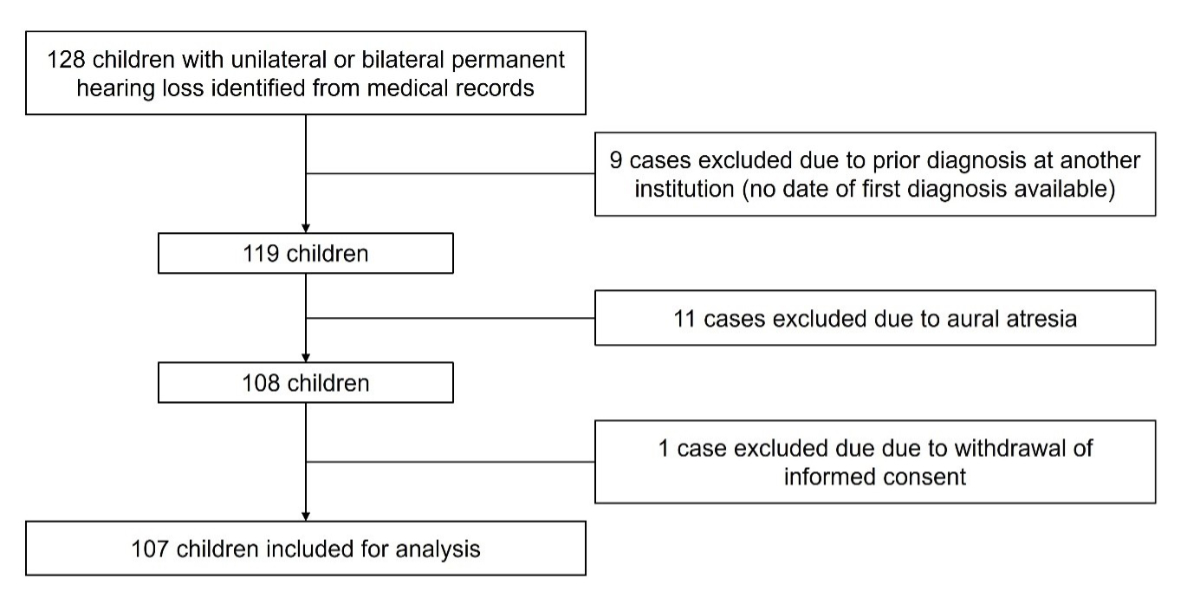
Figure 1Flow chart of the study population.
DOI: https://doi.org/https://doi.org/10.57187/s.4014
Paediatric hearing loss is considered to be an invisible disability as most of the children affected appear physically healthy and behave normally, especially at an early age. Without proper testing, the condition may remain undiagnosed in many individuals and therefore untreated for years, leading to detrimental effects on the child’s speech-language development, social and educational development, and quality of life [1–4]. There also seems to be a critical period for psycholinguistic development [5]. These negative consequences can largely be prevented by adequate treatment [6–10]. Therefore timing is crucial, as early diagnosis and treatment have been shown to improve outcomes [11–13].
It has been estimated that in more than 80% of cases, permanent paediatric hearing loss is already present at birth [10], and the prevalence of paediatric hearing loss continues to increase with age until adolescence, as cases with late-onset, progressive and acquired hearing loss accumulate [14–16]. These latter cases are usually noticed during routine examinations when reduced response, speech-language delay and hearing impairment in difficult listening situations raise questions regarding the child’s hearing ability.
Before universal newborn hearing screening was implemented, hearing loss was mostly diagnosed between the ages of two and five years when speech and language delays became evident [17, 18]. Universal newborn hearing screening programmes may have decreased the average age at diagnosis of hearing loss worldwide [12], but methods, screening algorithms, responsibilities and legal bases vary widely between countries [19, 20]. In our experience, some children are diagnosed at an even older age. However, to the best of our knowledge, there is no solid data available on age at diagnosis of paediatric permanent hearing loss in Switzerland.
In this retrospective cohort study, we therefore aimed to assess the median age of children at diagnosis of permanent unilateral and bilateral hearing loss and to describe the relationship between age at diagnosis and other factors such as hearing loss type, WHO grade and screening status. Data was from children who were diagnosed at the tertiary referral centre of Eastern Switzerland between January 2014 and December 2019.
We conducted a retrospective cohort study using diagnostic and descriptive data extracted from medical records and the central hearing test database of our institution (Division of Paediatric Audiology at the Cantonal Hospital of St Gallen). The study period was from 1 January 2014 to 31 December 2019.
Paediatric patients whose hearing loss was diagnosed at the Division of Paediatric Audiology at the Cantonal Hospital of St Gallen (the tertiary referral centre for Eastern Switzerland) during the study period were eligible. Patients were included if they were younger than 18 years at the time of diagnosis, if they were born in Switzerland, and if unilateral or bilateral permanent hearing loss (hearing threshold average of 500, 1000, 2000, and 4000 Hz; >26 dB hearing loss) was diagnosed for the first time at the Division of Paediatric Audiology at the Cantonal Hospital of St Gallen. Patients were excluded if hearing loss was deemed temporary (e.g. otitis media with effusion), if hearing loss had already been diagnosed at other institutions and no age at first diagnosis was available, and if aural atresia was present (obvious from physical appearance). Due to the expected low number of cases, a planned sample size was not calculated.
Descriptive data collected was the type of hearing loss (sensorineural, conductive, mixed, auditory neuropathy), the grade of hearing loss according to the WHO classification of 1991 [21] (I: slight 26–40 dB, II: moderate 41–60 dB, III: severe 61–80 dB, IV: profound including deafness ≥81 dB), the status of the newborn hearing screening (no information available, passed, failed, documented non-participation) and other information such as path of referral (maternity hospital, children’s hospital, paediatrician, ear-nose-throat specialist) and place of residence (cantons of Appenzell Innerrhoden, Appenzell Ausserrhoden, Principality of Liechtenstein, Graubünden, St Gallen, Thurgau, Zurich).
The primary endpoint was the age at diagnosis of permanent unilateral or bilateral hearing loss. The time of diagnosis was defined as the date on which a confirmatory auditory brainstem response (ABR) test or a pure tone hearing test was performed. In addition, the relationship between the descriptive parameters mentioned above and the primary endpoint was assessed.
Statistics were purely descriptive. Type of hearing loss, WHO grade, status of newborn hearing screening, path of referral and place of residence were described using counts and percentages in relation to the total number of cases and separately for unilateral and bilateral hearing loss. Kaplan-Meier curves were used to describe the median age at diagnosis (including interquartile range [IQR]) of the total study population and differentiated into unilateral and bilateral cases. The median age at diagnosis was additionally calculated for subgroups for type of hearing loss, WHO grade, status of hearing screening and path of referral. Histograms were used to illustrate the distribution of age at diagnosis (overall and by laterality). Finally, Kaplan-Meier curves were explicitly used to draw cumulative distribution functions for the variables age at diagnosis and WHO grade (overall and by laterality). Statistical analysis was performed with the statistics software R [22] using packages ggsurvfit, ggpubr, survival and dplyr.
Ethical approval was sought from the Ethics Committee of Eastern Switzerland. For cases diagnosed before 31 December 2018, the Ethics Committee waived the requirement to obtain informed consent; for cases after 1 January 2019, written consent was required and obtained from the parents.
A total of 128 eligible children with unilateral or bilateral permanent hearing loss were identified from medical records. Nine cases were subsequently excluded because hearing loss had been diagnosed at another institution before the study period and a date of first diagnosis was not available. Eleven cases with aural atresia were excluded, as the physical appearance made the hearing loss obvious. One case had to be excluded from further analysis as the parents did not consent. In total, 107 children with permanent hearing loss were included for analysis (figure 1).

Figure 1Flow chart of the study population.
Bilateral hearing loss (69.2%, n = 74) was more common than unilateral hearing loss (30.8%, n = 33). Sensorineural hearing loss was by far the most frequent type of hearing loss, detected in 91.6% (n = 98) of children, followed by conductive, mixed hearing loss and auditory neuropathy in, respectively, 3.7% (n = 4), 2.8% (n = 3) and 1.9% (n = 2) of cases. The proportions were comparable in the subgroups of unilateral and bilateral cases (table 1).
By WHO grade, the largest group was formed by those with grade II hearing loss (30.8%, n = 33), followed by grade IV (28%, n = 30), grade I (27.1%, n = 29) and grade III (14%, n = 15). While more bilateral cases were found in WHO grades I to III, proportions of unilateral and bilateral cases were comparable for grade IV cases (table 1).
Table 1Patient characteristics and descriptive data of the study population. All values correspond to the number of cases. The denominator used in all percentages is the total number of cases (n = 107).
| Descriptive data | Unilateral | Bilateral | Total | |
| Type of hearing loss | Sensorineural | 32 (29.9%) | 66 (61.7%) | 98 (91.6%) |
| Conductive | 1 (0.9%) | 3 (2.8%) | 4 (3.7%) | |
| Mixed | 0 (0.0%) | 3 (2.8%) | 3 (2.8%) | |
| Auditory neuropathy | 0 (0.0%) | 2 (1.9%) | 2 (1.9%) | |
| All types | 33 (30.8%) | 74 (69.2%) | 107 (100%) | |
| WHO grade of hearing loss | I (slight, 26–40 dB) | 8 (7.5%) | 21 (19.6%) | 29 (27.1%) |
| II (moderate, 41–60 dB) | 6 (5.6%) | 27 (25.2%) | 33 (30.8%) | |
| III (severe, 61–80 dB) | 3 (2.8%) | 12 (11.2%) | 15 (14.0%) | |
| IV (profound incl. deafness, ≥81 dB) | 16 (15.0%) | 14 (13.1%) | 30 (28.0%) | |
| All grades | 33 (30.8%) | 74 (69.2%) | 107 (100%) | |
| Newborn hearing screening status | No information | 15 (14.0%) | 25 (23.4%) | 40 (37.4%) |
| Passed | 8 (7.5%) | 7 (6.5%) | 15 (14.0%) | |
| Failed | 10 (9.3%) | 40 (37.4%) | 50 (46.7%) | |
| Not done | 0 (0.0%) | 2 (1.9%) | 2 (1.9%) | |
| All screening results | 33 (30.8%) | 74 (69.2%) | 107 (100%) | |
| Origin of patient referral | Maternity hospital | 1 (0.9%) | 9 (8.4%) | 10 (9.3%) |
| Children’s hospital | 5 (4.7%) | 13 (12.1%) | 18 (16.8%) | |
| Paediatrician | 16 (15.0%) | 33 (30.8%) | 49 (45.8%) | |
| Ear-nose-throat specialist | 11 (10.3%) | 19 (17.8%) | 30 (28.0%) | |
| All referrals | 33 (30.8%) | 74 (69.2%) | 107 (100%) | |
| Place of residence | Appenzell Innerrhoden | 2 (1.9%) | 2 (1.9%) | 4 (3.7%) |
| Appenzell Ausserrhoden | 1 (0.9%) | 6 (5.6%) | 7 (6.5%) | |
| Principality of Liechtenstein | 1 (0.9%) | 2 (1.9%) | 3 (2.8%) | |
| Graubünden | 3 (2.8%) | 1 (0.9%) | 4 (3.7%) | |
| St Gallen | 18 (16.8%) | 43 (40.2%) | 61 (57.0%) | |
| Thurgau | 7 (6.5%) | 20 (18.7%) | 27 (25.2%) | |
| Zurich | 1 (0.9%) | 0 (0.0%) | 1 (0.9%) | |
| All places of residence | 33 (30.8%) | 74 (69.2%) | 107 (100%) | |
dB: decibel.
A total of 10 unilateral and 40 bilateral cases failed newborn screening, while 8 unilateral and 7 bilateral cases passed the test (table 1). Overall, the status of the newborn hearing screening was unknown in 37.4% of cases. In 2 bilateral cases, the test was documented as not having been performed (table 1).
Children were referred to our institution from the private sector (paediatrician 45.8% [n = 49], ear-nose-throat specialist 28.0% [n = 30]) and from hospitals (maternity clinic 9.3% [n = 10]; children’s hospital 16.8% [n = 18]). Of the ten cases referred from maternity clinics, only one had unilateral hearing loss (table 1). With respect to geographical regions, children were mainly referred from the canton of St Gallen and surrounding cantons as well as from the Principality of Liechtenstein. In all but one canton, the frequency of bilateral hearing loss was equal to or notably higher than that of unilateral cases. In the canton of Graubünden, however, more cases of unilateral hearing loss were observed (table 1).
The overall median age at diagnosis of hearing loss was 45.0 months (IQR 5.7–74.8). At 25.8 months (IQR 3.6–70.5), the median age at diagnosis of bilateral hearing loss was lower than that of unilateral cases (63.1 months [IQR 11.4–88.5]). An overview of the median (IQR) age at diagnosis for all cases as well as in subgroups by hearing loss type, WHO grade, screening status and path of referral is shown in table 2, separately for all, unilateral and bilateral cases. Frequencies and cumulative distribution functions are shown in figures 2 and 3.
Table 2Median age (in months) at diagnosis of paediatric hearing loss, overall and in subgroups by type, WHO grade, newborn hearing screening status and origin of patient referral. Medians are based on Kaplan-Meier estimations. Empty fields indicate non-observed values.
| Unilateral | Bilateral | Total | ||
| (n = 33) | (n = 74) | (n = 107) | ||
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Overall | 63.1 (11.4–88.5) | 25.8 (3.6–70.5) | 45.0 (5.7–74.8) | |
| By type | Sensorineural (n = 98) | 61.0 (10.1–82.0) | 28.0 (3.4–70.2) | 45.2 (5.6–71.3) |
| Conductive (n = 4) | 91.2 (91.2–91.2) | 84.3 (0.0–87.7) | 86.0 (42.1–89.4) | |
| Mixed (n = 3) | – | 11.1 (10.1–79.1) | 11.1 (10.1–79.1) | |
| Auditory neuropathy (n = 2) | – | 21.1 (13.5–28.8) | 21.1 (13.5–28.8) | |
| By grade | I, 26–40 dB (n = 29) | 71.9 (8.2–105.1) | 65.6 (11.1–131.6) | 65.6 (8.8–110.9) |
| II, 41–60 dB (n = 33) | 54.4 (48.3–63.1) | 52.5 (3.6–79.1) | 52.5 (11.4–73.3) | |
| III, 61–80 dB (n = 15) | 51.0 (6.6–63.3) | 17.1 (4.0–41.4) | 18.1 (5.3–51.0) | |
| IV, ≥81 dB (n = 30) | 65.7 (15.4–82.0) | 4.5 (2.2–6.0) | 9.6 (4.4–67.1) | |
| By screening status | No information (n = 40) | 71.3 (53.5–120.3) | 73.3 (62.2–107.8) | 72.3 (59.0–114.2) |
| Passed (n = 15) | 41.9 (9.0–63.7) | 45.4 (33.3–64.1) | 45.4 (16.1–64.1) | |
| Failed (n = 50) | 28.6 (7.4–67.1) | 4.0 (2.2–12.3) | 5.6 (2.4–21.7) | |
| Not done (n = 2) | – | 86.0 (84.3–87.7) | 86.0 (84.3–87.7) | |
| By origin of patient referral | Maternity hospital (n = 10) | 2.4 (2.4–2.4) | 2.4 (1.9–2.6) | 2.4 (1.9–2.6) |
| Children’s hospital (n = 18) | 7.4 (6.6–48.3) | 5.3 (2.2–50.7) | 7.0 (2.7–50.7) | |
| Paediatrician (n = 49) | 69.2 (39.2–105.1) | 37.8 (11.1–79.1) | 55.9 (13.5–87.8) | |
| Ear-nose-throat specialist (n = 30) | 58.9 (49.9–70.5) | 62.2 (6.0–84.5) | 60.5 (13.1–70.5) | |
IQR: interquartile range; dB: decibel.
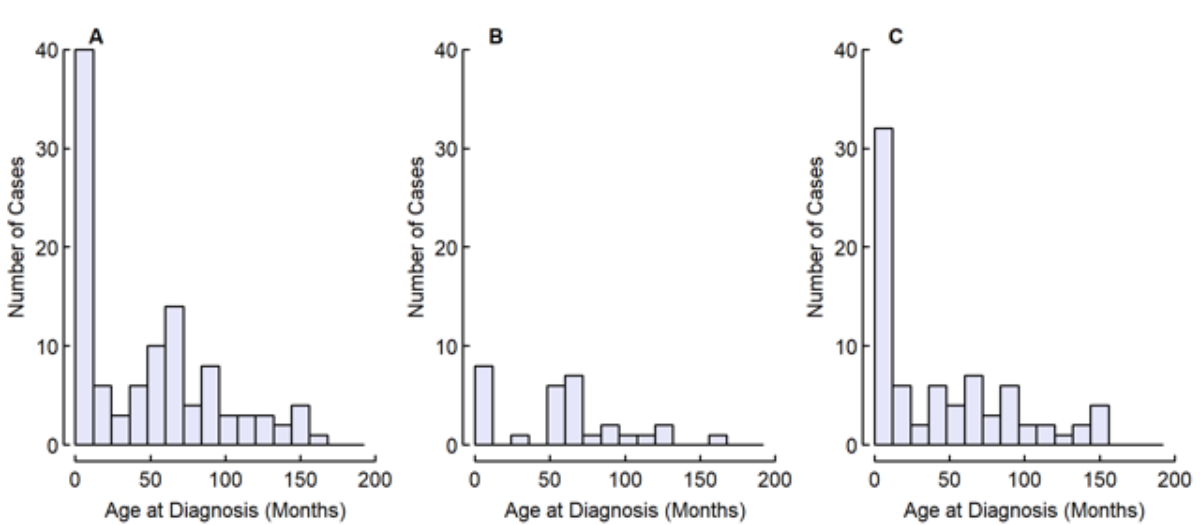
Figure 2Distribution of age at diagnosis of all children (A, n = 107), of children with unilateral hearing loss (B, n = 33) and of children with bilateral hearing loss (C, n = 74).
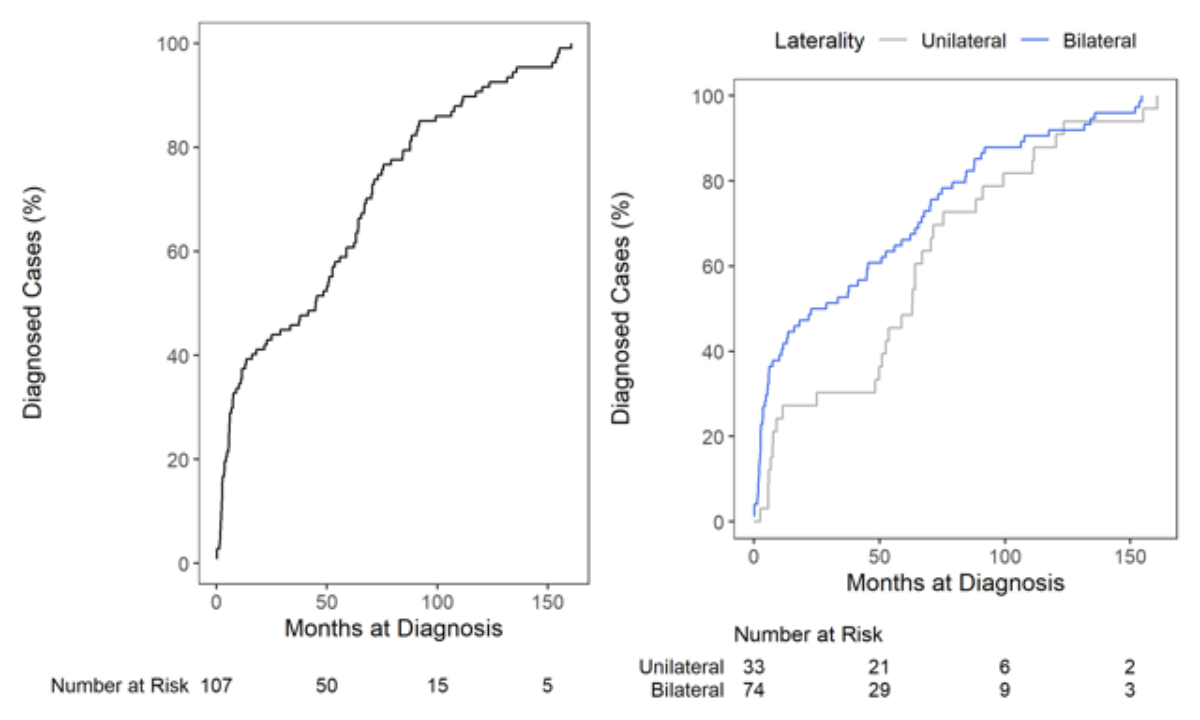
Figure 3Kaplan-Meier cumulative distribution functions of age at diagnosis (overall: left; unilateral and bilateral: right), including patients at risk.
Cases with conductive hearing loss were diagnosed later, at a median of 86.0 months (IQR 42.1–89.4) when compared to sensorineural (median 45.2 months [IQR 5.6–71.3]), auditory neuropathy (median 21.1 months [IQR 13.5–28.8]) and mixed hearing loss (median 11.1 months [IQR 10.1–79.1]). As shown in figure 4 and table 2, the overall median age at diagnosis was lower with higher WHO grades of hearing loss (grade IV: 9.6 months [IQR 4.4–67.1] vs grade I: 65.6 months [IQR 8.8–110.9]). This also applied for the subgroup of bilateral cases but not for unilateral cases. In both groups, median age at diagnosis was highest for WHO grade I hearing loss (unilateral: 71.9 months [IQR 8.2–105.1]; bilateral: 65.6 months [IQR 11.1–131.6]).
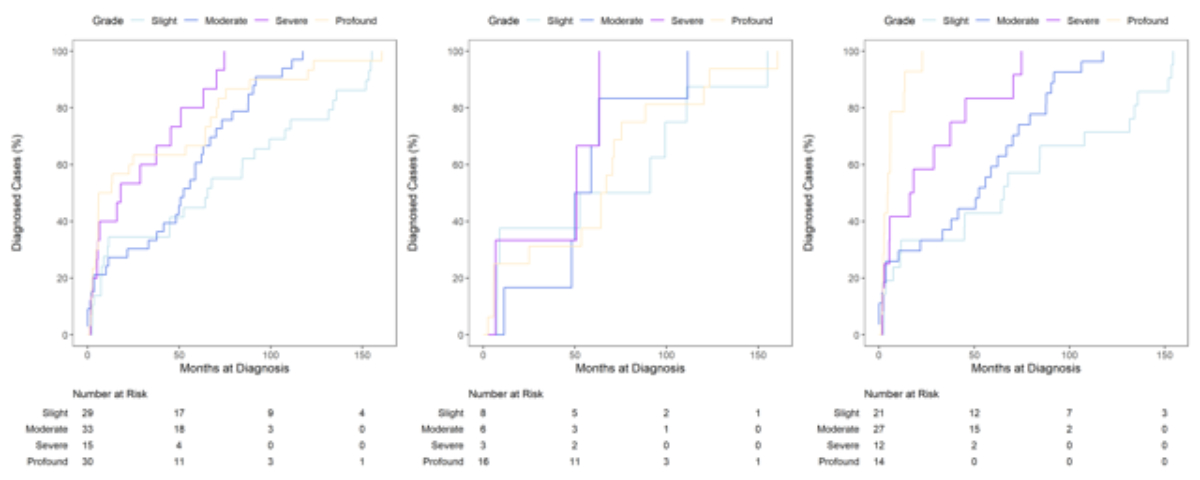
Figure 4Kaplan-Meier cumulative distribution functions of age at diagnosis by WHO grade (overall: left, unilateral: centre, bilateral: right), including patients at risk.
The median age at diagnosis of hearing loss in children with a documented failed newborn hearing screening was 5.6 months (IQR 2.4–21.7). In children who passed the initial newborn hearing screening, the median age at diagnosis was 45.4 months (IQR 16.1–64.1). Without a documented newborn hearing screening status, the median age at diagnosis was 72.3 months (IQR 59.0–114.2), and in children whose non-participation in the screening was documented, median age at diagnosis was 86.0 months (IQR 84.3–87.7). If the screening status was “no information” or “passed”, the median ages at diagnosis were similar in the unilateral and bilateral groups. If the screening status was “failed”, the median age at diagnosis was 4.0 months (IQR 2.2–12.3) for bilateral cases and 28.6 months (IQR 7.4–67.1) for unilateral cases.
Children referred from maternity hospitals were diagnosed earlier (median 2.4 months [IQR 1.9–2.6]) than children referred from children’s hospitals (median 7.0 months [IQR 2.7–50.7]), from paediatricians (median 55.9 months [IQR 13.5–87.8]) and from ear-nose-throat specialists (median 60.5 months [IQR 13.1–70.5]). Median ages at diagnosis were similar in unilateral and bilateral cases when children were referred from maternity hospitals and children’s hospitals. However, median ages at diagnosis were higher in referrals from paediatricians in the unilateral group (69.2 months [IQR 39.2–105.1]) and in referrals from ear-nose-throat specialists in the bilateral group (62.2 months [IQR 6.0–84.5]).
The median overall age at diagnosis of paediatric hearing loss was 45.0 months. When viewed in isolation, this number is high and requires further analysis. During the observation period of the study, however, only children with bilateral hearing loss were detected by the universal newborn hearing screening programme in Switzerland, as the test was considered passed with the presence of oto-acoustic emissions in only one ear. Accordingly, age distributions show that bilateral cases were mostly diagnosed at a young age while the peak for diagnosis of unilateral cases occurred only after 50 months.
For unilateral hearing loss, the median age at diagnosis was 63.1 months. This relatively high value might be explained by the algorithm of newborn hearing screening applicable during the observation period of the study. At this time, it was believed that one-ear hearing is sufficient for normal speech development. Therefore, unilateral hearing loss was usually not diagnosed at birth but later in life. However, children with unilateral hearing loss typically have hearing problems with background noise, which might increase depending on the environment (preschool vs school). This might lead to a delayed diagnosis, possibly explaining the second peak in the fifth and sixth year of life in unilateral cases. Another explanation for delayed diagnosis could be related to the cause of hearing loss, as late-onset, genetic, progressive and acquired causes are more frequently observed in unilateral cases [23, 24]. The phenomenon of late diagnosis of unilateral hearing loss has also been described by others. In US, Taiwanese and Dutch studies, the median ages at diagnosis were 52.8 months [23], 50.3 months [25] and 39.6 months [26], respectively. Therefore, the relatively high median age at diagnosis for unilateral hearing loss observed in our study is comparable to published data from other countries before universal newborn hearing screening was implemented.
For bilateral hearing loss, the median age at diagnosis was 25.8 months. The marked peak in the first year of life could be explained by cases of congenital hearing loss detected by newborn hearing screening. In our study, age at diagnosis of bilateral hearing loss is inversely related to the severity of hearing loss; in bilateral cases of WHO grade III and IV, the median ages at diagnosis were 17.1 and 4.5 months, respectively. Children with bilateral hearing loss of higher grades are usually detected by newborn hearing screening and tend to have more observable symptoms leading to earlier diagnosis [17, 27–29]. Nevertheless, even in bilateral cases, the age at diagnosis is relatively high in our cohort and performs unfavourably in terms of international recommendations [30, 31]. The literature provides variable numbers regarding the median age at diagnosis, ranging from 2.9, 3.0, 3.2 and 8.4 months, respectively, in German, US, French and Dutch studies [18, 26, 32, 33] to 20.5 and 20.2 months, respectively, in Italian and Saudi Arabian studies [34, 35]. Direct comparisons might be limited by differences in study design and screening rates. However, well established newborn hearing screening programmes with high screening rates have been demonstrated to significantly lower the median age at diagnosis [18, 36]. Considering the critical time period that is crucial for speech and language development, cases with bilateral WHO grade II and III hearing loss are diagnosed late, resulting in delayed treatment with hearing aids and, correspondingly, negative consequences for the affected children.
Children with a documented failed newborn hearing screening test were diagnosed at a median age of 4.0 months. This finding confirms that children who fail the newborn hearing screening test are in fact diagnosed early, which underlines the positive effect of newborn hearing screening. In our study, data concerning newborn hearing screening was incomplete due to the retrospective design of the study and inconsistent documentation of this information in medical records. While the analysis of our data does not allow us to clearly identify the cause of late diagnosis, certain mechanisms may be suggested by comparison with published literature data. Congenital hearing loss can be missed at birth if newborn hearing screening is not conducted, if children are lost to follow-up and if false negative screening results occur. According to a recent assessment in 47 countries and regions, screening rates are estimated to be approximately 96% [37]. A German study published in 2023 reported a screening rate of 98.7% [38], an Israeli study 98.7% [36] and an Australian study 96.2% [39]. The screening rate in Switzerland was not assessed in our study, but a survey from 2013 suggested that most newborns (97.9%) are screened in Switzerland [40]. According to the same study, 13% of newborns with a failed initial screening test were lost to follow-up [40]. International data show different rates of loss to follow-up after a failed initial hearing screen, but a systematic review comprising 53 articles from 2016 reported loss to follow-up rates of 20.5% [41].
It is questionable how stronger adherence to the newborn hearing screening algorithm can be achieved and whether a legal obligation would be advantageous over a mere recommendation. It should also be taken into account that slight hearing loss, hearing loss confined to specific frequency ranges and hearing loss due to auditory neuropathy spectrum disorders can be missed due to methodological limitations such as using oto-acoustic emissions for newborn hearing screening [42]. In addition, late-onset, progressive and acquired hearing loss are potential explanations for late diagnosis. Routine hearing evaluation by paediatricians, hearing testing when the hearing status of a child has to be questioned due to specific symptoms (e.g. reduced response, speech and language delay) and continued audiometric surveillance of children at risk of developing late-onset hearing loss (e.g. intrauterine CMV and other infections, positive family history, certain syndromes, meningitis) are important [16].
In our study, unilateral hearing loss was less common than bilateral hearing loss. This corresponds to the results of other studies showing a unilateral hearing loss in 20% to 42% of all hearing loss cases [24–26, 32]. Likewise, the distribution of type and grade of hearing loss in our study is also comparable to published data from other countries [18, 26, 43, 44].
While most children with bilateral hearing loss had a documented failed hearing screen (37.4%), a smaller proportion passed the hearing screen and possibly developed hearing loss after the newborn period (6.5%). In only 1.9% of cases, newborn hearing screening was not performed. Due to the retrospective nature of the study and inconsistent documentation of screening status in medical records, the status of newborn hearing screening remained unknown in 23.4% of the cases, limiting further analysis. Given the relatively high median age at diagnosis in this group, it can be assumed that the hearing loss of some of these children was missed at birth.
In our study, three quarters of cases were referred from the private sector (paediatricians; ear-nose-throat specialists), while the remaining cases were directly referred from hospitals (maternity clinics; children’s hospitals). This was surprising, as the algorithm of newborn hearing screening at that time required a full paediatric audiology assessment of those failing oto-acoustic emissions testing in both ears at the birth clinic. However, the distribution in our study corresponds to data from Zurich [40] that was comparable regarding the proportions of referrals from the private sector (slightly over 75%) and might be explained by the usual pattern of referrals at this time, possibly contributing to a delay in diagnosis.
To our knowledge, this is the first study on the age at diagnosis of paediatric permanent hearing loss in Switzerland. Moreover, our study demonstrated the positive effect of the newborn hearing screening programme on the age at diagnosis. The fact that our descriptive date is mostly consistent with published data suggests a high degree of representativeness of our study population.
The main limitation of our study is due to the retrospective design of the study and data quality. In particular, information regarding the status of newborn hearing screening was incomplete due to inconsistent documentation in medical records, thereby limiting further analysis. Moreover, information in other areas was also limited, often making it impossible to adequately differentiate between late diagnosis of congenital hearing loss and late-onset or even acquired hearing loss, thereby hindering the comparison with published data. Selection bias might have influenced our study, possibly impacting the external validity of the results. Therefore, additional prospective studies would be required to provide sound recommendations for improved diagnosis and screening of paediatric hearing loss.
Overall, the age at diagnosis of paediatric permanent hearing loss is variable. A considerably late diagnosis was found in some cases, especially in cases of bilateral hearing loss that should have been diagnosed by newborn hearing screening in congenital cases and in unilateral hearing loss. The median age at diagnosis was lower at higher WHO grades of bilateral hearing loss. Children with bilateral hearing loss and documented failed newborn hearing screen are diagnosed early, confirming the positive effect of the newborn hearing screening programme in Switzerland. Therefore, adherence to national newborn hearing screening recommendations is important. Newborn hearing screening should be conducted in all newborns, and failed results should be followed up appropriately. As hearing loss might be missed in the newborn period or might develop afterwards, an increased awareness of this issue is crucial during further childhood, and the hearing status as well as speech and language development should be regularly assessed or whenever required. It is likely that the newly issued revised national recommendations for bilateral newborn hearing screening in Switzerland will result in earlier diagnosis of permanent paediatric hearing loss, especially in unilateral cases. It will hopefully further raise awareness of the importance of a timely diagnosis of paediatric hearing loss in general.
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Tomblin JB, Harrison M, Ambrose SE, Walker EA, Oleson JJ, Moeller MP. Language Outcomes in Young Children with Mild to Severe Hearing Loss. Ear Hear. 2015 Nov-Dec;36 Suppl 1(0 1):76S-91S. doi: .
2. Moeller MP, Tomblin JB, Yoshinaga-Itano C, Connor CM, Jerger S. Current state of knowledge: language and literacy of children with hearing impairment. Ear Hear. 2007 Dec;28(6):740–53.
3. Moeller MP. Current state of knowledge: psychosocial development in children with hearing impairment. Ear Hear. 2007 Dec;28(6):729–39.
4. Roland L, Fischer C, Tran K, Rachakonda T, Kallogjeri D, Lieu JE. Quality of Life in Children with Hearing Impairment: Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 2016 Aug;155(2):208–19.
5. Kral A, Dorman MF, Wilson BS. Neuronal Development of Hearing and Language: Cochlear Implants and Critical Periods. Annu Rev Neurosci. 2019 Jul;42(1):47–65.
6. Moeller MP. Early intervention and language development in children who are deaf and hard of hearing. Pediatrics. 2000 Sep;106(3):E43.
7. Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998 Nov;102(5):1161–71.
8. Kennedy CR, McCann DC, Campbell MJ, Law CM, Mullee M, Petrou S, et al. Language ability after early detection of permanent childhood hearing impairment. N Engl J Med. 2006 May;354(20):2131–41.
9. Pimperton H, Kennedy CR. The impact of early identification of permanent childhood hearing impairment on speech and language outcomes. Arch Dis Child. 2012 Jul;97(7):648–53.
10. Pimperton H, Blythe H, Kreppner J, Mahon M, Peacock JL, Stevenson J, et al. The impact of universal newborn hearing screening on long-term literacy outcomes: a prospective cohort study. Arch Dis Child. 2016 Jan;101(1):9–15.
11. Yoshinaga-Itano C. Benefits of early intervention for children with hearing loss. Otolaryngol Clin North Am. 1999 Dec;32(6):1089–102. doi: https://doi.org/10.1016/S0030-6665(05)70196-1
12. Yoshinaga-Itano C, Manchaiah V, Hunnicutt C. Outcomes of Universal Newborn Screening Programs: systematic Review. J Clin Med. 2021 Jun;10(13):2784.
13. Ching TY. Is Early Intervention Effective in Improving Spoken Language Outcomes of Children With Congenital Hearing Loss? Am J Audiol. 2015 Sep;24(3):345–8.
14. Lieu JE, Kenna M, Anne S, Davidson L. Hearing Loss in Children: A Review. JAMA. 2020 Dec;324(21):2195–205.
15. Mehra S, Eavey RD, Keamy DG Jr. The epidemiology of hearing impairment in the United States: newborns, children, and adolescents. Otolaryngol Head Neck Surg. 2009 Apr;140(4):461–72.
16. Kenna MA. Acquired Hearing Loss in Children. Otolaryngol Clin North Am. 2015 Dec;48(6):933–53.
17. Kittrell AP, Arjmand EM. The age of diagnosis of sensorineural hearing impairment in children. Int J Pediatr Otorhinolaryngol. 1997 Jun;40(2-3):97–106. doi: https://doi.org/10.1016/S0165-5876(97)01506-1
18. Berger R, Goeze A, Müller-Mazzotta J, Hanschmann H, Kadaifciu B, Eroglu E. Frühzeitige Diagnose kindlicher Hörstörung durch Einführung des Neugeborenen Hörscreenings (UNHS) [Early diagnosis of infant hearing impairment after introduction of newborn hearing screening (UNHS)]. Laryngorhinootologie. 2012 Oct;91(10):637-40. German. doi: .
19. Kanji A, Khoza-Shangase K, Moroe N. Newborn hearing screening protocols and their outcomes: A systematic review. Int J Pediatr Otorhinolaryngol. 2018 Dec;115:104–9.
20. Neumann K, Mathmann P, Chadha S, Euler HA, White KR. Newborn Hearing Screening Benefits Children, but Global Disparities Persist. J Clin Med. 2022 Jan;11(1):271.
21. Report of the informal working group on prevention of deafness and hearing impairment programme planning, Geneva, 18-21 June 1991. Geneva: World Health Organization; 1991. Available from: http://www.who.int/iris/handle/10665/58839
22. R Core Team. (2023). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Availabe from: https://www.R-project.org/
23. Ghogomu N, Umansky A, Lieu JE. Epidemiology of unilateral sensorineural hearing loss with universal newborn hearing screening. Laryngoscope. 2014 Jan;124(1):295–300.
24. Fitzpatrick EM, Whittingham J, Durieux-Smith A. Mild bilateral and unilateral hearing loss in childhood: a 20-year view of hearing characteristics, and audiologic practices before and after newborn hearing screening. Ear Hear. 2014;35(1):10–8.
25. Hung YC, Chen PH, Lin TH, Lim TZ. Children With Unilateral Hearing Loss After Newborn Hearing Screening in Taiwan. Am J Audiol. 2022 Sep;31(3):646–55.
26. van Beeck Calkoen EA, Engel MS, van de Kamp JM, Yntema HG, Goverts ST, Mulder MF, et al. The etiological evaluation of sensorineural hearing loss in children. Eur J Pediatr. 2019 Aug;178(8):1195–205.
27. Finckh-Krämer U, Spormann-Lagodzinski ME, Nubel K, Hess M, Gross M. Wird die Diagnose bei persistierenden kindlichen Hörstörungen immer noch zu spät gestellt? [Is diagnosis of persistent pediatric hearing loss still made too late?]. HNO. 1998 Jun;46(6):598-602. German. doi: .
29. Harrison M, Roush J, Wallace J. Trends in age of identification and intervention in infants with hearing loss. Ear Hear. 2003 Feb;24(1):89–95.
29. Weichbold V, Nekahm-Heis D, Welzl-Müller K. Zehn Jahre Neugeborenen-Hörscreening in Osterreich. Eine Evaluierung [Evaluation of the Austrian Newborn Hearing Screening Program]. Wien Klin Wochenschr. 2005 Sep;117(18):641-6. German. doi: .
30. American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007 Oct;120(4):898–921.
31. WHO Hearing screening: considerations for implementation. Available from: https://www.who.int/publications/i/itei/9789240032767. Accessed 04/2024.
32. Dalzell L, Orlando M, MacDonald M, Berg A, Bradley M, Cacace A, et al. The New York State universal newborn hearing screening demonstration project: ages of hearing loss identification, hearing aid fitting, and enrollment in early intervention. Ear Hear. 2000 Apr;21(2):118–30.
33. Langagne T, Lévêque M, Schmidt P, Chays A. Universal newborn hearing screening in the Champagne-Ardenne region: a 4-year follow-up after early diagnosis of hearing impairment. Int J Pediatr Otorhinolaryngol. 2010 Oct;74(10):1164–70.
34. Canale A, Favero E, Lacilla M, Recchia E, Schindler A, Roggero N, et al. Age at diagnosis of deaf babies: a retrospective analysis highlighting the advantage of newborn hearing screening. Int J Pediatr Otorhinolaryngol. 2006 Jul;70(7):1283–9.
35. Alshawi YA, Al-Gazlan N, Alrawaf F, Almuhawas F. Value of Newborn Hearing Screening on Early Intervention in the Saudi Population and Review of International Records. Cureus. 2019 Oct;11(10):e5990.
36. Wasser J, Ari-Even Roth D, Herzberg O, Lerner-Geva L, Rubin L. Assessing and monitoring the impact of the national newborn hearing screening program in Israel. Isr J Health Policy Res. 2019 Mar;8(1):30.
37. Mackey AR, Bussé AM, Hoeve HL, Goedegebure A, Carr G, Simonsz HJ, et al.; EUS€REEN Foundation. Assessment of hearing screening programmes across 47 countries or regions II: coverage, referral, follow-up and detection rates from newborn hearing screening. Int J Audiol. 2021 Nov;60(11):831–40.
38. Thangavelu K, Martakis K, Feldmann S, Roth B, Herkenrath P, Lang-Roth R. Universal Newborn Hearing Screening Program: 10-Year Outcome and Follow-Up from a Screening Center in Germany. Int J Neonatal Screen. 2023 Oct;9(4):61.
39. Bailey HD, Bower C, Krishnaswamy J, Coates HL. Newborn hearing screening in Western Australia. Med J Aust. 2002 Aug;177(4):180–5.
40. Metzger D, Pezier TF, Veraguth D. Evaluation of universal newborn hearing screening in Switzerland 2012 and follow-up data for Zurich. Swiss Med Wkly. 2013 Dec;143:w13905.
41. Ravi R, Gunjawate DR, Yerraguntla K, Lewis LE, Driscoll C, Rajashekhar B. Follow-up in newborn hearing screening - A systematic review. Int J Pediatr Otorhinolaryngol. 2016 Nov;90:29–36.
42. Wroblewska-Seniuk KE, Dabrowski P, Szyfter W, Mazela J. Universal newborn hearing screening: methods and results, obstacles, and benefits. Pediatr Res. 2017 Mar;81(3):415–22.
43. Finckh-Krämer U, Spormann-Lagodzinski M, Gross M. German registry for hearing loss in children: results after 4 years. Int J Pediatr Otorhinolaryngol. 2000 Dec;56(2):113–27. doi: https://doi.org/10.1016/S0165-5876(00)00401-8
44. Rissmann A, Koehn A, Loderstedt M, Schwemmle C, Goetze G, Bartel S, et al. Population-based cross-sectional study to assess newborn hearing screening program in central Germany [Erratum in: Int J Pediatr Otorhinolaryngol. 2019 Apr;119:193]. Int J Pediatr Otorhinolaryngol. 2018 Apr;107:110–20.