direct effect on behavioural attitude and intention of use.
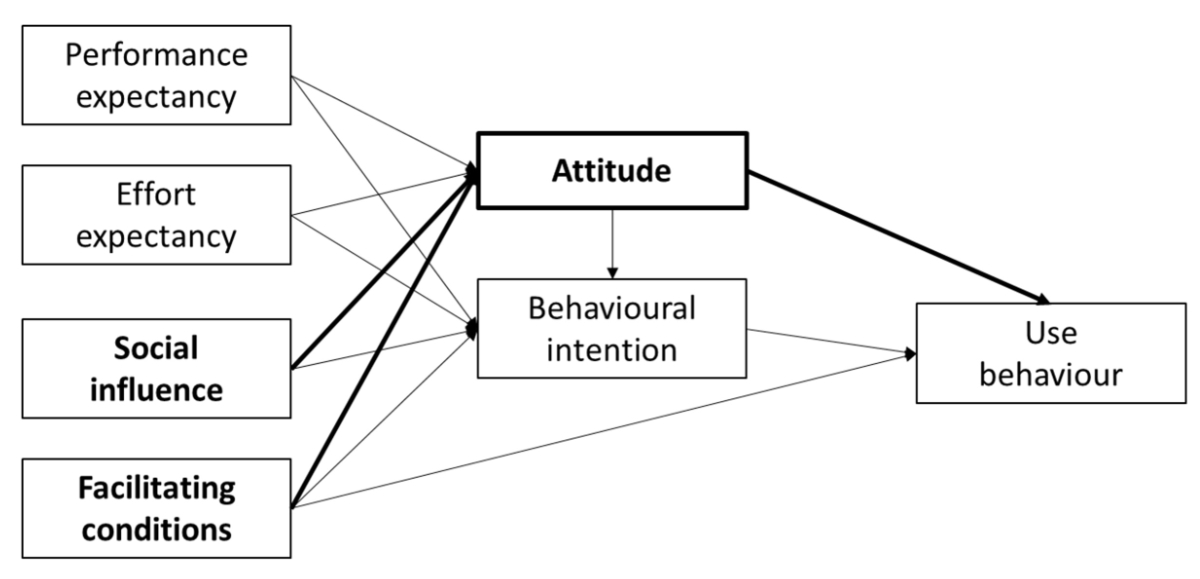
Figure 4Model of technology acceptance: different factors influencing use
behaviour; in bold the factors with the most important influence. Adapted from: Dwivedi YK,
Rana NP, Jeyaraj A, Clement M, Williams MD. Re-examining the Unified Theory of Acceptance
and Use of Technology (UTAUT): Towards a Revised Theoretical Model. Inf Syst Front.
2019;21(3):719–34 [83], https://doi.org/10.1007/s10796-017-9774-y, distributed under the terms of the Creative Commons CC BY license (https://creativecommons.org/share-your-work/cclicenses/).
Technology literacy – a neglected prevention path
As a prerequisite for active and healthy ageing, older people must also
be able to use the potential of technological innovations. But it is precisely
this ideal of autonomy that is put to the test in the case of dementia. Digital
literacy is therefore considered a key competence for active participation in
an increasingly digital society. The acceptance and the safe and beneficial use
of technologies are promoted by teaching digital skills to older people today
to prepare them for a possible future life with dementia.
Consequently, there is a pressing need for user-centred design
principles that prioritise intuitive interfaces and ease of use. This also
means the inclusion of people with dementia and their caregivers in the
developmental process of technologies to become real solutions instead of
obstacles.
Ethical considerations also loom large, particularly regarding privacy
and autonomy. The use of surveillance technologies, while bolstering safety,
concurrently raises questions about consent and the right to privacy. It is
imperative that ethical frameworks are developed to navigate these issues,
ensuring that the dignity of the individual with dementia is upheld and that motivation
and trust can be built in cognitive healthy periods of life.
In summary, the strategic use of technology in dementia care is
promising, offering tools that can improve safety, cognitive function,
independence and quality of life. However, the translation of these
technological advances into widespread clinical and home use must be approached
with caution. Interdisciplinary collaboration among technologists, healthcare
providers, patients and caregivers and inclusion of the target group of the
respective technology are essential to optimise the use of technology in
dementia care and to ensure that it serves as a bridge to a better quality of
life for those affected. This implies that approaches to shape the attitudes of
individuals for influencing intentions and behaviours will be an even more
important pathway for (primary, secondary and tertiary) prevention for a good
and self-determined life with dementia. But no matter how
good the new technology is, it will always have to serve the individual and
will never replace personal contact or human attention. Fostering the
motivation to use and an interest in new technologies in older adults can
however be an additional path of prevention for a self-determined life,
especially in case of a dementia diagnosis.
Conclusion
The
conference underscored the importance of a multidisciplinary approach for successfully
integrating new technologies in both clinical-translational research and
dementia prevention, diagnosis and care. Recent developments in the field of
clinical, neuropsychological and biomarker diagnosis, along with digital remote
assessment methods will substantially change both attitudes and clinical
practice regarding individual risk profiling, and early diagnosis and monitoring
of dementia diseases. This will allow for personalised counselling and tailored
lifestyle, pharmacological and psychosocial interventions, and improve the
quality of care. Upcoming disease-modifying interventions such as anti-amyloid
antibodies will require precise identification of the targeted pathology and assessment
of treatment-related risks. While these innovations represent major steps
forwards, their implementation also comes with important challenges to be
addressed including questions on ethical aspects, healthcare system
preparedness and adaptation, resource availability, technology literacy,
acceptance and appropriate use.
Acknowledgments
The authors thank OM Pharma, Merz, Eisai,
Biogen, Roche for supporting the Dementia Summit 2023.
Julius Popp, MD
Department
of Adult Psychiatry and Psychotherapy
Psychiatric
University Hospital Zurich and University of Zurich
Lenggstrasse
31
CH-8032
Zurich
julius.popp[at]uzh.ch
References
1. Alzheimer Schweiz. Demenz in der Schweiz 2024. Zahlen und Fakten. 2024; Available
from: https://www.alzheimer-schweiz.ch/fileadmin/dam/Alzheimer_Schweiz/Dokumente/Ueber_Demenz/Zahlen-Fakten/Factsheet_DemenzCH_2024_DE.pdf
2. Kheirouri S, Alizadeh M. MIND diet and cognitive performance in older adults: a systematic
review. Crit Rev Food Sci Nutr. 2022;62(29):8059–77. doi: https://doi.org/10.1080/10408398.2021.1925220
3. Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A
2 year multidomain intervention of diet, exercise, cognitive training, and vascular
risk monitoring versus control to prevent cognitive decline in at-risk elderly people
(FINGER): a randomised controlled trial. Lancet. 2015 Jun;385(9984):2255–63. doi: https://doi.org/10.1016/S0140-6736(15)60461-5
4. Barnes LL, Dhana K, Liu X, Carey VJ, Ventrelle J, Johnson K, et al. Trial of the MIND
Diet for Prevention of Cognitive Decline in Older Persons. N Engl J Med. 2023 Aug;389(7):602–11.
doi: https://doi.org/10.1056/NEJMoa2302368
5. Gates EJ, Bernath AK, Klegeris A. Modifying the diet and gut microbiota to prevent
and manage neurodegenerative diseases. Rev Neurosci. 2022 Mar;33(7):767–87. doi: https://doi.org/10.1515/revneuro-2021-0146
6. Xiao S, Chan P, Wang T, Hong Z, Wang S, Kuang W, et al. A 36-week multicenter, randomized,
double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium
oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimers Res Ther. 2021 Mar;13(1):62.
doi: https://doi.org/10.1186/s13195-021-00795-7
7. Grammatikopoulou MG, Goulis DG, Gkiouras K, Theodoridis X, Gkouskou KK, Evangeliou A,
et al. To Keto or Not to Keto? A Systematic Review of Randomized Controlled Trials
Assessing the Effects of Ketogenic Therapy on Alzheimer Disease. Adv Nutr. 2020 Nov;11(6):1583–602.
doi: https://doi.org/10.1093/advances/nmaa073
8. Fortier M, Castellano CA, St-Pierre V, Myette-Côté É, Langlois F, Roy M, et al. A
ketogenic drink improves cognition in mild cognitive impairment: results of a 6-month
RCT. Alzheimers Dement. 2021 Mar;17(3):543–52. doi: https://doi.org/10.1002/alz.12206
9. Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in
Alzheimer disease. Arch Neurol. 2006 Sep;63(9):1312–7. doi: https://doi.org/10.1001/archneur.63.9.1312
10. Rosano C, Newman A, Santanasto A, Zhu X, Goodpaster B, Miljkovic I. Increase in skeletal
muscular adiposity and cognitive decline in a biracial cohort of older men and women.
J Am Geriatr Soc. 2023 Sep;71(9):2759–68. doi: https://doi.org/10.1111/jgs.18419
11. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based
recommendations for optimal dietary protein intake in older people: a position paper
from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013 Aug;14(8):542–59. doi: https://doi.org/10.1016/j.jamda.2013.05.021
12. Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of
a vitamin D and leucine-enriched whey protein nutritional supplement on measures of
sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled
trial. J Am Med Dir Assoc. 2015 Sep;16(9):740–7. doi: https://doi.org/10.1016/j.jamda.2015.05.021
13. Frisoni GB, Altomare D, Ribaldi F, Villain N, Brayne C, Mukadam N, et al. Dementia
prevention in memory clinics: recommendations from the European task force for brain
health services. Lancet Reg Health Eur. 2023 Jan;26:100576. doi: https://doi.org/10.1016/j.lanepe.2022.100576
14. Livingston G, Huntley J, Liu KY, Costafreda SG, Selbæk G, Alladi S, et al. Dementia
prevention, intervention, and care: 2024 report of the Lancet standing Commission.
Lancet. 2024 Aug;404(10452):572–628. doi: https://doi.org/10.1016/S0140-6736(24)01296-0
15. Ballesteros S, Kraft E, Santana S, Tziraki C. Maintaining older brain functionality:
A targeted review. Neurosci Biobehav Rev. 2015 Aug;55:453–77. doi: https://doi.org/10.1016/j.neubiorev.2015.06.008
16. Meng X, Fang S, Zhang S, Li H, Ma D, Ye Y, et al. Multidomain lifestyle interventions
for cognition and the risk of dementia: A systematic review and meta-analysis. Int
J Nurs Stud. 2022 Jun;130:104236. doi: https://doi.org/10.1016/j.ijnurstu.2022.104236
17. Brioschi Guevara A, Bieler M, Altomare D, Berthier M, Csajka C, Dautricourt S, et
al. Protocols for cognitive enhancement. A user manual for Brain Health Services-part
5 of 6. Alzheimers Res Ther. 2021 Oct;13(1):172. doi: https://doi.org/10.1186/s13195-021-00844-1
18. Sokolov AA, Collignon A, Bieler-Aeschlimann M. Serious video games and virtual reality
for prevention and neurorehabilitation of cognitive decline because of aging and neurodegeneration.
Curr Opin Neurol. 2020 Apr;33(2):239–48. doi: https://doi.org/10.1097/WCO.0000000000000791
19. Belleville S, Cuesta M, Bieler-Aeschlimann M, Giacomino K, Widmer A, Mittaz Hager AG,
et al. Rationale and protocol of the StayFitLonger study: a multicentre trial to measure
efficacy and adherence of a home-based computerised multidomain intervention in healthy
older adults. BMC Geriatr. 2020 Aug;20(1):315. doi: https://doi.org/10.1186/s12877-020-01709-2
20. Belleville S, Cuesta M, Bieler-Aeschlimann M, Giacomino K, Widmer A, Mittaz Hager AG,
et al. Pre-frail older adults show improved cognition with StayFitLonger computerized
home-based training: a randomized controlled trial. Geroscience. 2023 Apr;45(2):811–22.
doi: https://doi.org/10.1007/s11357-022-00674-5
21. Leidi-Maimone B, Notter-Bielser ML, Laouadi MH, Perrin S, Métraux H, Damian D, et
al. How non-drug interventions affect the quality of life of patients suffering from
progressive cognitive decline and their main caregiver. Aging (Albany NY). 2020 Jun;12(11):10754–71.
doi: https://doi.org/10.18632/aging.103291
22. Chatton P, Martins M, Carlier S, Bieler-Aeschlimann M. [Long COVID : which neuropsychological
intervention? The example applied at the Leenaards Memory Centre]. Rev Med Suisse.
2023 May;19(827):979–83. doi: https://doi.org/10.53738/REVMED.2023.19.827.979
23. Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, et al.; Cache
County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms
in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008 Feb;23(2):170–7.
doi: https://doi.org/10.1002/gps.1858
24. Sheikh F, Ismail Z, Mortby ME, Barber P, Cieslak A, Fischer K, et al.; PROMPT registry
investigators. Prevalence of mild behavioral impairment in mild cognitive impairment
and subjective cognitive decline, and its association with caregiver burden. Int Psychogeriatr.
2018 Feb;30(2):233–44. doi: https://doi.org/10.1017/S104161021700151X
25. Spiegl K, Luttenberger K, Graessel E, Becker L, Scheel J, Pendergrass A. Predictors
of institutionalization in users of day care facilities with mild cognitive impairment
to moderate dementia. BMC Health Serv Res. 2021 Sep;21(1):1009. doi: https://doi.org/10.1186/s12913-021-07017-8
26. Peters ME, Schwartz S, Han D, Rabins PV, Steinberg M, Tschanz JT, et al. Neuropsychiatric
symptoms as predictors of progression to severe Alzheimer’s dementia and death: the
Cache County Dementia Progression Study. Am J Psychiatry. 2015 May;172(5):460–5. doi: https://doi.org/10.1176/appi.ajp.2014.14040480
27. Showraki A, Murari G, Ismail Z, Barfett JJ, Fornazzari L, Munoz DG, et al. Cerebrospinal
Fluid Correlates of Neuropsychiatric Symptoms in Patients with Alzheimer’s Disease/Mild
Cognitive Impairment: A Systematic Review. J Alzheimers Dis. 2019;71(2):477–501. doi: https://doi.org/10.3233/JAD-190365
28. Bloniecki V, Zetterberg H, Aarsland D, Vannini P, Kvartsberg H, Winblad B, et al. Are
neuropsychiatric symptoms in dementia linked to CSF biomarkers of synaptic and axonal
degeneration? Alzheimers Res Ther. 2020 Nov;12(1):153. doi: https://doi.org/10.1186/s13195-020-00718-y
29. Rabl M, et al. Plasma neurofilament light, glial fibrillary acid protein, and phosphorylated
tau 181 as biomarkers for neuropsychiatric symptoms and related clinical disease progression.
Res Sq, 2024 Mar 22:rs.3.rs-4116836. doi: 10.21203/rs.3.rs-4116836/v1
30. Rabl M, Clark C, Dayon L, Bowman GL, Popp J. Blood plasma protein profiles of neuropsychiatric
symptoms and related cognitive decline in older people. J Neurochem. 2023 Jan;164(2):242–54.
doi: https://doi.org/10.1111/jnc.15715
31. Mroczek M, Clark C, Dayon L, Bowman GL, Popp J. Cerebrospinal Fluid Proteome Alterations
Associated with Neuropsychiatric Symptoms in Cognitive Decline and Alzheimer’s Disease.
Cells. 2022 Mar;11(6):1030. doi: https://doi.org/10.3390/cells11061030
32. Clark C, Richiardi J, Maréchal B, Bowman GL, Dayon L, Popp J. Systemic and central
nervous system neuroinflammatory signatures of neuropsychiatric symptoms and related
cognitive decline in older people. J Neuroinflammation. 2022 May;19(1):127. doi: https://doi.org/10.1186/s12974-022-02473-3
33. Rabl M, Clark C, Dayon L, Popp J; Alzheimer’s Disease Neuroimaging Initiative. Neuropsychiatric
symptoms in cognitive decline and Alzheimer’s disease: biomarker discovery using plasma
proteomics. J Neurol Neurosurg Psychiatry. 2024 Sep;•••:jnnp-2024-333819. 10.1136/jnnp-2024-333819
34. Jotterand F, et al. (Jotterand F, Ienca M, Wangmo T, Elger B, editors). Intelligent
assistive technologies for dementia: clinical, ethical, social, and regulatory implications.
USA: Oxford University Press; 2019. doi: https://doi.org/10.1093/med/9780190459802.001.0001
35. Ienca M, Fabrice J, Elger B, Caon M, Scoccia Pappagallo A, Kressig RW, et al. Intelligent
assistive technology for Alzheimer’s disease and other dementias: a systematic review.
J Alzheimers Dis. 2017;56(4):1301–40. doi: https://doi.org/10.3233/JAD-161037
36. Ienca M, Jotterand F, Vică C, Elger B. Social and assistive robotics in dementia care:
ethical recommendations for research and practice. Int J Soc Robot. 2016;8(4):565–73.
doi: https://doi.org/10.1007/s12369-016-0366-7
37. Javeed A, Dallora AL, Berglund JS, Ali A, Ali L, Anderberg P. Machine Learning for
Dementia Prediction: A Systematic Review and Future Research Directions. J Med Syst.
2023 Feb;47(1):17. doi: https://doi.org/10.1007/s10916-023-01906-7
38. Ienca M, Wangmo T, Jotterand F, Kressig RW, Elger B. Ethical design of intelligent
assistive technologies for dementia: a descriptive review. Sci Eng Ethics. 2018 Aug;24(4):1035–55.
doi: https://doi.org/10.1007/s11948-017-9976-1
39. Cote, A.C., et al., Evaluation of Wearable Technology in Dementia: A Systematic Review
and Meta-Analysis. Front Med (Lausanne), 2021 Jan 11:7:501104. doi: 10.3389/fmed.2020.501104. eCollection 2020.
40. Buchman DZ, Wadhawan S. A global vision for neuroethics needs more social justice:
brain imaging, chronic pain, and population health inequalities. AJOB Neurosci. 2019;10(3):130–2.
doi: https://doi.org/10.1080/21507740.2019.1632966
41. Goodman MS, Ackermann N, Bowen DJ, Panel D, Thompson VS. Reaching consensus on principles
of stakeholder engagement in research. Prog Community Health Partnersh. 2020;14(1):117–27.
doi: https://doi.org/10.1353/cpr.2020.0014
42. Wangmo T, Lipps M, Kressig RW, Ienca M. Ethical concerns with the use of intelligent
assistive technology: findings from a qualitative study with professional stakeholders.
BMC Med Ethics. 2019 Dec;20(1):98. doi: https://doi.org/10.1186/s12910-019-0437-z
43. Ienca M, Schneble C, Kressig RW, Wangmo T. Digital health interventions for healthy
ageing: a qualitative user evaluation and ethical assessment. BMC Geriatr. 2021 Jul;21(1):412.
doi: https://doi.org/10.1186/s12877-021-02338-z
44. American Psychiatric Association, DSM-5 Task Force. Diagnostic and statistical manual
of mental disorders: DSM-5. 5th ed. American Psychiatric Publishing, Inc.; 2013. 10.1176/appi.books.9780890425596
45. Romero HR, Monsch AU, Hayden KM, Plassman BL, Atkins AS, Keefe RS, et al. TOMMORROW
neuropsychological battery: german language validation and normative study. Alzheimers
Dement (N Y). 2018 Jul;4(1):314–23. 10.1016/j.trci.2018.06.009
46. Chen C, Ding S, Wang J. Digital health for aging populations. Nat Med. 2023 Jul;29(7):1623–30.
doi: https://doi.org/10.1038/s41591-023-02391-8
47. Berron D, Glanz W, Clark L, Basche K, Grande X, Güsten J, et al. A remote digital
memory composite to detect cognitive impairment in memory clinic samples in unsupervised
settings using mobile devices. NPJ Digit Med. 2024 Mar;7(1):79. doi: https://doi.org/10.1038/s41746-024-00999-9
48. Cognat E, Mouton Liger F, Troussière AC, Wallon D, Dumurgier J, Magnin E, et al.;
ePLM network. What is the clinical impact of cerebrospinal fluid biomarkers on final
diagnosis and management in patients with mild cognitive impairment in clinical practice?
Results from a nation-wide prospective survey in France. BMJ Open. 2019 May;9(5):e026380.
doi: https://doi.org/10.1136/bmjopen-2018-026380
49. Rabinovici GD, Carrillo MC, Apgar C, Gareen IF, Gutman R, Hanna L, et al. Amyloid
Positron Emission Tomography and Subsequent Health Care Use Among Medicare Beneficiaries
With Mild Cognitive Impairment or Dementia. JAMA Neurol. 2023 Nov;80(11):1166–73.
doi: https://doi.org/10.1001/jamaneurol.2023.3490
50. Popp J, Georgescu D, Bürge M, Mundwiler-Pachlatko E, Bernasconi L, Felbecker A. [Biomarkers
for the diagnosis of cognitive impairment - Recommendations from the Swiss Memory
Clinics]. Rev Med Suisse. 2022 Dec;18(808):2400–5. 10.53738/REVMED.2022.18.808.2400
51. Popp, J., et al., [Biomarkers for the diagnosis of cognitive impairment - Recommendations
from the Swiss Memory Clinics]. Praxis (Bern 1994), 2022. 111(13): p. 738-744. doi:
10.1024/1661-8157/a003913
52. Popp J, Oikonomidi A, Tautvydaitė D, Dayon L, Bacher M, Migliavacca E, et al. Markers
of neuroinflammation associated with Alzheimer’s disease pathology in older adults.
Brain Behav Immun. 2017 May;62:203–11. doi: https://doi.org/10.1016/j.bbi.2017.01.020
53. Clark C, Gholam M, Zullo L, Kerksiek A, Castelao E, von Gunten A, et al. Plant sterols
and cholesterol metabolism are associated with five-year cognitive decline in the
elderly population. iScience. 2023 Apr;26(6):106740. doi: https://doi.org/10.1016/j.isci.2023.106740
54. Clark C, Dayon L, Masoodi M, Bowman GL, Popp J. An integrative multi-omics approach
reveals new central nervous system pathway alterations in Alzheimer’s disease. Alzheimers
Res Ther. 2021 Apr;13(1):71. doi: https://doi.org/10.1186/s13195-021-00814-7
55. Clark C, Rabl M, Dayon L, Popp J. The promise of multi-omics approaches to discover
biological alterations with clinical relevance in Alzheimer’s disease. Front Aging
Neurosci. 2022 Dec;14:1065904. doi: https://doi.org/10.3389/fnagi.2022.1065904
56. Hansson O, Blennow K, Zetterberg H, Dage J. Blood biomarkers for Alzheimer’s disease
in clinical practice and trials. Nat Aging. 2023 May;3(5):506–19. doi: https://doi.org/10.1038/s43587-023-00403-3
57. Pontecorvo MJ, Lu M, Burnham SC, Schade AE, Dage JL, Shcherbinin S, et al. Association
of Donanemab Treatment With Exploratory Plasma Biomarkers in Early Symptomatic Alzheimer
Disease: A Secondary Analysis of the TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA
Neurol. 2022 Dec;79(12):1250–9. doi: https://doi.org/10.1001/jamaneurol.2022.3392
58. Leuzy A, Mattsson-Carlgren N, Palmqvist S, Janelidze S, Dage JL, Hansson O. Blood-based
biomarkers for Alzheimer’s disease. EMBO Mol Med. 2022 Jan;14(1):e14408. doi: https://doi.org/10.15252/emmm.202114408
59. van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in Early
Alzheimer’s Disease. N Engl J Med. 2023 Jan;388(1):9–21. doi: https://doi.org/10.1056/NEJMoa2212948
60. Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, et al.; TRAILBLAZER-ALZ
2 Investigators. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ
2 Randomized Clinical Trial. JAMA. 2023 Aug;330(6):512–27. doi: https://doi.org/10.1001/jama.2023.13239
61. Sperling R. Lecanemab for the Treatment of Early Alzheimer’s Disease: The Extension
of Efficacy Results from Clarity AD. in 16 th Clinical Trials on Alzheimer’s Disease
(CTAD). 2023. Boston, MA (USA)
62. O’Gorman J. Pooled ENGAGE/EMERGE integrated placebo-controlled period and long-term
extension (LTE) topline results: slower clinical progression at Week 134 in aducanumab-treated
patients that became amyloid PET negative at Week 78. in 16 th Clinical Trials on
Alzheimer’s Disease (CTAD). 2023. Boston, MA (USA)
63. Salloway S, Chalkias S, Barkhof F, Burkett P, Barakos J, Purcell D, et al. Amyloid-Related
Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With
Early Alzheimer Disease. JAMA Neurol. 2022 Jan;79(1):13–21. doi: https://doi.org/10.1001/jamaneurol.2021.4161
64. Honig LS, Barakos J, Dhadda S, Kanekiyo M, Reyderman L, Irizarry M, et al. ARIA in
patients treated with lecanemab (BAN2401) in a phase 2 study in early Alzheimer’s
disease. Alzheimers Dement (N Y). 2023 Mar;9(1):e12377. doi: https://doi.org/10.1002/trc2.12377
65. Yu C, Sommerlad A, Sakure L, Livingston G. Socially assistive robots for people with
dementia: systematic review and meta-analysis of feasibility, acceptability and the
effect on cognition, neuropsychiatric symptoms and quality of life. Ageing Res Rev.
2022 Jun;78:101633. doi: https://doi.org/10.1016/j.arr.2022.101633
66. Wright J. Inside Japan’s long experiment in automating elder care. 2023; Available
from: https://www.technologyreview.com/2023/01/09/1065135/japan-automating-eldercare-robots/
67. Bate GL, Kirk C, Rehman RZ, Guan Y, Yarnall AJ, Del Din S, et al. The Role of Wearable
Sensors to Monitor Physical Activity and Sleep Patterns in Older Adult Inpatients:
A Structured Review. Sensors (Basel). 2023 May;23(10):4881. 10.3390/s23104881
68. Cortés OL, Piñeros H, Aya PA, Sarmiento J, Arévalo I. Systematic review and meta-analysis
of clinical trials: in-hospital use of sensors for prevention of falls. Medicine (Baltimore).
2021 Oct;100(41):e27467. doi: https://doi.org/10.1097/MD.0000000000027467
69. Owens AP, Krebs C, Kuruppu S, Brem AK, Kowatsch T, Aarsland D, et al. Broadened assessments,
health education and cognitive aids in the remote memory clinic. Front Public Health.
2022 Dec;10:1033515. doi: https://doi.org/10.3389/fpubh.2022.1033515
70. Brill E, Klusmann-Weisskopf V, Klöppel S. Wenn Werte motivieren. Schweiz Arzteztg.
2023;104(40):34–5.
71. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She
was probably able to ambulate, but I’m not sure”. JAMA. 2011 Oct;306(16):1782–93.
doi: https://doi.org/10.1001/jama.2011.1556
72. Adams D, Wolfe AJ, Warren J, Laberge A, Richards AC, Herzer K, et al. Initial Findings
From an Acute Hospital Care at Home Waiver Initiative. JAMA Health Forum. 2023 Nov;4(11):e233667.
doi: https://doi.org/10.1001/jamahealthforum.2023.3667
73. Shepperd S, Iliffe S, Doll HA, Clarke MJ, Kalra L, Wilson AD, et al. Admission avoidance
hospital at home. Cochrane Database Syst Rev. 2016 Sep;9(9):CD007491.
74. Shepperd S, Butler C, Cradduck-Bamford A, Ellis G, Gray A, Hemsley A, et al. Is Comprehensive
Geriatric Assessment Admission Avoidance Hospital at Home an Alternative to Hospital
Admission for Older Persons?: A Randomized Trial. Ann Intern Med. 2021 Jul;174(7):889–98.
doi: https://doi.org/10.7326/M20-5688
75. Bates DW, Levine DM, Salmasian H, Syrowatka A, Shahian DM, Lipsitz S, et al. The Safety
of Inpatient Health Care. N Engl J Med. 2023 Jan;388(2):142–53. doi: https://doi.org/10.1056/NEJMsa2206117
76. England N. NHS delivers 10,000 virtual ward beds target with hundreds of thousands
of patients treated at home. 2023 oct 12; Available from: https://www.england.nhs.uk/2023/10/nhs-delivers-10000-virtual-ward-beds-target-with-hundreds-of-thousands-of-patients-treated-at-home/
77. Ouchi K, Liu S, Tonellato D, Keschner YG, Kennedy M, Levine DM. Home hospital as a
disposition for older adults from the emergency department: benefits and opportunities.
J Am Coll Emerg Physicians Open. 2021 Jul;2(4):e12517. doi: https://doi.org/10.1002/emp2.12517
78. Chu JN, Kaplan C, Lee JS, Livaudais-Toman J, Karliner L. Increasing Telehealth Access
to Care for Older Adults During the COVID-19 Pandemic at an Academic Medical Center:
Video Visits for Elders Project (VVEP). Jt Comm J Qual Patient Saf. 2022 Mar;48(3):173–9.
doi: https://doi.org/10.1016/j.jcjq.2021.11.006
79. Huang G, Oteng SA. Gerontechnology for better elderly care and life quality: a systematic
literature review. Eur J Ageing. 2023 Jun;20(1):27. doi: https://doi.org/10.1007/s10433-023-00776-9
80. Lindqvist E, PerssonVasiliou A, Hwang AS, Mihailidis A, Astelle A, Sixsmith A, et
al. The contrasting role of technology as both supportive and hindering in the everyday
lives of people with mild cognitive deficits: a focus group study. BMC Geriatr. 2018 Aug;18(1):185.
doi: https://doi.org/10.1186/s12877-018-0879-z
81. Sixsmith A, Mihailidis A, Simeonov D. Aging and Technology: Taking the Research into
the Real World. Public Policy Aging Rep. 2017;27(2):74–8. doi: https://doi.org/10.1093/ppar/prx007
82. Seifert A, Charness N. Digital transformation of everyday lives of older Swiss adults:
use of and attitudes toward current and future digital services. Eur J Ageing. 2022 Jan;19(3):729–39.
doi: https://doi.org/10.1007/s10433-021-00677-9
83. Dwivedi YK, Rana NP, Jeyaraj A, Clement M, Williams MD. Re-examining the Unified Theory
of Acceptance and Use of Technology (UTAUT): Towards a Revised Theoretical Model.
Inf Syst Front. 2019;21(3):719–34. doi: https://doi.org/10.1007/s10796-017-9774-y
84. Ienca M, Kressig RW, Jotterand F, Elger B. Proactive Ethical Design for Neuroengineering,
Assistive and Rehabilitation Technologies: the Cybathlon Lesson. J Neuroeng Rehabil.
2017 Nov;14(1):115. doi: https://doi.org/10.1186/s12984-017-0325-z



