Surveillance of varicella-associated paediatric hospitalisations and complications
in Switzerland from 2021 to 2023
DOI: https://doi.org/https://doi.org/10.57187/s.3872
Noëlle Staldera,
Ulrich Heiningerab,
Michael Buettcheracde
a Medical Faculty, University of Basel, Basel, Switzerland
b Paediatric Infectious Diseases and Vaccinology,
University Children’s Hospital Basel (UKBB), Basel, Switzerland
c Paediatric Infectious Diseases Unit, Department of
Paediatrics, Children’s Hospital of Central Switzerland, Lucerne, Switzerland
d Paediatric Pharmacology and Pharmacometrics Research
Centre at University Children’s Hospital Basel (UKBB), Basel, Switzerland
e Faculty of Health Sciences and Medicine, University
of Lucerne, Lucerne, Switzerland
Summary
AIM: To prospectively assess varicella zoster virus-associated
disease burden in hospitalised children 0–16 years of age prior to the
introduction of universal varicella vaccination in Switzerland.
METHODS: We performed an observational,
prospective surveillance study. Anonymised data (clinical
characteristics, diagnostics, treatment and outcome) of hospitalised children were
available from monthly active case reporting by 29 paediatric clinics and
hospitals to the Swiss Paediatric Surveillance Unit from July 2021 to June
2023.
RESULTS: During the 2-year study period, 239
children were hospitalised with varicella (n = 224; 94%) or herpes zoster (n = 15;
6%). Mean age was 5 years, median 4.7 years (range 0–16 years). In 13 patients, varicella
was
concomitant and not the primary reason for hospitalisation. 199 patients (83%)
were primary healthy, 138 were male (58%). Mean duration of hospitalisation for
varicella patients was 5.7 days. Of the 224 children with varicella, 211 (94%)
were primary hospitalised due to varicella, 120 (54%) had acute skin
complications (including 52 Streptococcus pyogenes infections), 29 (13%)
musculoskeletal and 27 (12%) neurological complications. Two patients (1%) had
ischaemic strokes. 33 (14%) patients (32 with varicella and 1 with herpes
zoster) required intensive care treatment (mean duration 3.5 days). 40 patients
with varicella (18%) required surgical interventions. Two (1%) patients died. The
calculated hospitalisation
incidence rate was 7.2 per 100,000 for children and the calculated hospitalisation
rate was 12.6 per 10,000 cases.
CONCLUSIONS: Varicella is associated with considerable morbidity, particularly in
primary healthy children. Complications affecting the skin (mainly secondary
bacterial infections), musculoskeletal and neurological systems are the main
reasons for hospitalisation and may cause death even in previously healthy,
immunocompetent children. The baseline burden of disease presented herein will permit
evaluation of the impact of universal varicella vaccination, introduced in
Switzerland in January 2023.
Introduction
Varicella zoster virus (VZV) infection is a well-known
and highly infectious childhood disease. Primary infection causes chickenpox,
clinically presenting as a characteristic pruritic rash with macular, papular,
vesicular and crusted skin lesions which may appear simultaneously. After
primary infection, VZV persists lifelong in sensory nerve ganglia and when
reactivated presents as shingles, with a characteristic dermatomal
distribution, also known as herpes zoster [1].
Most people think of varicella as a benign childhood disease. However,
worldwide, numerous studies have demonstrated a relevant amount of morbidity
including bacterial and neurological complications leading to hospitalisation
in children with or without underlying chronic conditions [2–5].
In Switzerland, the last prospective surveillance
study on VZV-associated hospitalisations of children was conducted during a 3-year
period from 2000 to 2003 and included 335 cases and a hospitalisation rate of
13 per 10,000. The median age of patients was 3.5 years. Secondary bacterial
infections, central nervous system involvement and pneumonitis were the most
common complications. Overall, 319 complications were recorded. Intensive care
unit (ICU) treatment was documented in 11 (3%) patients, 12 (4%) experienced sequelae
and 3 died [6].
Since January 2023, the Federal Office of Public
Health (FOPH) has recommended universal varicella vaccination (UVV) for
infants. The preferred immunisation is a quadrivalent measles, mumps, rubella
and varicella (MMRV) vaccine with the first dose administered at 9 months and
the second dose at 12 months of age. The FOPH also recommend a catch-up
vaccination for all individuals 1–40 years of age who have not had varicella or
two varicella vaccinations yet [7]. In
addition, the FOPH and the Federal Commission for Vaccination (EKIF) have
recommended vaccination against herpes zoster with the adjuvanted subunit
vaccine Shingrix® since 2021. This is for healthy people aged ≥65 years, patients
with immunodeficiency aged ≥50 years and patients with severe immunodeficiency
aged ≥18 years [8].
The aim of this study was to assess the burden of VZV-associated
hospitalisations and complications in children (0–16 years) over a 2-year
period from 2021 to 2023 in Switzerland. This will allow future evaluation of
the impact of universal varicella vaccination in Switzerland.
Methods
Study design
We are conducting an observational, prospective
surveillance study, which started in July 2021 and is planned to continue until
June 2027, on reported VZV hospitalisations in children by use of the Swiss Paediatric
Surveillance Unit (SPSU) in Switzerland to assess VZV-associated disease burden
in hospitalised children aged 0–16 years prior to the introduction of universal
varicella vaccination in Switzerland. All 29 Swiss paediatric clinics are
members of the Swiss Paediatric Surveillance Unit, participate in the study and
report their cases monthly to the Swiss Paediatric Surveillance Unit [9]. This current
analysis refers to the
initial 2-year period (July 2021 to June 2023) without a follow-up.
The study protocol was prepared by authors MB and UH
and approved by the Ethics Committee Northwestern and Central Switzerland
(EKNZ) (project number: 2021-00211). It can be accessed by contacting the
corresponding author (MB). According to article 34 a of the HFG
(swissethics.ch), the need for individual consent was waived by the Ethics Committee.
Case definition
Hospitalised children and adolescents aged ≤16 years
(in the text described as “children”) with clinical manifestations of VZV infection
(ICD-10: B01.-), i.e. varicella or herpes zoster.
Study population
All children who meet the case definition regardless
of underlying chronic conditions or other comorbidities are eligible for
reporting. Patients with VZV infection who were primarily hospitalised for a
different diagnosis were also included. After reporting a case to the Swiss Paediatric
Surveillance Unit, the centres receive an anonymised questionnaire (Supplementary
1. Appendix Questionnaire). Questionnaire data are collected in a central
database (secuTrial®),
set up by the clinical trial unit (CTU) of Cantonal Hospital Lucerne. MB,
UH and the database managers are the only individuals who had access. Data
cleaning was performed by MB and NS. Missing data were correctly addressed and
considered as missing or unknown.
With regards to VZV-associated stroke cases, reports
via Swiss Paediatric Surveillance Unit were supplemented by a second source of
information, the Swiss Neuropaediatric Stroke Registry (SNSR)
(https://snpsr.neuropaediatrie.ch/).
To record and describe the epidemiological situation in Switzerland, place of
residence (Switzerland or Not-Switzerland) was recorded, not patient’s race or
ethnicity. Analyses were performed for three subgroups, primary healthy
patients, immunocompromised patients and patients with underlying disease other
than immunodeficiency. The quantitative variable age was assigned to four age
categories. The categories were chosen by analogy with similar studies from
other countries to ensure comparability [3–6, 10–23].
Other quantitative variables were not further categorised.
Statistical analysis
Statistical analysis was performed with IBM SPSS
Statistics 29.0.1.0 (171). We used data editor functions, prepared and cleaned
data and performed data transformation with arithmetic and statistical
functions (mean, median, range). The functions are described in chapters 5, 7
and 8 of “IBM SPSS Statistics 29 Core System User’s Guide” [24]. For the incidence
calculation, population
data were adopted from the Federal Office of Statistics [25]. We extracted age-specific
numbers of inhabitants from
population figures for 2021 and 2022 in Switzerland for the calculation of mean
age. For the estimated number of varicella cases per year, we used varicella
incidence numbers from a systematic review on estimated age-specific seroprevalence
and annual
varicella incidence per 100,000 in Switzerland and extrapolated this data to the
mean inhabitants stated above [16].
This resulted in an estimated number of 85,398 varicella cases per year. We
calculated an annual hospitalisation incidence per 100,000 inhabitants by using
the calculation path (# hospitalised
patients / # inhabitants) × 100,000 where # hospitalised patients was the mean
age-specific number of hospitalised varicella patients with a residence in
Switzerland during the 2-year study period, yielding a figure of (108 / 1,493,157)
× 100,000 = 7.2 per 100,000.
The calculation of the annual hospitalisation rate per 10,000 varicella
patients was (# hospitalised
patients / estimated # varicella cases per year) × 10,000 or (108 / 85,398) × 10,000 = 12.6 per 10,000
varicella patients. The
definition for # hospitalised patients remained the same. The
varicella hospitalisation incidence refers to Swiss residents aged 0 to 16
years while the annual hospitalisation rate refers to the estimated varicella
cases per year. For further illustration, see table 1.
Table 1Annual varicella hospitalisation incidence
and hospitalisation rate by age groups. Data from: Bollaerts K, Riera-Montes M, Heininger
U, Hens N, Souverain A, Verstraeten
T, et al. A systematic review of varicella seroprevalence in European countries
before universal childhood immunization: deriving incidence from seroprevalence
data. Epidemiol Infect. 2017;145(13):2666–77.
https://doi.org/10.1017/S0950268817001546
| Age group |
Number of inhabitants* |
Seroprevalence (%)** |
Varicella incidence per 100,000** |
Estimated number of varicella cases per year*** |
Number of hospitalised patients**** |
Annual hospitalisation incidence per 100,000 |
Annual hospitalisation rate per 10,000 |
| <9 months |
84,520 |
36% |
7368 |
32,166 |
12 |
14.2 |
19.9 |
| 9 months – 4 years |
352,048 |
52 |
14.8 |
| 5–9 years |
447,381 |
59% |
11,798 |
52,782 |
41 |
9.2 |
7.8 |
| 10–16 years |
609,028 |
0.7% |
74### |
450 |
3 |
0.5 |
66.7 |
| Total |
1,493,157 |
96.5% |
|
85,398 |
108 |
7.2 |
12.6 |
Tools
ChatGPT 3.5 (Version 10.01.2024) was used to improve
scientific English in the discussion section. We used the prompt Please improve
the text in respect to the scientific language but keep the contents. The
text output was revised and corrected by the authors regarding the accuracy of
content. We used the STROBE cohort reporting checklist guidelines before paper
submission [26].
Results
During the 2-year study period, 239
hospitalised patients were reported (237 only to the Swiss Paediatric Surveillance
Unit, 1 only to the Swiss Neuropaediatric Stroke Registry and 1 to both
surveillance systems). Varicella was the primary admission reason for more than
two thirds of all varicella patients. For only a few patients, a different
diagnosis was the primary reason for
their hospital admission: pneumopathy (n = 6),
epilepsy (n = 2), neuroblastoma (n = 1), perforated appendicitis (n = 1), renal
failure (n = 1), syndrome of inappropriate antidiuretic hormone secretion
(SIADH) (n = 1) and a new diagnosis of dilated cardiomyopathy (n = 1). All
herpes zoster patients were hospitalised due to their clinical herpes zoster
presentation.
Immunocompromised patients were defined as patients
with primary immunodeficiency or under immunosuppressive therapy and possible
other chronic disease. Underlying chronic diseases were skin disorders, cancer
or leukaemia, genetic syndromes, cardiovascular diseases, HIV, inflammatory
bowel disease, congenital neurological disorder, lung disorder, nephrotic
syndrome, epilepsy, vasculitis, sickle cell disease and haemolytic anaemia.
Skin disorders, cancer or leukaemia, genetic syndromes and cardiovascular
diseases were most common over the 2-year study period. There were no
significant differences between the two years of observation in the distribution
of the underlying chronic diseases. Figure 1 shows that less than one fifth
(40/239) of all hospitalised patients had any underlying chronic disease
including immunodeficiency. Only 5 (2%) of all immunocompromised patients had
no underlying chronic disease other than their immunodeficiency.
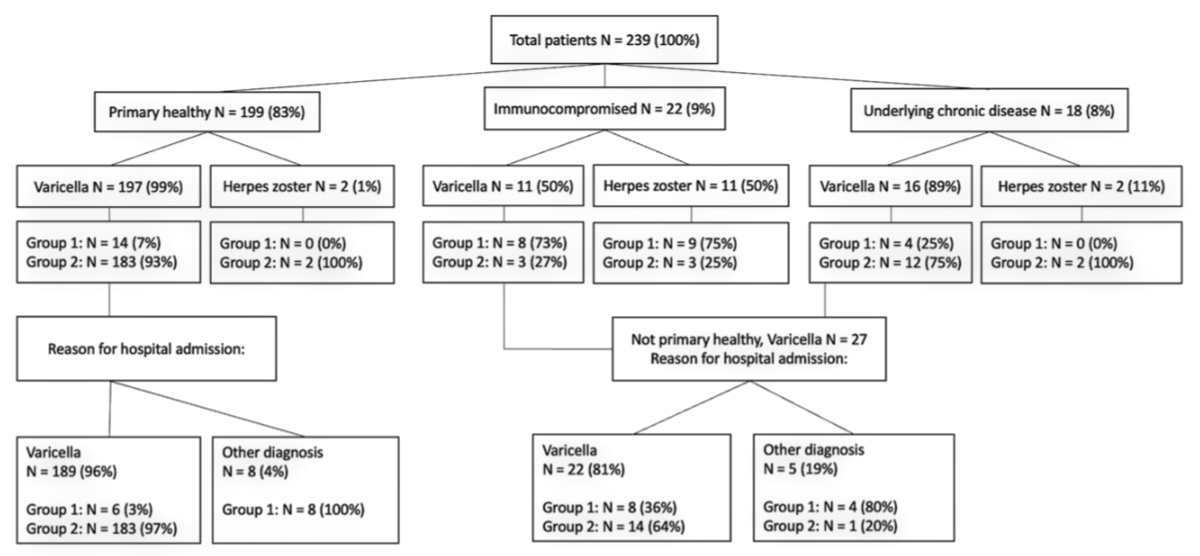
Figure 1Study
flow chart of varicella zoster virus (VZV)-associated hospitalisations. Underlying
chronic disease: excluding immunocompromised host who are presented separately
in the figure. Group 1: no complications. Group 2: with complications. Other
diagnosis: varicella was not primary reason for hospitalisation. All patients
hospitalised due to another diagnosis had concomitant varicella.
Table 2 shows the general characteristics of primary
healthy patients and those with underlying chronic illnesses including
immunodeficiency. For
immunocompromised patients and patients with an underlying chronic disease
other than immunodeficiency, median age was higher. Patients’ residence was for
nearly all patients in Switzerland. No patient had received vaccination prior to
hospitalisation. VZV exposure was mostly unknown or via contact with family
members regardless of patients’ immune status and other chronic comorbidities.
Almost half of all hospitalisations occurred in children aged 9 months to 4
years old. Four primary healthy patients were hospitalised with
varicella due to their young age which the treating physicians considered a
risk factor for a complicated course of chickenpox. Of these, two were neonates
and two were 2 months old. However, none of them experienced a complication and
they were discharged within five days. The mean interval between the onset of
rash and hospitalisation was 4.95 days(median 4, range −1–61) and the mean duration
of all
varicella hospitalisations was 5.7 days (median 4, range 0–33). Patients with
herpes zoster, if not primary healthy, stayed longer in hospital than patients
with varicella. In general, immunocompromised patients stayed longer in
hospital than primary healthy patients. In contrast to herpes zoster, varicella
patients with underlying chronic disease other than immunodeficiency were
hospitalised for a shorter period than primary healthy varicella patients.
Table 2General characteristics. Study year 1 (July 2021–June
2022). Study year 2 (July 2022–June 2023).
|
Study year 1 |
Study year 2 |
Total study
period |
Primary
healthy |
Immunocompromised |
Underlying
chronic disease other than immunodeficiency |
| Patients (n) |
51 |
188 |
239 |
199 |
22 |
18 |
| Female sex, n (%) |
22 (43%) |
79 (42%) |
101 (42%) |
80 (40%) |
10 (45%) |
9 (50%) |
| Age (in years) |
Mean |
4.3 |
5.2 |
5.0 |
4.6 |
7.9 |
6.2 |
| Median |
3.6 |
4.8 |
4.7 |
4.6 |
7.0 |
6.9 |
| Range |
0–14 |
0–16 |
0–16 |
0–15 |
1–16 |
0–12 |
| Age group
distribution, n (%) |
<9 months |
10 (20%) |
15 (8%) |
25 (10%) |
22 (11%) |
0 (0%) |
3 (17%) |
| 9 months – 4 years |
27 (53%) |
83 (44%) |
110 (46%) |
97 (49%) |
9 (41%) |
4 (22%) |
| 5–9 years |
8 (16%) |
78 (41%) |
86 (36%) |
73 (37%) |
5 (23%) |
8 (44%) |
| 10–16 years |
6 (12%) |
12 (6%) |
18 (8%) |
7 (4%) |
8 (36%) |
3 (17%) |
| Residence, n (%) |
Switzerland |
49 (96%) |
182 (97%) |
231 (97%) |
192 (97%) |
22 (100%) |
17 (94%) |
| Not-Switzerland |
2 (4%) |
6 (3%) |
8 (3%) |
7 (4%) |
0 (0%) |
1 (6%) |
| Varicella
vaccination history, n (%) |
Yes |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
| No |
48 (94%) |
176 (94%) |
224 (94%) |
187 (94%) |
20 (91%) |
17 (94%) |
| Unknown |
3 (6%) |
12 (6%) |
15 (6%) |
12 (6%) |
2 (9%) |
1 (6%) |
| Varicella
zoster virus exposure*, n (%) |
Within family |
17 (37%) |
62 (35%) |
79 (35%) |
68 (35%) |
5 (45%) |
6 (38%) |
| Outside family |
5 (11%) |
14 (8%) |
19 (8%) |
13 (7%) |
4 (36%) |
2 (13%) |
| Unknown |
24 (52%) |
103 (58%) |
126 (56%) |
116 (59%) |
2 (18%) |
8 (50%) |
| Varicella, n |
|
|
224 |
197 |
11 |
16 |
| – Hospitalisation
days |
Mean |
|
|
5.7 |
5.8 |
6.3 |
3.4 |
| Median |
|
|
4 |
4 |
5 |
3 |
| Range |
|
|
0–33 |
0–33 |
2–16 |
1–10 |
| Herpes zoster,
n |
|
|
15 |
2 |
11 |
2 |
| – Hospitalisation
days |
Mean |
|
|
8.4 |
2.5 |
9.1 |
10.5 |
| Median |
|
|
8 |
2.5 |
8 |
10.5 |
| Range |
|
|
2–16 |
2–3 |
3–16 |
10–11 |
Figure 2 shows that few varicella cases were reported
during study year 1 with a nationwide outbreak during study year 2 from
December 2022 to May 2023. Cantons with the highest number of varicella hospitalisations
in their children’s hospitals and clinics were Zurich (n = 49), Vaud (n = 29),
Lucerne (n = 24), Basel (n = 22) and Bern (n = 20).
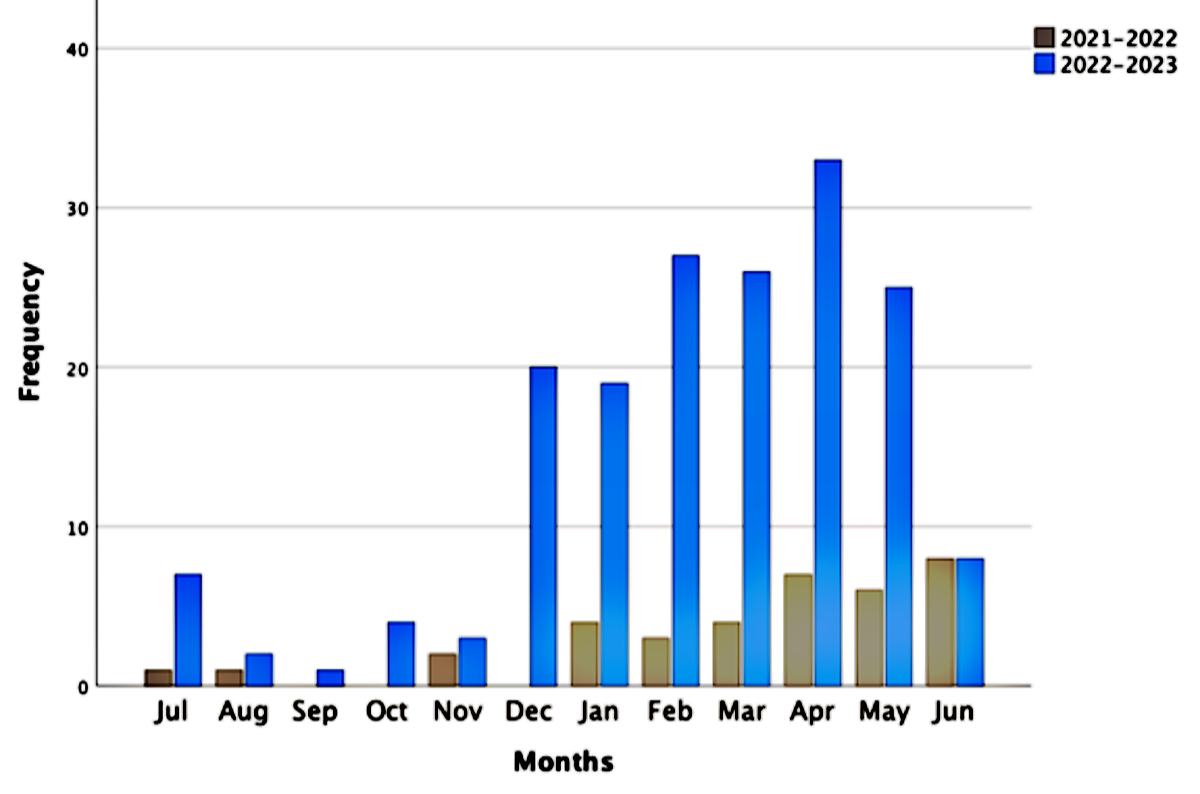
Figure 2Seasonality of varicella hospitalisations.
Varicella as primary reason for hospitalisation: study year one n = 45; study
year two: n = 179.
Table 3 demonstrates complications from
varicella for the total study period. Complications
secondary to varicella were the most common reason for hospitalisation in the majority
of patients, most frequently skin disorders. From April 2022 to July 2023,
invasive infections with group A Streptococcus were reported in 52 varicella
cases. Musculoskeletal complications were the second most common and
neurological complications the third. Several patients experienced more than
one complication. Over 90% of primary healthy children experienced
complications compared to less than a third of immunocompromised patients and
three quarters of patients with an underlying chronic disease other than
immunodeficiency. Severe complications with admission to the ICU appeared only
in primary healthy children. Typical were skin infections, focal purulent
collection, musculoskeletal and neurological disorders and lung involvement
with pneumonia or pneumonitis. And fulminant varicella with multiorgan failure,
sepsis or death only occurred in primary healthy children as well. Two patients
had a varicella-associated ischaemic stroke. Two deaths occurred. One 2-year-old patient
with varicella and a
fulminant pneumococcal sepsis already died on day of admission in the emergency
department. The other fatal case was also two years old, admitted with
varicella in septic shock, who also died in the emergency unit shortly after
arrival. Clinical circumstances and microbiology revealed Streptococcus
pyogenes as the cause of the lethal sepsis.
Table 3Complications in 224 patients with varicella.
n = 224 patients. Total: total study period (July 2021 – June 2023).
| Varicella patients, n (%) |
Total |
Primary healthy |
Immunocompromised |
Underlying chronic
disease other than immunodeficiency |
| 224 (100%) |
197 (100%) |
11 (100%) |
16 (100%) |
| Complication,
n (%) |
198 (88%) |
183 (93%) |
3 (27%) |
12 (75%) |
| Skin infection2 |
120 (61%) |
111 (61%) |
1 (33%) |
8 (67%) |
| – Invasive Group A Streptococcal infection3 |
52 (43%) |
47 (42%) |
1 (33%) |
4 (50%) |
| Musculoskeletal4 |
29 (15%) |
26 (14%) |
0 (0%) |
3 (25%) |
| Neurological5 |
27 (14%) |
25 (14%) |
1 (33%) |
2(17%) |
| Focal purulent collection6 |
21 (11%) |
19 (10%) |
0 (0%) |
2 (17%) |
| Fulminant, with multiorgan failure |
19 (10%) |
19 (10%) |
0 (0%) |
0 (0%) |
| Pneumopathy7 |
16 (8%) |
15 (8%) |
1 (33%) |
0 (0%) |
| Sepsis |
13 (7%) |
13 (7%) |
0 (0%) |
0 (0%) |
| Haematological8 |
7 (4%) |
5 (3%) |
0 (0%) |
2 (17%) |
| Lymphadenitis |
5 (3%) |
5 (3%) |
0 (0%) |
0 (0%) |
| Keratitis |
2 (1%) |
2 (1%) |
0 (0%) |
0 (0%) |
| Hepatitis |
1 (0.5%) |
0 (0%) |
0 (0%) |
1 (8%) |
| Reye’s syndrome |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
| Deaths, n |
2 |
2 |
0 |
0 |
| ICU admission, n (%) |
32 (16%) |
32 (17%) |
0 (0%) |
0 (0%) |
| Varicella complication of
ICU patients, n (%) |
| Skin |
10 (31%) |
10 (31%) |
0 (0%) |
0 (0%) |
| Musculoskeletal4 |
10 (31%) |
10 (31%) |
0 (0%) |
0 (0%) |
| Focal purulent collection |
7 (22%) |
7 (22%) |
0 (0%) |
0 (0%) |
| Fulminant, with multiorgan failure |
7 (22%) |
7 (22%) |
0 (0%) |
0 (0%) |
| Pneumonia |
6 (19%) |
6 (19%) |
0 (0%) |
0 (0%) |
| Toxic shock syndrome |
6 (19%) |
6 (19%) |
0 (0%) |
0 (0%) |
| Neurological5 |
5 (16%) |
5 (16%) |
0 (0%) |
0 (0%) |
| Septic shock |
2 (6%) |
2 (6%) |
0 (0%) |
0 (0%) |
| Cardiovascular9 |
1 (3%) |
1 (3%) |
0 (0%) |
0 (0%) |
| Haematological8 |
1 (3%) |
1 (3%) |
0 (0%) |
0 (0%) |
| SIADH |
1 (3%) |
1 (3%) |
0 (0%) |
0 (0%) |
| Patients with surgical intervention,
n (%) |
40 (18%) |
38 (19%) |
0 (0%) |
2 (13%) |
Among the 15 patients with herpes zoster,
13 (87%) had an underlying chronic disease. Seven patients (47%) developed
complications of whom five were not primary healthy. Three patients had complications
that involved the central nervous system (meningitis n = 2, encephalitis n = 1).
Two patients had skin complications, namely cellulitis (n = 1) and invasive
group A streptococcal infection (n = 1). One patient had elevated liver
enzyme values (AST/ALT) in the laboratory and in one patient pneumonia was
detected on X-ray.
ICU treatment was necessary for 33 (14%)
patients, 32 (14%) with varicella and 1 (7%) with herpes zoster. For varicella
and herpes zoster patients, the mean length of stay in the ICU was 3.5 days
(median 2, range 0–14). The herpes zoster patient stayed in the ICU for 6 days and
improved status after infection. For varicella patients, the mean length of stay
was 3.4 days (median 2, range 0–14). Skin complications like cellulitis and
soft tissue abscess and musculoskeletal complications were the most common
reason for ICU admission (n = 10). Most varicella patients in the ICU
(91%) did not experience sequelae or die.
In total, 214 varicella patients had a favourable
outcome, i.e. had recovered (n = 117) or improved when discharged (n = 97).
Only 8 patients experienced sequelae, mainly remaining skin defects (n = 5),
residual loss of the vestibulocochlear nerve (n = 1), pain (n = 1) or ataxia (n
= 1). With regards to herpes zoster, 13 of 15 patients had a favourable
outcome. Six patients recovered and seven had improved status when discharged; only
two had sequelae, one a right peripheral facial nerve palsy and the other pain.
During hospitalisation, cerebrospinal fluid was
obtained from 22 (9%) patients. They were 2 times positive with varicella
zoster virus evidence. Table 4 shows the number and type of pathogens from
blood cultures and other site cultures for varicella patients. In total, 56 Streptococcus
pyogenes, 15 Staphylococcus aureus and 16 other bacteria were
reported. None of the 15 herpes zoster patients had a positive blood culture.
Table 4Pathogen detection in blood cultures and
cultures from other sites. Blood cultures in 151 (63%) of 239 patients. Other-site
cultures in 81 (35%) of 239 patients.
| Pathogen |
Positive blood
culture |
Positive other-site
culture |
| n (%) |
18 (100%) |
63 (100%) |
| Streptococcus
pyogenes |
10 (56%) |
41 (65%) |
| Others |
6 (33%) |
9 (14%) |
| Staphylococcus
aureus |
2 (11%) |
7 (11%) |
| Streptococcus
pyogenes and Staphylococcus
aureus |
0 (0%) |
5 (8%) |
| Staphylococcus
aureus and others |
0 (0%) |
1 (2%) |
Table 5 lists
therapies for varicella and herpes zoster patients. Varicella patients were
prescribed more antibiotic therapy while herpes zoster patients received more
antiviral therapy. All immunocompromised patients received intravenous
antiviral therapy. Antifungal therapy was only prescribed once for an
immunocompromised herpes zoster patient.
Table 5Therapies listed for varicella and herpes
zoster patients for the total study period.
| Number of patients, n (%) |
Varicella |
Primary healthy |
Immunocompromised |
Underlying chronic disease other than
immunodeficiency |
Herpes zoster |
Primary healthy |
Immunocompromised |
Underlying chronic disease other than
immunodeficiency |
| n = 224 |
n = 197 |
n = 11 |
n = 16 |
n = 15 |
n = 2 |
n = 11 |
n = 2 |
| Antiviral therapy |
44 (20%) |
29 (15%) |
11 (100%) |
4 (25%) |
14 (93%) |
1 (50%) |
10 (91%) |
2 (100%) |
| – Intravenous* |
40 (18%) |
25 (13%) |
11 (100%) |
4 (25%) |
14 (93%) |
1 (50%) |
11 (100%) |
2 (100%) |
| – Oral** |
25 (11%) |
12 (6%) |
9 (82%) |
4 (25%) |
11 (73%) |
0 (0%) |
10 (91%) |
1 (50%) |
| Antibiotic therapy |
168 (75%) |
156 (79%) |
4 (36%) |
8 (50%) |
8 (53%) |
2 (100%) |
5 (45%) |
1 (50%) |
| – Intravenous |
165 (74%) |
155 (79%) |
3 (27%) |
7 (44%) |
8 (53%) |
2 (100%) |
5 (46%) |
1 (50%) |
| – Oral |
134 (60%) |
123 (62%) |
3 (27%) |
8 (50%) |
3 (20%) |
2 (100%) |
1 (9%) |
0 (0%) |
| Antifungal therapy |
|
|
|
|
|
|
|
|
| – Intravenous |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
1 (7%) |
0 (0%) |
1 (9%) |
0 (0%) |
| – Oral |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
| Other treatment |
25 (11%) |
19 (10%) |
1 (9%) |
5 (31%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
With data from a systematic review on varicella
prevalence in European countries before universal childhood vaccination,
population data from Switzerland and the Swiss Paediatric Surveillance Unit reports,
we estimated the number of varicella cases per year and calculated the annual hospitalisation
incidence per 100,000 inhabitants and the annual hospitalisation rate per 10,000
varicella patients by age groups and present them in table 1 [16, 25]. Only children with varicella and
resident in Switzerland were included in these calculations (n = 216). The
calculated annual varicella hospitalisation incidence for our 2-year study
period was 7.2 per 100,000 and the estimated annual hospitalisation rate 12.6
per 10,000.
Discussion
This observational prospective 2-year surveillance
study, encompassing reports from all Swiss children’s hospitals within the Swiss
Paediatric Surveillance Unit, elucidates the morbidity rate of varicella,
particularly manifesting in previously healthy (>80%) children. The study
shows a comprehensive portrayal of hospitalisations and associated
complications of varicella in Switzerland, prior to the implementation of universal
varicella vaccination recommendations. Furthermore, it illustrates the seasonal
and yearly variations in varicella epidemiology with a nationwide outbreak
during the second study year from December 2022 to May 2023. Paediatric
varicella hospitalisation frequencies increased 4- to 7-fold in these months
compared to the first study year. The seasonal distribution of varicella hospitalisations
adhered to its well-established pattern, with a peak incidence during the
winter and spring months [2, 11, 12, 14, 17, 18].
Since 2005, there have been no descriptive national epidemiological studies
pertaining to varicella-related hospitalisations. Hereby we give an up-to-date
epidemiological investigation on varicella morbidity and mortality in children
in Switzerland.
The annual hospitalisation rate of 13 per 10,000 remains
consistent with the findings of the preceding prospective surveillance study
conducted in Switzerland in 2005. Looking at European studies and a study from
New Zealand, characterised by either the absence of universal varicella
vaccination or low vaccination coverage, the annual varicella hospitalisation
incidence ranges from 0.82 to 29.5 per 100,000 [2–5,
11, 13, 14, 19]. Our calculated annual varicella hospitalisation
incidence, quantified at 7.2 per 100,000, falls within this broader range. This
incidence closely aligns with figures reported in the Netherlands (6.8 per 100,000)
and New Zealand (8.6 per 100,000) and underscores the idea of comparability of
varicella-related hospitalisation rates across nations with analogous
demographic and infrastructural characteristics [3,
19].
Comparative analysis of baseline characteristics among
patients revealed that the sex ratio (male:female) was often almost evenly
distributed [5, 13, 17, 27]. Our cohort
manifested a slight male predominance (58%).
The median age within our cohort, 4.7 years, diverges
by 1 to 3 years from the reported median age in other studies [2–4, 11, 13, 14, 19].
The peak frequency of hospitalisations
was observed in patients aged 9 months to 4 years. This aligns with findings
from several studies, thus illustrating a recognised pattern of elevated
varicella hospital admission rates within younger age cohorts. These
observations challenge the conventional presumption that severe complications
are exclusive to older children, thereby highlighting the vulnerability of
younger age groups to varicella-related complications [2–5, 11, 13].
The majority of children requiring hospitalisation due
to varicella were primary healthy. Comparative analyses with studies conducted
in Germany, the Netherlands, Belgium and Ireland reveal proportions of hospital
admissions for primary healthy patients ranging from 61% to 96.3% [2–5, 11]. In our
2-year study period, 83% of
all observed hospitalised patients and 88% of the varicella patient subset were
healthy. These findings substantiate the assumption that primary healthy
children are at risk of severe varicella infections.
Our investigation revealed an augmented occurrence of
invasive Group A Streptococcus (iGAS) infections concomitant with varicella
compared to pre-COVID-19 years. Specifically, in the second study year, 49
cases of iGAS infections were registered. This noteworthy increase aligns with
findings reported in a Dutch study [28].
Other European countries documented an increase of iGAS as well. Cameron et al.
documented a notable proportion of infectious complications involving iGAS [11]. McCarthy
et al. observed a rise in iGAS
infections too [5]. These collective
observations underscore the complex interplay between varicella and iGAS
infections, emphasising the need for heightened vigilance and surveillance in
monitoring such infectious dynamics.
Prevalent underlying chronic diseases in other
European studies include congenital anomalies, skin disorders and pneumopathies
[2, 3, 11]. We found these categories in
our study too; however skin disorders, particularly atopic dermatitis, cancer
or leukaemia, genetic syndromes and cardiovascular diseases were the most
common. Notably, hospitalisations occurred primarily due to complications from
varicella. Varicella complications commonly involve the skin, central nervous
system and lungs, with variations in the order and frequency of affected organ
systems across studies. Our investigation showed predominant skin infections,
followed by musculoskeletal and neurological complications. The observed
spectrum of complications in our study aligns with that of comparable studies
from other countries [2–5, 11, 13, 14, 17, 19].
During our analysed period, no patient had a history of prior VZV vaccination.
All patients admitted to the ICU were primary healthy. The severity of
complications concentrating in primary healthy patients has been described in
similar studies [4, 11, 19]. Further, the
ICU admission rate at 14% for all patients in our study falls within the range
observed in European countries and New Zealand (2.5% to 24%) [4, 5, 11, 19].
The fatality rate, observed at 4.5% in 2021–2022 and
0.5% in 2022–2023, closely mirrors the outcomes reported from Switzerland in
2005 and therefore underscores the notion of an unchanged epidemiology of
varicella in Switzerland [6]. Fatalities
resulting from varicella hospitalisations are documented in various studies [2, 4,
11, 13].
As a limitation, the interpretative scope of our
findings is constrained by the abbreviated 2-year study period, marked by
fluctuations in the frequency of varicella-related hospitalisations. An
extended observation period will provide a more nuanced and accurate
description of the epidemiological dynamics. Comparative analyses with other
studies are circumscribed by variations in case definitions and methodological
approaches. The absence of a follow-up questionnaire or a capture-recapture
analysis introduces the potential for underestimating the true burden of
disease. Assignment errors may have transpired during the codification process,
emphasising the need for cautious interpretation.
Nations that have already instituted universal
varicella vaccination strategies have consistently reported a decline in hospitalisations
[12, 15, 18, 21–23, 29–31]. Universal
varicella vaccination has been proposed and implemented as a measure against
severe complications, thereby diminishing the overall burden on healthcare
resources and reducing hospitalisation rates [4,
5, 11, 19].
Data sharing statement
Anonymised study data can be shared on request by contacting the corresponding
author (MB). Apart from software and tools described above in the analysis and
tools subheadings, no additional software libraries, frameworks or packages
were used in this study.
Acknowledgments
We would like to thank the study nurses involved at
the Research Centre of the Children’s Hospital of Central Switzerland (Ms
Janine Stritt, Ms Marisa Hostettler and Ms Katja Hrup) in helping with data
entry and coordination of CRF’s with the respective centres. We would like to
thank all the reporting centres: Paediatric Clinic, Cantonal Hospital, Aarau; Paediatric
Clinic, Cantonal Hospital, Baden; University Children’s Hospital Basel, UKBB,
Basel; Istituto Pediatrico della Svizzera Italiana, Bellinzona; University
Clinic for Paediatrics, Bern; Neonatology, University Clinic for Paediatrics,
Bern; Children’s Hospital Wildermeth, Biel; Clinic for Paediatrics and
Adolescent Medicine, Cantonal Hospital, Chur; Service de Pédiatrie, Hôpital du
Jura, Delémont; Service de Pédiatrie, Hôpital Cantonal, Fribourg; Hôpital des
Enfants, HUG, Genève; Service de Pédiatrie, CHUV, Lausanne; Hôpital de l’Enfance,
Lausanne; Division de Néonatologie, CHUV, Lausanne; Paediatric Clinic, Cantonal
Hospital, Lucerne; Service de Pédiatrie, Hôpital de Zone, Morges; Clinic for
Children and Adolescents, Cantonal Hospital, Münsterlingen; Département de
Pédiatrie, Hôpital Pourtalès, Neuchâtel; Service de Pédiatrie, Centre
hospitalier, Rennaz; Neonatology, Clinic for Obstetrics and Gynecology, St
Gallen; Paediatric Clinic, Eastern Switzerland Children’s Hospital, St Gallen;
Service de Pédiatrie, CHCVs, Sion; Paediatric Clinic, Spitalzentrum Oberwallis,
Visp; Children’s Clinic, Kantonsspital, Winterthur; Service de Pédiatrie, eHnV,
Yverdon; Paediatrics/Neonatology, Zollikerberg; University Children’s Hospital,
Zurich; Clinic for Children and Adolescents, Triemli Hospital, Zurich;
Neonatology, University Women’s Hospital, Zurich for their continuous
participation in the study.
We thank the representatives of the paediatric units
within the Swiss Paediatric Surveillance Unit: Florence Barbey,
François Cachat, Livia Dülli, Simon Fluri, Mathias Gebauer, Silke Grupe, Ulla
Jochumsen, Eva Kellner, Ute Kerr, Guido Laube, Bernard Laubscher, Andreas
Malzacher, Jane McDougall, Stefan Minocchieri, Vincent Muehlethaler, Anita
Niederer, Anne Pittet, Christa Relly, Michel Russo, Fabiola Stollar, Andreas
Wörner, Jonas Zeller and all the dedicated physicians for taking care of
the patients and helping to complete the questionnaires.
We also thank the Swiss Neuropaediatric Stroke
Registry coordinators for reviewing their cases for varicella zoster virus
associated stroke cases and reporting a further case.
We would also like to acknowledge the administrative
support provided by Daniela Beeli, Mirjam Mäusezahl and Fabien Tschagellar,
from the Federal Office of Public Health Switzerland.
Swiss Paediatric Surveillance Unit site
coordinators: Florence Barbey, François Cachat, Livia Dülli, Simon Fluri,
Mathias Gebauer, Silke Grupe, Ulla Jochumsen, Eva Kellner, Ute Kerr, Guido
Laube, Bernard Laubscher, Andreas Malzacher, Jane McDougall, Stefan
Minocchieri, Vincent Muehlethaler, Anita Niederer, Anne Pittet, Christa Relly,
Michel Russo, Fabiola Stollar, Andreas Wörner, Jonas Zeller and the Swiss
Paediatric Surveillance Unit, https://www.spsu.ch/en/home
Disclaimer: The paper represents the views of the
authors and not an official position of any institution or funder.
Source of support: Case registration and data
provision by the Swiss Paediatric Surveillance Unit.
Michael Buettcher
Paediatric Infectious Diseases Unit
Department of Paediatrics
Children’s Hospital of Central Switzerland
CH-6000 Lucerne
Michael.Buettcher[at]luks.ch
References
1. Heininger U, Seward JF. Varicella. Lancet. 2006 Oct;368(9544):1365–76. doi: https://doi.org/10.1016/S0140-6736(06)69561-5
2. Liese JG, Grote V, Rosenfeld E, Fischer R, Belohradsky BH, v Kries R; ESPED Varicella
Study Group. The burden of varicella complications before the introduction of routine
varicella vaccination in Germany. Pediatr Infect Dis J. 2008 Feb;27(2):119–24. 10.1097/INF.0b013e3181586665
3. van Lier A, van der Maas NA, Rodenburg GD, Sanders EA, de Melker HE. Hospitalization
due to varicella in the Netherlands. BMC Infect Dis. 2011 Apr;11(1):85. 10.1186/1471-2334-11-85
4. Blumental S, Sabbe M, Lepage P; Belgian Group for Varicella. Varicella paediatric
hospitalisations in Belgium: a 1-year national survey. Arch Dis Child. 2016 Jan;101(1):16–22.
10.1136/archdischild-2015-308283
5. McCarthy KN, Ó Maoldomhnaigh C, Butler KM, Gavin PJ. Varicella Related Hospital Admissions
in Ireland. Ir Med J. 2019 Aug;112(7):966.
6. Bonhoeffer J, Baer G, Muehleisen B, Aebi C, Nadal D, Schaad UB, et al. Prospective
surveillance of hospitalisations associated with varicella-zoster virus infections
in children and adolescents. Eur J Pediatr. 2005 Jun;164(6):366–70. 10.1007/s00431-005-1637-8
7. Bundesamt für Gesundheit. Neue Empfehlungen zur Impfung gegen Varizellen (Windpocken).
BAG-Bulletin. 2022 Oct 31;44.
8. Bundesamt für Gesundheit. Neue Empfehlungen zur Impfung gegen Herpes zoster: impfstoff
Shingrix. BAG-Bulletin. 2021 Nov 22;47:8–15.
9. Swiss Paediatric Surveillance Unit (SPSU). Internet: https://www.spsu.ch/en/home
10. Riera-Montes M, Bollaerts K, Heininger U, Hens N, Gabutti G, Gil A, et al. Estimation
of the burden of varicella in Europe before the introduction of universal childhood
immunization. BMC Infect Dis. 2017 May;17(1):353. 10.1186/s12879-017-2445-2
11. Cameron JC, Allan G, Johnston F, Finn A, Heath PT, Booy R. Severe complications of
chickenpox in hospitalised children in the UK and Ireland. Arch Dis Child. 2007 Dec;92(12):1062–6.
10.1136/adc.2007.123232
12. Streng A, Grote V, Rack-Hoch A, Liese JG. Decline of Neurologic Varicella Complications
in Children During the First Seven Years After Introduction of Universal Varicella
Vaccination in Germany, 2005-2011. Pediatr Infect Dis J. 2017 Jan;36(1):79–86. doi: https://doi.org/10.1097/INF.0000000000001356
13. Fontoura-Matias J, Moreira RS, Reis-Melo A, Freitas A, Azevedo I. Varicella Admissions
in Children and Adolescents in Portugal: 2000-2015. Hosp Pediatr. 2021 Aug;11(8):856–64.
10.1542/hpeds.2020-004275
14. Pierik JG, Gumbs PD, Fortanier SA, Van Steenwijk PC, Postma MJ. Epidemiological characteristics
and societal burden of varicella zoster virus in the Netherlands. BMC Infect Dis.
2012 May;12(1):110. 10.1186/1471-2334-12-110
15. Waye A, Jacobs P, Tan B. The impact of the universal infant varicella immunization
strategy on Canadian varicella-related hospitalization rates. Vaccine. 2013 Oct;31(42):4744–8.
10.1016/j.vaccine.2013.08.022
16. Bollaerts K, Riera-Montes M, Heininger U, Hens N, Souverain A, Verstraeten T, et al. A
systematic review of varicella seroprevalence in European countries before universal
childhood immunization: deriving incidence from seroprevalence data. Epidemiol Infect.
2017 Oct;145(13):2666–77. 10.1017/S0950268817001546
17. Bernal JL, Hobbelen P, Amirthalingam G. Burden of varicella complications in secondary
care, England, 2004 to 2017. Euro Surveill. 2019 Oct;24(42):1900233. doi: https://doi.org/10.2807/1560-7917.ES.2019.24.42.1900233
18. Spackova M, Muehlen M, Siedler A. Complications of varicella after implementation
of routine childhood varicella vaccination in Germany. Pediatr Infect Dis J. 2010 Sep;29(9):884–6.
10.1097/INF.0b013e3181e2817f
19. Wen SC, Best E, Walls T, Dickson N, McCay H, Wilson E. Prospective surveillance of
hospitalisations associated with varicella in New Zealand children. J Paediatr Child
Health. 2015 Nov;51(11):1078–83. 10.1111/jpc.12937
20. van Lier A, van Erp J, Donker GA, van der Maas NA, Sturkenboom MC, de Melker HE. Low
varicella-related consultation rate in the Netherlands in primary care data. Vaccine.
2014 Jun;32(28):3517–24. 10.1016/j.vaccine.2014.04.034
21. Heywood AE, Wang H, Macartney KK, McIntyre P. Varicella and herpes zoster hospitalizations
before and after implementation of one-dose varicella vaccination in Australia: an
ecological study. Bull World Health Organ. 2014 Aug;92(8):593–604. 10.2471/BLT.13.132142
22. Sheridan SL, Quinn HE, Hull BP, Ware RS, Grimwood K, Lambert SB. Impact and effectiveness
of childhood varicella vaccine program in Queensland, Australia. Vaccine. 2017 Jun;35(27):3490–7.
10.1016/j.vaccine.2017.05.013
23. Streng A, Grote V, Carr D, Hagemann C, Liese JG. Varicella routine vaccination and
the effects on varicella epidemiology - results from the Bavarian Varicella Surveillance
Project (BaVariPro), 2006-2011. BMC Infect Dis. 2013 Jul;13(1):303. 10.1186/1471-2334-13-303
24. IBM. IBM SPSS Statistics 29 Core System User's Guide.
25. Sozialdienst und Migration. Ständige Wohnbevölkerung nach Geschlecht und Alter, 1860-2022.
Available from: https://www.pxweb.bfs.admin.ch/pxweb/de/px-x-0102030000_101/px-x-0102030000_101/px-x-0102030000_101.px
26. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)
statement: guidelines for reporting observational studies. Lancet. 2007 Oct;370(9596):1453–7.
doi: https://doi.org/10.1016/S0140-6736(07)61602-X
27. Grote V, von Kries R, Springer W, Hammersen G, Kreth HW, Liese J. Varicella-related
deaths in children and adolescents—Germany 2003-2004. Acta Paediatr. 2008 Feb;97(2):187–92.
10.1111/j.1651-2227.2007.00595.x
28. de Gier B, Marchal N, de Beer-Schuurman I, Te Wierik M, Hooiveld M, de Melker HE,
et al.; ISIS-AR Study Group; GAS Study group; Members of the GAS study group; Members
of the ISIS-AR study group. Increase in invasive group A streptococcal (Streptococcus pyogenes) infections (iGAS) in young children in the Netherlands, 2022. Euro Surveill. 2023 Jan;28(1):2200941.
doi: https://doi.org/10.2807/1560-7917.ES.2023.28.1.2200941
29. Hirose M, Gilio AE, Ferronato AE, Ragazzi SL. Impacto da vacina varicela nas taxas
de internações relacionadas à varicela: revisão de dados mundiais [The impact of varicella
vaccination on varicella-related hospitalization rates: global data review]. Rev Paul
Pediatr. 2016 Sep;34(3):359–66. 10.1016/j.rpped.2015.12.006
30. Quinn HE, Gidding HF, Marshall HS, Booy R, Elliott EJ, Richmond P, et al.; PAEDS (Paediatric
Active Enhanced Disease Surveillance) Network. Varicella vaccine effectiveness over
10 years in Australia; moderate protection from 1-dose program. J Infect. 2019 Mar;78(3):220–5.
10.1016/j.jinf.2018.11.009
31. Marshall HS, Clarke M, Heath C, Quinn H, Richmond PC, Crawford N, et al.; PAEDS Investigators.
Severe and Complicated Varicella and Associated Genotypes 10 Years After Introduction
of a One-Dose Varicella Vaccine Program. J Infect Dis. 2019 Jan;219(3):391–9. 10.1093/infdis/jiy518

