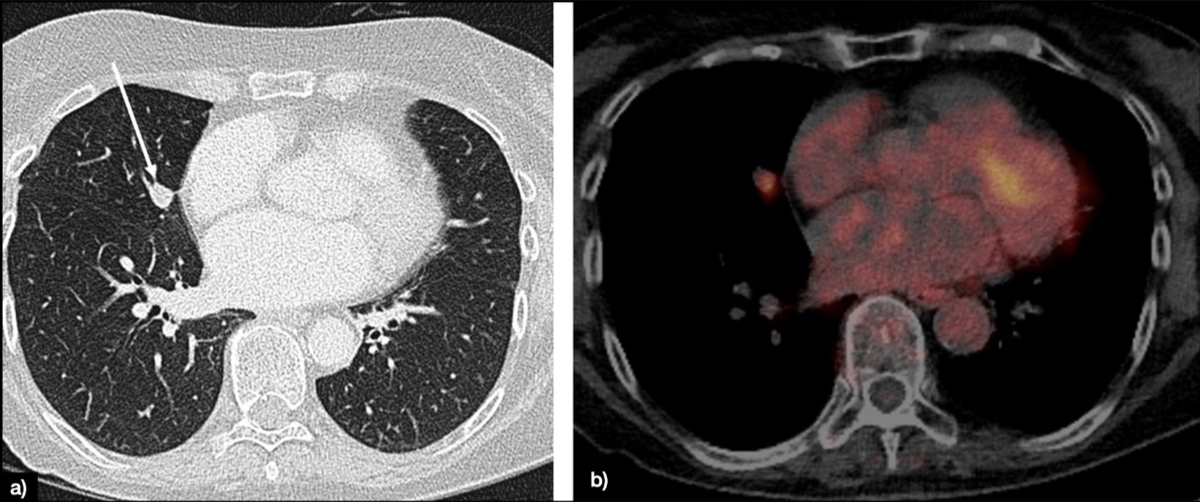
Figure 1Lung solid nodule of 12 mm in the middle lobe (white arrow), classified as Lung-RADS 4A (suspicious) (A). The patient underwent PET-CT, showing fluorodeoxyglucose (FDG) avidity only of the nodule (B).
DOI: https://doi.org/https://doi.org/10.57187/s.3843
Lung cancer is one of the most common cancers and the leading cause of cancer death worldwide in both men and women [1]. In Switzerland, on average, around 2700 men and 1800 women are diagnosed with lung cancer and approximately 2000 men and 1200 women die from lung cancer every year. This cancer was the most common in men, accounting for 21.3% of all cancer deaths [2], 2021.
Based on the National Lung Screening Trial (NLST) [3] and Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) trial data [4], efforts are being made throughout Europe, including Switzerland, to introduce national screening programmes to improve long-term survival by detecting early-stage lung cancer.
The increasing awareness and ongoing stage shift have implications for imaging-related non-invasive diagnosis and staging, requiring highly specific and sensitive imaging methods that allow a better patient-centred strategy towards curative surgery. Characterisation of tumour genomic abnormalities and clinical applications of anticancer agents that can effectively target these abnormalities have transformed treatment approaches and have brought precision therapy into the mainstream of lung cancer care [5]. This has implications on how to use imaging for tumour follow-up assessment beyond the general use of response evaluation criteria in solid tumours (RECIST), which might not be the tool of choice in the future [6].
Currently, different guidelines regarding postoperative follow-up are available [7, 8], but new tissue-sparing surgeries as well as non-surgical procedures (stereotactic body radiotherapy, radiofrequency ablation) require adaptation of the follow-up scheme and new imaging strategies [9].
This white paper aims to give an overview of the current advances in imaging in terms of diagnosis, staging, treatment and follow-up of patients, along with appropriate recommendations.
Chest radiographs are still frequently used to screen for pulmonary anomalies. However, substantial limitations of this method are the limited accuracy for small lung lesions, reduced accuracy for part-solid and ground-glass lesions, the dependency of lesion location, the reduced spatial resolution and the difficulty of assessing the mediastinal structures; foremost potential lymph node involvement [10, 11]. An overview of the pros and cons of the various modalities is given in table 1.
Table 1Summary of strengths and weaknesses of each imaging modality.
| Imaging modality | Strengths | Weaknesses |
| Conventional X-ray | Rapidity | Low accuracy for small lesions |
| Availability | Low accuracy for non-solid and partially solid lesions | |
| Low-cost | Difficulty of assessing lymph nodes | |
| Low radiation dose | ||
| Computed tomography | Early detection of lung lesions | Low specificity for normal-sized lymph nodes |
| Reproducibility | ||
| Availability | ||
| Positron emission tomography-Computed tomography | Characterisation of lung nodules | Lack of sensitivity for brain metastases |
| Detection of positive lymph nodes (and guide for invasive assessment) | ||
| Detection of distant metastases | ||
| Magnetic resonance imaging | Quantitative imaging | Breathing artefacts |
| Expensive | ||
| Time-consuming | ||
| High-end technology | ||
| Ultrasound | Assessment of parietal pleural and chest wall invasion | Low visibility of deep structures |
| Interventional radiology | Image-guided biopsies | Post-procedural complications |
| Percutaneous thermal ablation |
Computed tomography overcomes the limitations of conventional X-ray and since the dissemination of the results of the National Lung Screening Trial in 2011, low-dose chest CT has been promoted as the mainstay of lung cancer screening [12]. Compared with chest radiography, the CT screening group showed a reduced mortality from lung cancer by 20% [12]. In contrast to the promising results from the National Lung Screening Trial, the first European screening studies did not demonstrate an improved survival in a screening cohort. For instance, a pooled analysis of the Detection and Screening of Early Lung Cancer by Novel Imaging Technology and Molecular Essays (DANTE) trial and the Multicentric Italian Lung Detection (MILD) trial did not show decreased mortality in the screening cohorts [13, 14]. Although the results were not statistically significant, both trials as well as the Danish Lung Cancer Screening Trial (DLCST) detected more cancers in the CT screening population. The heterogeneous results from these studies can be attributed to the study design which was underpowered to evaluate reduced mortality. With the results from the NELSON trial however, a significant reduction in lung cancer mortality was found. Based on these results, a general recommendation for CT screening in high-risk populations is under discussion.
In general, suspicious lesions on CT imaging are characterised by irregular, spiculated borders and heterogeneous morphology. Lung cancers tend to occur in the upper lobes and the apical segments of the lower lobes. For a comprehensive risk stratification, additional factors such as age, smoking history and a family history of lung cancer should be considered as well. Based on the overall risk assessment, further recommendations on the management of lesions can be provided, such as follow-up intervals, additional positron emission tomography (PET)-CT and/or invasive work-up (CT-guided biopsy, endobronchial ultrasound).
The clinical staging of lung cancer (currently in its 8th edition [120]) rests on either CT or CT in conjunction with PET. The local tumour extent is mainly determined by multiplanar CT measurements. In cases with bronchial obstruction, PET-CT can help to distinguish the lesion boundaries from atelectasis. Lymph node assessment in CT is mainly based on size criteria. Although lymph nodes vary in size depending on their location, a general threshold is deemed to be 1 cm in short-axis diameter. Additional criteria comprise texture (i.e. enhancement patterns) and shape. PET-CT is more sensitive for the nodal assessment and the depiction of distant metastasis. If metastatic spread to the brain is suspected, additional MR imaging is required to determine the M stage (figure 1).

Figure 1Lung solid nodule of 12 mm in the middle lobe (white arrow), classified as Lung-RADS 4A (suspicious) (A). The patient underwent PET-CT, showing fluorodeoxyglucose (FDG) avidity only of the nodule (B).
Lymph node assessment in CT is mainly based on size criteria. Although short axis thresholds vary depending on the location, a general threshold is deemed to be 1 cm in short-axis diameter. Additional criteria comprise texture (i.e. enhancement patterns) and shape [15, 16]. By comparison, PET-CT is more sensitive for the nodal assessment and the depiction of distant metastasis. In suspected tumour spread to the brain, additional MR imaging is required. An overview of the pros and cons of the various modalities is given in table 1. There are no established protocols for follow-up surveillance imaging after curative therapy, but it is advisable to consider annual imaging for at least the initial 5 years.
Several meta-analyses demonstrate high sensitivity (ca. 90%) and overall good specificity (ca. 78%) of fluorodeoxyglucose F 18 (18F-FDG) (PET/CT for the assessment of unclear lung lesions [17–19]. The diagnostic accuracy of the PET/CT is strongly positively associated with the intensity of 18F-FDG uptake in lesions [20]. The sensitivity is also higher for larger lesions (e.g. ca. 96% for lesions larger than 10 mm) [21] and vice versa. Small malignant lesions at the boundaries of the spatial resolution of PET scanners (2–6 mm, depending on the performance of the scanners) are often rated false-negatively [22]. The same applies to malignant lesions with low 18F-FDG uptake such as bronchoalveolar cell carcinoma or carcinoid tumours. The lower rates of specificity are caused by accumulation of 18F-FDG in reactive and inflammatory tissues. Whole-body 18F-FDG PET/CT is a powerful method for the detection of lymph nodal or distant metastases of non-small cell lung cancer (NSCLC) and, in this regard, is superior than CT alone (figure 2).
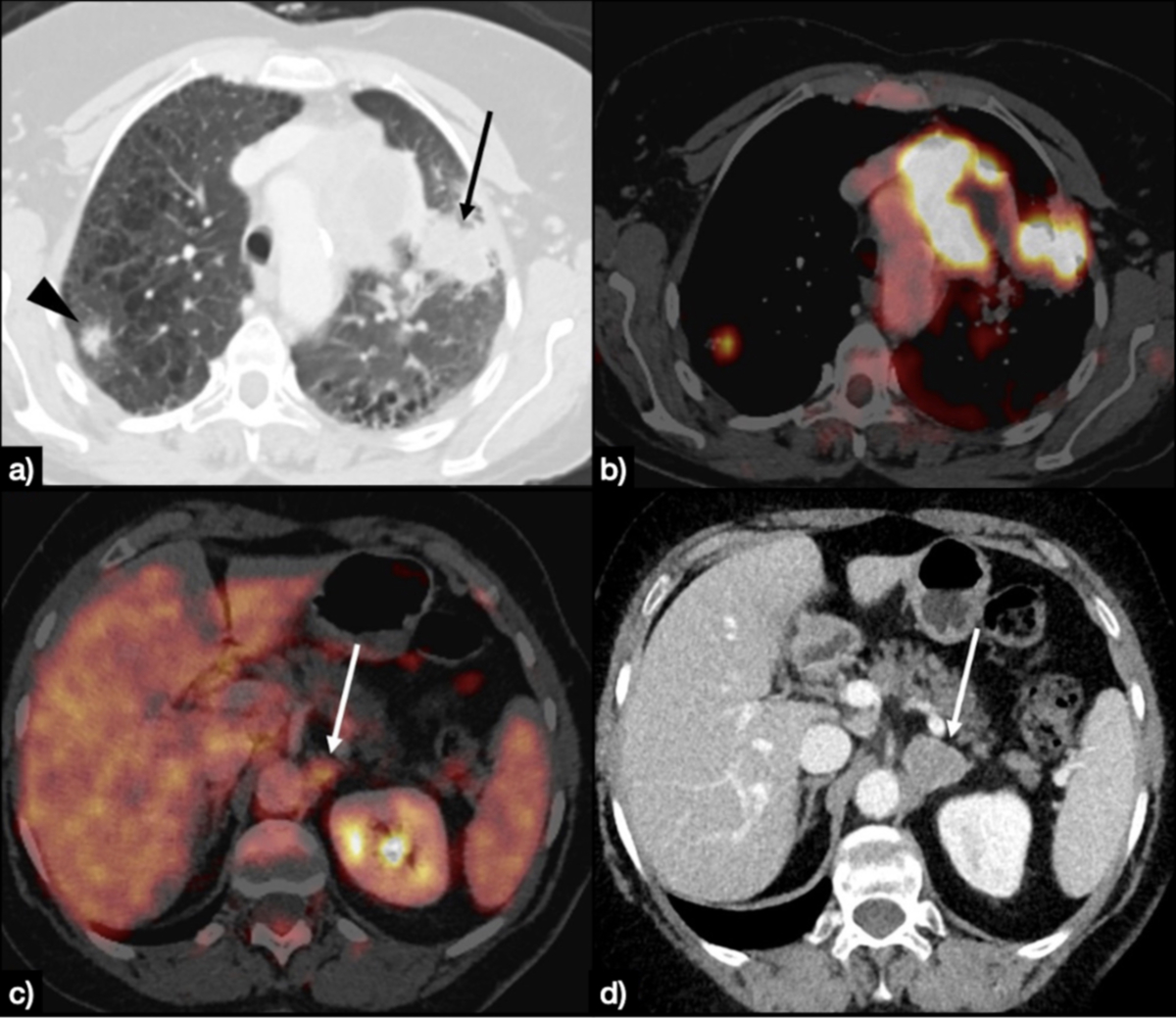
Figure 2Large solid mass of the left upper lung (black arrow), with concomitant contralateral nodule (black arrowhead) (A), fluorodeoxyglucose (FDG)-avid on PET-CT (B). PET-CT also raised suspicion of a lesion in the left adrenal (white arrow in panel C), that was confirmed as an adrenal lesion at the subsequent CT scan, as an enlargement of the gland (white arrow in panel D).
Different meta-analyses show a sensitivity of 74–85% and a specificity of 85–92% for differentiation between a N0–1 status and a N2–3 status [18, 23–26]. The sensitivity and specificity of the 18F-FDG PET/CT for metastases of non-small cell lung cancer in CT-morphologically non-suspicious lymph nodes are 70% and 94%, respectively [25]. In a randomised prospective clinical trial, futile surgical treatments could be reduced by 50% when 18F-FDG PET/CT was performed prior to the surgical intervention [27]. Another clinical trial demonstrated that the number of invasive tests, especially of mediastinoscopies and thoracotomies, is significantly reduced by prior 18F-FDG PET/CT [27, 28]. For the detection of distant metastases of non-small cell lung cancer in 18F-FDG PET/CT, a meta-analysis revealed a sensitivity of 93% and a specificity of 96% [29]. The majority of distant metastases are found in osseous structures and the adrenal glands [20]. Since brain tissue shows a physiologically high 18F-FDG uptake, the sensitivity for the detection of brain metastases with 18F-FDG PET/CT is significantly reduced [30]. 18F-FDG PET/CT is frequently able to identify additional sites of small cell lung cancer (SCLC) thereby changing the tumour stage from “limited disease” to “extensive disease” [31, 32].
For primary staging of small cell lung cancer, 18F-FDG PET/CT demonstrated superior performance (sensitivity of 93%) when compared with conventional imaging modalities (CT and bone scan, sensitivity: 79%) [31]. Specificity was 100% for both PET/CT and conventional methods [31]. In several studies, 18F-FDG PET/CT changed the initially planned therapy in 8–17% of patients [31, 33, 34]. The performance of 18F-FDG PET/CT regarding the N status of small cell lung cancer is supposed to be similar to that of non-small cell lung cancer. In a meta-analysis, 18F-FDG PET/CT showed a sensitivity of 99% and a specificity of 89% regarding the detection of recurrent local cancer after initial surgery [19]. Further studies confirmed these results [35, 36]. Besides the detection of recurrent local tumour, 18F-FDG PET/CT also provides an excellent tool for the simultaneous detection of distant metastases as described above. Response evaluation is recommended after 2–3 cycles of chemotherapy or immunotherapy, using the same initial radiographic investigation that demonstrated tumour lesions (level of evidence IV, grade of recommendation B). The same procedure and timing (every 6–9 weeks) should be applied for the response evaluation in patients treated with targeted therapies and/or immunotherapy. Follow-up with PET is not routinely recommended, due to its high sensitivity and relatively low specificity.
An overview of the pros and cons of the various modalities is given in table 1.
Magnetic resonance imaging (MRI) [37] offers improved soft tissue contrast as well as the ability to image without the use of ionising radiation and is routinely used in cancer imaging at multiple disease sites (e.g. prostate cancer, liver tumours, etc.). Unfortunately, several challenges have so far impeded any widespread adoption of MRI in detection or staging of lung cancer, most importantly breathing artefacts due to comparably long scan times and an inferior signal-to-noise ratio due to the low proton density of air. However, technical improvements over the last few years (for example, shorter examination times resulting in single breath-hold examinations) have increased interest in the use of MRI in lung cancer; furthermore, some promising pilot studies have been published, suggesting that MRI may be of value in detecting lung nodules ≥6 mm [38], and may be used in follow-up studies after prior CT imaging [39].
MRI also offers the ability to quantify (patho-)physiological parameters of the tumour, thus potentially gaining increased insight into the tumour microenvironment. For example, Razek et al. [40] showed that the apparent diffusion coefficient (ADC) of diffusion-weighted imaging in lung cancer was correlated with the tumour’s pathological grade and the presence of metastatic lymph nodes, while Chang et al. demonstrated a potential value of dynamic contrast-enhanced MRI in the assessment of treatment response in a small group of 11 patients [41]. While these results are certainly promising, the use of MRI in lung cancer detection and staging is currently mostly limited to clinical trials. However, given the increasing availability of MR as part of PET/MR and the greater technical performance of clinical MR scanners, MRI may be of value in routine clinical practice in the future. An overview of the pros and cons of the various modalities is given in table 1.
Ultrasound plays a minor role in the diagnosis, staging and follow-up of lung tumours. Nevertheless, transthoracic ultrasound could be used as an additional tool in specific settings such as assessing parietal pleural and chest wall invasion [42]. An overview of the pros and cons of the various modalities is given in table 1.
The Fleischner Society 2017 Guidelines recommend tissue sampling in solid, noncalcified lung nodules larger than 8 mm either by minimally invasive surgery, transbronchial endoscopic access or transthoracic needle biopsy [15]. CT-guided lung biopsy, performed under local anaesthesia, is an established method with an excellent diagnostic accuracy ranging from 80% to 90% [43]. Major complications are rare; however, the risk of pneumothorax ranges from 10% to 40% with chest tube insertion in 5% to 15% of cases [44]. Tumour seeding through the needle tract represents a very rare complication with a prevalence reported in the literature between 0.012% and 0.061% [45].
The current European Society of Medical Oncology guidelines consider surgery as the standard of care for early-stage (Stage IA) non-small cell lung cancer [46, 47]. In non-operable patients, accounting for about 20% to 30% of patients at time of diagnosis [48, 49], stereotactic body radiotherapy is recommended with thermal ablation being a reasonable alternative with curative intent [50]. Recent studies showed comparable outcomes in stage IA non-small cell lung cancer for thermal ablation compared to stereotactic body radiotherapy [51–55]. With thermal ablative techniques, 1-year local tumour control and overall survival rates of 77–85% and 78–91% can be achieved [56, 57]. The most common complication after thermal ablation is pneumothorax with a chest tube insertion rate of about 20% [56]. Serious adverse events such as systemic air embolism are reported in less than 1% of cases [56, 57]. Moreover, thermal ablation has multiple advantages [58, 59]:
In thermal ablation, tissue destruction is achieved either through application of heat or cold. The most studied technique so far is radiofrequency ablation (figure 3). Other thermal ablative techniques include microwave ablation and cryoablation, both with comparable results to radiofrequency ablation [56, 60]. Cryoablation in particular has shown promising results in recent years with the multicentre, prospective SOLSTICE trial and long-term results by the prospective ECLIPSE trial with local tumour control rates of 85–90% [61, 62]. One of the main advantages of cryoablation is the painless ablation technique, which makes general anaesthesia obsolete [63]. In addition, pleural tumours can be treated without postoperative pain. Further comparative studies have to be performed to compare the effectiveness of the two techniques.
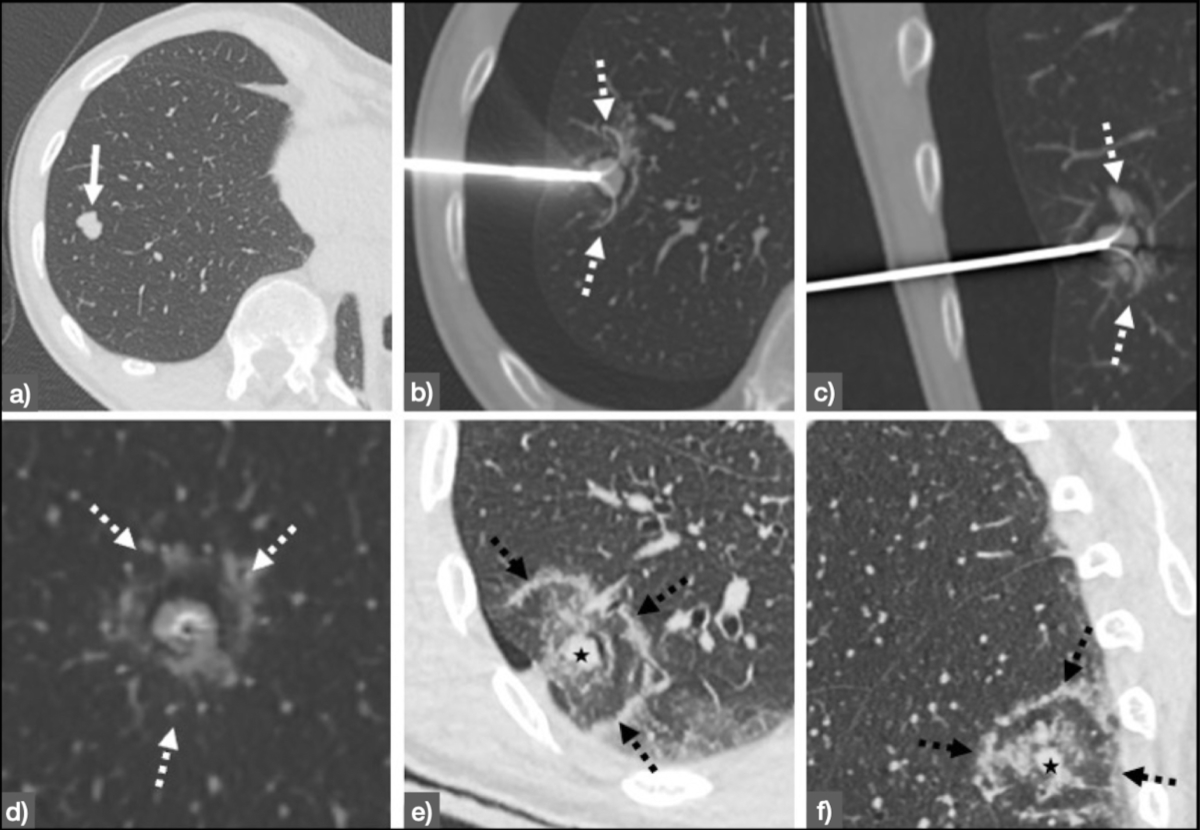
Figure 3Radiofrequency ablation of histologically proven early-stage (Stage IA) non-small cell lung cancer [46] in a 65-year-old patient. Panel A shows the planning CT depicting the tumour (white arrow) before intervention. Panels B (axial), C (sagittal) and D (coronal) show the deployable radiofrequency ablation (RFA) needle (LeVeen 3.5 cm, Boston Scientific, Natick, MA, USA) in place covering the entire lesion (white dotted arrows). Panels E (axial) and F (coronal) show the control scan immediately after the procedure, in which the ablation margin (black dotted arrows) is clearly visible with sufficient distance to the treated tumour (black star). After one year, no signs of local recurrence were detected.
In the context of screening, the radiological report should include details about imaging parameters like the radiation dose. It should also provide a concise overview of the screening results along with specific management suggestions, as well as any additional findings [64]. Standardised templates are preferred to ensure uniform reporting and guideline adherence [65]. Further, the use of a common terminology is recommended for data collection, quality control and increased efficiency [66, 67]. Each nodule should be described with its location (lobe, segment), size (determined on lung window images and reported as the average diameter), attenuation (soft tissue, type of calcification, fat), morphology (solid, non-solid and part-solid), margins (smooth, lobulated, spiculated) and compared to preliminary examinations (growth, change of composition) [68]. For unification reasons, nodules need to be classified by an established classification system such as Lung-RADS 1.1 [69].
For lung cancer screening, the American College of Radiology (ACR) proposed in the CT Screening Reporting and Data System [70] the category “S”, where the radiologist can mention non-lung cancer findings that he or she believes are clinically relevant [69]. We propose that a similar tag be used in imaging reports of lung cancer patients to outline findings that are not directly related to the patients’ malignancy but of potential relevance for the patients’ further work-up. The ACR Incidental Findings Committee has published a set of recommendations for managing incidentally detected lung findings on thoracic CT [71] to assist the radiologist in this important task.
The tumour, node, metastasis (TNM) classification system for lung cancer was introduced in its 8th edition by the Union for International Cancer Control (UICC) in 2016 and came into effect in January 2017 with authorisation by the American Joint Committee on Cancer (AJCC) in January 2018. The changes made to the 7th edition were based on recommendations from the International Association for the Study of Lung Cancer [72] Staging Project, including the analysis of an international database of 94,708 patients from 46 sites and 19 countries. The UICC recommends the inclusion of the TNM classification in the data reporting. The classification applies to carcinomas of the lung including non-small cell lung cancer, small cell lung cancer and bronchopulmonary carcinoid tumours, but does not apply to sarcomas and other rare tumours of the lung. The classification consists of a clinical and a histopathological part. The clinical classification (cTNM) is essential for selecting and evaluating the therapy. It is based on evidence acquired before treatment, from physical examination, imaging (CT and PET-CT), endoscopy, biopsy, surgical exploration and other relevant examinations. The pathological classification (pTNM) represents a postsurgical classification, used to guide adjuvant therapy and provide additional data to estimate the patient’s prognosis. Compared to the cTNM, it is supplemented or modified by data from surgery and pathological examination.
Major changes introduced by the TNM 8th edition were: tumour size cut points in every T category, based on 1 cm intervals up to 5 cm, thus creating new IA subgroups (IA1, IA2, IA3); the classification of main bronchus involvement as T2 (with subsequent removal of the 2 cm distance from the carina as a limit between pT2 and pT3 tumours); classification of partial and total atelectasis as T2; categorisation of diaphragm invasion as T4. Mediastinal pleural invasion was removed from the criteria of T3 definition due to infrequent use. No changes were made to the N categories, though the new TNM categories led to further subgrouping of the III stage category (IIIA, IIIB, IIIC), corresponding to different treatments and outcomes [73]. Likewise, a new M1b descriptor was introduced for patients with a single extrathoracic metastatic lesion in a single organ, because they have better survival and different treatment options, compared with those with multiple extrathoracic lesions (M1c) [74].
The UICC also defined prognostic factors, subcategorised into tumour-related, host-related and environment-related factors. For example, tumour-related factors for surgically resected non-small cell lung cancer patients are T category, N category or extracapsular nodal extension; patient-related factors for surgically resected non-small cell lung cancer patients are parameters such as weight loss and/or performance status; environment-related factors for non-small cell lung cancer patients are resection margins or adequacy of mediastinal dissection.
New promising prognostic factors such as tumour-related molecular/biological markers as well as quality-of-life assessments have been named and might be addressed in future TNM editions.
RECIST (response evaluation criteria in solid tumours) are objective criteria used for evaluation of cancer therapy response in patients included in clinical trials. The latest and currently used version of RECIST (v1.1) was published in 2009. According to Eisenhauer et al. [75], lesions to be followed during treatment are defined at baseline (no more than 4 weeks before the beginning of the treatment) and separated as target lesions (up to 5 lesions with maximum diameter >10 mm and/or lymph nodes with short axis >15 mm) and non-target lesions (consisting of measurable lesions not meeting the target lesion criteria and non-measurable lesions such as effusions and bony lesions). The sum of the diameters of target lesions is compared to the nadir value to define complete response (disappearance of lesions), partial response (at least a 30% decrease), stable disease (changes insufficient to define partial response or progressive disease) or progressive disease (at least a 20% increase). The appearance of one or more new lesions is always considered progressive disease. Non-target lesions are evaluated qualitatively. Immunotherapy aims to enhance the immunological response of patients to the cancer cells. The effects of these therapies may lead to a response, to a pseudo-progression or, in a limited number of cases, to a so-called hyperprogressive disease, representing a rapid progression after starting the treatment [76]. Considering hyperprogressive disease and pseudoprogression, the RECIST working group developed the immune response evaluation criteria in solid tumours (iRECIST) [77], where the first assessment of progressive disease is considered unconfirmed until reassessment, usually after 6–8 weeks.
From lung cancer screening trials, we know that the best predictors of malignancy are nodule size and volume doubling time [78–80]. In a screening setting, the recommended cut-off for malignant tumours would be a volume doubling time of <600 days [81] – although the range of volume doubling time for malignant tumours is larger (50–800 days), especially due to the slow growth of less frequent adenocarcinomas [82]. For tumour restaging and follow-up CT exams, there is no need for volumetry since the RECIST criteria define the response to chemotherapy. However, volumetry may be helpful in the assessment of lung nodules in patients with primary cancers outside the lung, a screening setting or the appearance of a new lung nodule in a healed cancer patient. There is substantial inter-observer variability among volume software and radiologists; therefore, it is mandatory to use the same software or radiologist with the same reconstruction filter of the CT images (hard/soft) to reduce measurement errors [83]. The nodule volume can be calculated manually by the Schwartz formula (volume doubling time = [t log2] / [log Vt/V0], where t is the time between scans, Vt is the second volume, and V0 is the first volume) or more simply by using an online calculator.
Annual screening has already been proven to reduce lung cancer mortality in large trials [4, 12, 84], therefore annual screening should be preferred. Nevertheless, the MILD trial provides original evidence that prolonged screening beyond five years with every 2 years low-dose computerised tomography (LDCT) can achieve a lung cancer mortality reduction comparable to annual LDCT in subjects with a negative baseline examination [85, 86]. In the future, scores based on individual risk assessments will further stratify the screening follow-ups [87].
Precision medicine is the integration of genetics, clinics, imaging and environmental patient features with the aim of finding the most suitable treatment for each individual patient, with maximum benefit and limited toxicity [5].
In lung cancer this concept may be currently exemplified by targeted therapy and immunotherapy. Targeted therapy is based on the use of drugs specifically directed against oncogenic driver mutations. The two currently most used drugs of this category are directed to the epidermal growth factor receptor mutations and anaplastic lymphoma kinase (ALK) rearrangements. Evidence already exists supporting the use of alternative therapeutic agents for specific target alterations (e.g. BRAF, ROS1, among others). At some point during these therapies, an acquired resistance may present, and imaging may play a pivotal role for its early identification [5, 88]. Furthermore, radiomics is a recently introduced field of research that may well represent the role of imaging in lung cancer precision medicine. Radiomics refers to the extraction of qualitative and quantitative information from digital images and to perform a correlation with clinical data with or without associated gene expression, in order to support evidence-based clinical decision-making [89]. Research studies show that CT radiomics features may be helpful in more patient-specific selection of therapy, based on the correlation of these characteristics with mutational status and prognostication [90–92].
In the context of precision medicine, liquid biopsy must also be mentioned as a crucial non-invasive technique for managing lung cancer. Liquid biopsy is a non-invasive technique critical for managing lung cancer. It analyses circulating tumour DNA (ctDNA) and circulating tumour cells (CTCs) in the blood to detect cancer-specific genetic mutations, such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase and KRAS. This method allows for early detection and continuous monitoring of the disease, providing insights into treatment effectiveness and resistance [93]. Liquid biopsies also help detect minimal residual disease, which could be beneficial in the future for selecting patients who may benefit from adjuvant treatment after surgery. Additionally, they help monitor cancer recurrence post-treatment. Although not a complete replacement for traditional biopsies, liquid biopsies are safer, less invasive and a valuable complement to enhance lung cancer management [94].
Artificial intelligence refers to specific algorithms driven by existing data that allow objects to be predicted or classified. With the exponential growth of the computational power of microchips over the last decades, linear and logistic regression algorithms have given way to complex algorithms based on machine learning, such as decision trees, support vector machines and Bayesian networks. Furthermore, deep learning, which uses several layers of machine learning algorithms, paved the way to even more advanced analysis, such as artificial neural networks [95], convolutional networks, recurrent neural networks, long-term/short-term memory and generative adversarial networks [96]. Supervised learning systems are considered the most accurate models for training algorithms, requiring researchers to label patient data with inputs and outputs, often used to predict survival, cancer risk, nodule detection and nodule characteristics. Unsupervised learning does not require data labelling and is used to identify associations between samples. There are also semi-supervised learning models and reinforcement learning models, the latter using a reward function to adjust the algorithm.
By supporting diagnosis and predicting clinical outcomes, artificial intelligence can play a central role in shaping individual patient management. The most direct contributions of artificial intelligence to precision medicine are drug selection, prediction of treatment response [97] and estimation of survival [98]. Artificial intelligence-based models can also anticipate treatment-related toxicity, as recently documented for radiation-induced pneumonitis [99, 100]. Nevertheless, many technical challenges hamper the widespread implementation of artificial intelligence-based models. The reproducibility and standardisation of artificial intelligence methodology are critical aspects that need to be refined [101], and these account for the publication of the Image Biomarker Standardization Initiative [102] and the introduction of the radiomic quality score [103].
Augmented reality partially falls within the scope of artificial intelligence systems, and is currently underway in generating holograms for more precise surgical planning as well as for teaching purposes. As mentioned above, low-dose CT enables early detection of lung cancer and increases the survival of lung cancer patients. Recently, the LUNA16 challenge was set up, where several algorithms were tested, with the best algorithm providing a sensitivity of more than 95% at fewer than 1.0 false-positive per scan over a database of 888 CT scans [104]. Artificial intelligence-based screening models represent promising tools for the role of a second reader, as they detected up to 70% of lung cancers not detected by the radiologist, but did not detect about 20% of the lung cancers initially identified by the radiologist [105]. Moreover, once a nodule is detected, artificial intelligence can be used to predict the histopathological characteristics [106] and to stratify the risk of malignancy [107]. In the International Symposium on Biomedical Imaging (ISBI) 2018 Lung Nodule Malignancy Prediction Challenge [108], the top five participants used deep learning models with area under the curve (AUC) between 0.87 and 0.91 without significant differences. Another recent artificial intelligence model achieved an accuracy of 93%, with a sensitivity of 82% and a precision of 84% [109]. In addition, artificial intelligence-based models can distinguish between small cell lung cancer and non-small cell lung cancer [110], differentiate non-small cell lung cancer subtypes [111], identify specific molecular features (i.e. Ki-67, anaplastic lymphoma kinase, PDL1 or EGFR expression) through radiomic analysis [112].
The role of the pulmonologist is critical in detecting patients at risk of lung cancer early, in diagnosing suspected lung cancer patients and in evaluating disease extent as well as treating lung cancer [113]. Diagnosis and definition of disease extent and staging are not only crucial in determining prognosis but also necessary to direct treatment strategies [114]. Pulmonary interventions offer less invasive diagnostic and staging possibilities for the assessment of lung cancer patients. Following radiological imaging, bronchoscopy allows for cytopathological and histopathological sampling, which can be used to determine the precise type of cancer and thus guide treatment strategies. Direct biopsy (e.g. forceps) can be used for visible endobronchial lesions, while radioscopic or endobronchial ultrasound-guided transbronchial forceps biopsy can help to establish diagnosis in peripheral pulmonary lesions. Various techniques are available for diagnostic purposes (e.g. needle techniques endobronchial ultrasound-needle aspiration, endoscopic ultrasound-needle aspiration and combined endobronchial ultrasound/endoscopic ultrasound-needle aspiration) [115]. For staging purposes, endobronchial and endoscopic oesophageal ultrasound-guided transbronchial needle aspiration has replaced surgical mediastinal nodal staging as the initial procedure [116]. Pleural effusion puncture can determine stage IV lung cancer. Treatment decisions are based on the underlying lung cancer type, staging and comorbidities. The pulmonologist evaluates lung functional performance to evaluate operability and determine risk before possible other treatment approaches (e.g. chemotherapy and radiation therapy). The treatment decision and treatment administration is usually decided in a multidisciplinary setting where the pulmonologist plays a pivotal role for patients with lung cancer.
Promising and significant developments have recently occurred in the treatment of lung carcinoma, in part due to modern oncology. This is also reflected in the current histopathological subtyping of lung carcinoma, which now distinguishes numerous tumour entities leading to different treatment approaches. In addition to the histopathological and molecular characteristics of the tumour, the prognosis is also determined by the patient’s sex, general condition and concomitant diseases. The three modalities for treating lung cancer remain surgery, radiation and systemic therapy, which are increasingly recommended in a patient-orientated and multimodality approach due to the aforementioned development. A curative therapy claim exists for non-small cell lung cancer [117] in early and, in some cases, advanced stages. However, for the majority of stage IIIB/C and IV patients, therapy is not curative. In recent years, drug development has led to a significant improvement in the prognosis of many patients thanks to immune checkpoint and kinase inhibitors in combination with predictive biomarkers. Important advances have also been made in the surgical treatment of lung cancer thanks to technical advances in minimally invasive surgery. Efforts to resect tumours in a way that spares lung tissue are also likely to advance patient-centred tumour therapy. Other developments in therapy include local endoscopic and percutaneous interventional therapy and new options in palliative care. The current staging of non-small cell lung cancer is based on the TNM classification and the UICC8 criteria [118–120]. Due to the diversity of treatment strategies, the very heterogeneous stage IIIA with ipsilateral mediastinal lymph node involvement is additionally classified according to Robinson [121]. As a rule, cytological or histological confirmation should be performed if N2 or N3 metastasis is suspected. For this purpose, further clarification by endobronchial or endo-oesophageal ultrasound (EBUS/EUS) is primarily indicated. If this does not lead to a diagnosis, surgical biopsy by video-assisted mediastinoscopy / video-assisted mediastinoscopic lymphadenectomy (VAM/VAMLA) or video-assisted thoracoscopy (VATS) is another diagnostic option [118, 122, 123]. Therapeutic advances have necessitated the development of new pathological classifications of lung cancer [124]. One reason for this is the importance of molecular testing in addition to histopathological diagnosis, which must also be feasible from small tissue samples. If there are no primary contraindications for surgery, neither from the tumour situation nor from comorbidities, the expected postoperative lung function and the perioperative cardiovascular risk are crucial for planning the anatomical lung resection. Clarifying algorithms for determining cardiopulmonary reserve have been established, for example, by the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) [125]. The recommendations for local therapy with curative intent apply to the entire group of non-small cell lung cancers. For systemic therapy without curative intent, recommendations are differentiated by histological, immunological and genetic markers. With the advent of systemic molecular targeted therapy, checkpoint inhibitors and multimodality multidisciplinary therapy, even patients with metastatic disease and especially patients with oligometastatic tumour stage can achieve survival greater than 5 years. The main method of cure is surgery. A prerequisite for this treatment option is the performance of an anatomical lung resection. The current standard of minimal expansion is lobectomy [126]. If resectability for a minimally invasive surgical approach is given (cT1–3, cN0–1), lobectomy should be performed minimally invasive, uniportally or multiportally video-assisted (uniportal or multiportal VATS lobectomy). This is now the standard procedure for stage I tumours and is associated with less postoperative morbidity and physical impairment [127]. For tumours ≤2 cm in diameter, anatomical segment resection is an alternative to lobectomy. Currently, data are available from the Japanese JCOG0802 trial, in which n = 1106 stage IA patients were randomly assigned to lobectomy or anatomical segment resection [128]. The 5-year survival rate without recurrence was not different between the two groups, 87.9% and 88.0%, respectively. However, the 5-year survival rate showed a significant advantage in favour of segment resection (94.3% versus 91.1%). This advantage was primarily due to lower mortality from second malignancies and a higher rate of curative therapy for second malignancies in the segmental group. In both groups combined, 4.9% of patients died from their primary lung cancer and 7.8% from another cause of death, primarily second malignancy, during the observation period (median 7.3 years) [128]. For central tumour location, the larger resection extents of pneumonectomy or the more technically challenging but parenchyma-sparing sleeve resections are available. The mortality after pneumonectomy is two to three times higher than after lobectomy, due in part to the greater loss of lung parenchyma and the associated burden on the right heart. The goal of complete removal of the hilar and mediastinal lymph nodes during tumour surgery is to improve prognosis by accurately determining tumour stage (N status) as a basis for stage-appropriate postoperative therapy. There is no evidence of increased postoperative morbidity or mortality associated with radical mediastinal lymphadenectomy. Even in PET-negative mediastinum, systematic intraoperative lymph node dissection reveals tumour-involved lymph nodes in 10–16%, depending on tumour location and size [129].
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. MFC reports grants from CSL Behring and consulting fees from Boehringer Ingelheim, MSD, Pfizer, GSK, Syndax and AstraZeneca, all paid to her institution. The other authors did not disclose any potential conflicts of interest related to the content of this manuscript.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 Mar;65(2):87–108. doi: https://doi.org/10.3322/caac.21262
2. N. K. N. Bundesamt für Statistik (BFS). Kinderkrebsregister (KiKR), Schweizerischer Krebsbericht 2021, in Stand und Entwicklungen, Bundesamt für Statistik, Neuchatel, 2021. Available at: https://www.nkrs.ch/assets/files/publications/Krebsbericht2021/1177-2100-de.pdf
3. Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, et al.; National Lung Screening Trial Research Team. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013 Sep;369(10):920–31. doi: https://doi.org/10.1056/NEJMoa1208962
4. de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020 Feb;382(6):503–13. doi: https://doi.org/10.1056/NEJMoa1911793
5. Park H, Sholl LM, Hatabu H, Awad MM, Nishino M. Imaging of Precision Therapy for Lung Cancer: Current State of the Art. Radiology. 2019 Oct;293(1):15–29. doi: https://doi.org/10.1148/radiol.2019190173
6. Ko CC, Yeh LR, Kuo YT, Chen JH. Imaging biomarkers for evaluating tumor response: RECIST and beyond. Biomark Res. 2021 Jul;9(1):52. doi: https://doi.org/10.1186/s40364-021-00306-8
7. Rubins J, Unger M, Colice GL; American College of Chest Physicians. Follow-up and surveillance of the lung cancer patient following curative intent therapy: ACCP evidence-based clinical practice guideline (2nd edition). Chest. 2007 Sep;132(3 Suppl):355S–67S. doi: https://doi.org/10.1378/chest.07-1390
8. Subramanian M, Liu J, Greenberg C, Schumacher J, Chang GJ, McMurry TL, et al. Imaging Surveillance for Surgically Resected Stage I Non-Small Cell Lung Cancer: Is More Always Better? J Thorac Cardiovasc Surg. 2019 Mar;157(3):1205–1217.e2. doi: https://doi.org/10.1016/j.jtcvs.2018.09.119
9. Billè A, Ahmad U, Woo KM, Suzuki K, Adusumilli P, Huang J, et al. Detection of Recurrence Patterns After Wedge Resection for Early Stage Lung Cancer: Rationale for Radiologic Follow-Up. Ann Thorac Surg. 2016 Oct;102(4):1067–73. doi: https://doi.org/10.1016/j.athoracsur.2016.04.056
10. Shah PK, Austin JH, White CS, Patel P, Haramati LB, Pearson GD, et al. Missed non-small cell lung cancer: radiographic findings of potentially resectable lesions evident only in retrospect. Radiology. 2003 Jan;226(1):235–41. doi: https://doi.org/10.1148/radiol.2261011924
11. Quekel LG, Kessels AG, Goei R, van Engelshoven JM. Miss rate of lung cancer on the chest radiograph in clinical practice. Chest. 1999 Mar;115(3):720–4. doi: https://doi.org/10.1378/chest.115.3.720
12. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al.; National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011 Aug;365(5):395–409. doi: https://doi.org/10.1056/NEJMoa1102873
13. Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Ceresoli G, et al.; DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009 Sep;180(5):445–53. doi: https://doi.org/10.1164/rccm.200901-0076OC
14. Infante M, Sestini S, Galeone C, Marchianò A, Lutman FR, Angeli E, et al. Lung cancer screening with low-dose spiral computed tomography: evidence from a pooled analysis of two Italian randomized trials. Eur J Cancer Prev. 2017 Jul;26(4):324–9. doi: https://doi.org/10.1097/CEJ.0000000000000264
15. MacMahon H, Naidich DP, Goo JM, Lee KS, Leung AN, Mayo JR, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017 Jul;284(1):228–43. doi: https://doi.org/10.1148/radiol.2017161659
16. Rizzo S, Radice D, Femia M, De Marco P, Origgi D, Preda L, et al. Metastatic and non-metastatic lymph nodes: quantification and different distribution of iodine uptake assessed by dual-energy CT. Eur Radiol. 2018 Feb;28(2):760–9. doi: https://doi.org/10.1007/s00330-017-5015-5
17. Somatostatin receptor physiology and targets for somatostatin analogue therapy. Abstracts of the Young Investigator Meeting. 31 October-2 November 2002, Barcelona, Spain, Eur J Endocrinol, vol. 148 Suppl 1, p. 50 pages, Jan 2003. Available at: https://www.ncbi.nlm.nih.gov/pubmed/12602334
18. Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001 Feb;285(7):914–24. doi: https://doi.org/10.1001/jama.285.7.914
19. Hellwig D, Ukena D, Paulsen F, Bamberg M, Kirsch CM, Onko PE; Onko-PET der Deutschen Gesellschaft fur Nuklearmedizin. Metaanalyse zum Stellenwert der Positronen-Emissions-Tomographie mit F-18-Fluorodesoxyglukose (FDG-PET) bei Lungentumoren. Diskussionsbasis der deutschen Konsensus-Konferenz Onko-PET 2000 - [Meta-analysis of the efficacy of positron emission tomography with F-18-fluorodeoxyglucose in lung tumors. Basis for discussion of the German Consensus Conference on PET in Oncology 2000]. Pneumologie. 2001 Aug;55(8):367–77. doi: https://doi.org/10.1055/s-2001-16201
20. Ung YC, Maziak DE, Vanderveen JA, Smith CA, Gulenchyn K, Lacchetti C, et al.; Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. 18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a systematic review. J Natl Cancer Inst. 2007 Dec;99(23):1753–67. doi: https://doi.org/10.1093/jnci/djm232
21. Fischer BM, Mortensen J, Højgaard L. Positron emission tomography in the diagnosis and staging of lung cancer: a systematic, quantitative review. Lancet Oncol. 2001 Nov;2(11):659–66. doi: https://doi.org/10.1016/S1470-2045(01)00555-1
22. Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. Evaluation of F-18 fluorodeoxyglucose (FDG) PET scanning for pulmonary nodules less than 3 cm in diameter, with special reference to the CT images. Lung Cancer. 2004 Jul;45(1):19–27. doi: https://doi.org/10.1016/j.lungcan.2004.01.009
23. Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003 Jan;123(1 Suppl):137S–46S. doi: https://doi.org/10.1378/chest.123.1_suppl.137S
24. Birim O, Kappetein AP, Stijnen T, Bogers AJ. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg. 2005 Jan;79(1):375–82. doi: https://doi.org/10.1016/j.athoracsur.2004.06.041
25. Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA; American College of Chest Physicians. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007 Sep;132(3 Suppl):202S–20S. doi: https://doi.org/10.1378/chest.07-1362
26. Hellwig D, Baum RP, Kirsch C. FDG-PET, PET/CT and conventional nuclear medicine procedures in the evaluation of lung cancer: a systematic review. Nuklearmedizin. 2009;48(2):59–69. doi: https://doi.org/10.3413/nukmed-0217
27. van Tinteren H, Hoekstra OS, Smit EF, van den Bergh JH, Schreurs AJ, Stallaert RA, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002 Apr;359(9315):1388–93. doi: https://doi.org/10.1016/S0140-6736(02)08352-6
28. Herder GJ, Kramer H, Hoekstra OS, Smit EF, Pruim J, van Tinteren H, et al.; POORT Study Group. Traditional versus up-front [18F] fluorodeoxyglucose-positron emission tomography staging of non-small-cell lung cancer: a Dutch cooperative randomized study. J Clin Oncol. 2006 Apr;24(12):1800–6. doi: https://doi.org/10.1200/JCO.2005.02.4695
29. Mayor S. NICE issues guidance for diagnosis and treatment of lung cancer. BMJ. 2005 Feb;330(7489):439. doi: https://doi.org/10.1136/bmj.330.7489.439-b
30. Marom EM, McAdams HP, Erasmus JJ, Goodman PC, Culhane DK, Coleman RE, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999 Sep;212(3):803–9. doi: https://doi.org/10.1148/radiology.212.3.r99se21803
31. Fischer BM, Mortensen J, Langer SW, Loft A, Berthelsen AK, Petersen BI, et al. A prospective study of PET/CT in initial staging of small-cell lung cancer: comparison with CT, bone scintigraphy and bone marrow analysis. Ann Oncol. 2007 Feb;18(2):338–45. doi: https://doi.org/10.1093/annonc/mdl374
32. Brink I, Schumacher T, Mix M, Ruhland S, Stoelben E, Digel W, et al. Impact of [18F]FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2004 Dec;31(12):1614–20. doi: https://doi.org/10.1007/s00259-004-1606-x
33. Kut V, Spies W, Spies S, Gooding W, Argiris A. Staging and monitoring of small cell lung cancer using [18F]fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET). Am J Clin Oncol. 2007 Feb;30(1):45–50. doi: https://doi.org/10.1097/01.coc.0000239095.09662.19
34. Niho S, Fujii H, Murakami K, Nagase S, Yoh K, Goto K, et al. Detection of unsuspected distant metastases and/or regional nodes by FDG-PET [corrected] scan in apparent limited-disease small-cell lung cancer. Lung Cancer. 2007 Sep;57(3):328–33. doi: https://doi.org/10.1016/j.lungcan.2007.04.001
35. Vansteenkiste J, Fischer BM, Dooms C, Mortensen J. Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol. 2004 Sep;5(9):531–40. doi: https://doi.org/10.1016/S1470-2045(04)01564-5
36. Hellwig D, Gröschel A, Graeter TP, Hellwig AP, Nestle U, Schäfers HJ, et al. Diagnostic performance and prognostic impact of FDG-PET in suspected recurrence of surgically treated non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2006 Jan;33(1):13–21. doi: https://doi.org/10.1007/s00259-005-1919-4
37. Khaji RA, Kabwebwe VM, Mringo AG, Nkwabi TF, Bigio J, Mergenthaler C, et al. Factors Affecting Motivation among Key Populations to Engage with Tuberculosis Screening and Testing Services in Northwest Tanzania: A Mixed-Methods Analysis. Int J Environ Res Public Health. 2021 Sep;18(18):9654. doi: https://doi.org/10.3390/ijerph18189654
38. Meier-Schroers M, Homsi R, Skowasch D, Buermann J, Zipfel M, Schild HH, et al. Lung cancer screening with MRI: results of the first screening round. J Cancer Res Clin Oncol. 2018 Jan;144(1):117–25. doi: https://doi.org/10.1007/s00432-017-2521-4
39. Darçot E, Jreige M, Rotzinger DC, Gidoin Tuyet Van S, Casutt A, Delacoste J, et al. Comparison Between Magnetic Resonance Imaging and Computed Tomography in the Detection and Volumetric Assessment of Lung Nodules: A Prospective Study. Front Med (Lausanne). 2022 Apr;9:858731. doi: https://doi.org/10.3389/fmed.2022.858731
40. Razek AA, Fathy A, Gawad TA. Correlation of apparent diffusion coefficient value with prognostic parameters of lung cancer. J Comput Assist Tomogr. 2011;35(2):248–52. doi: https://doi.org/10.1097/RCT.0b013e31820ccf73
41. Chang YC, Yu CJ, Chen CM, Hu FC, Hsu HH, Tseng WY, et al. Dynamic contrast-enhanced MRI in advanced nonsmall-cell lung cancer patients treated with first-line bevacizumab, gemcitabine, and cisplatin. J Magn Reson Imaging. 2012 Aug;36(2):387–96. doi: https://doi.org/10.1002/jmri.23660
42. Bandi V, Lunn W, Ernst A, Eberhardt R, Hoffmann H, Herth FJ. Ultrasound vs. CT in detecting chest wall invasion by tumor: a prospective study. Chest. 2008 Apr;133(4):881–6. doi: https://doi.org/10.1378/chest.07-1656
43. Han Y, Kim HJ, Kong KA, Kim SJ, Lee SH, Ryu YJ, et al. Diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: A systematic review and meta-analysis. PLoS One. 2018 Jan;13(1):e0191590. doi: https://doi.org/10.1371/journal.pone.0191590
44. Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 2017 Jan;27(1):138–48. doi: https://doi.org/10.1007/s00330-016-4357-8
45. Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol. 2011 Jun;196(6):W678-82. doi: https://doi.org/10.2214/AJR.10.4659
46. Navani N, Fisher DJ, Tierney JF, Stephens RJ, Burdett S, Burdett S, et al.; NSCLC Meta-analysis Collaborative Group. The Accuracy of Clinical Staging of Stage I-IIIa Non-Small Cell Lung Cancer: An Analysis Based on Individual Participant Data. Chest. 2019 Mar;155(3):502–9. doi: https://doi.org/10.1016/j.chest.2018.10.020
47. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017 Jul;28 suppl_4:iv1–21.
48. Donington J, Ferguson M, Mazzone P, Handy J Jr, Schuchert M, Fernando H, et al.; Thoracic Oncology Network of the American College of Chest Physicians and the Workforce on Evidence-Based Surgery of the Society of Thoracic Surgeons. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest. 2012 Dec;142(6):1620–35. doi: https://doi.org/10.1378/chest.12-0790
49. Videtic GM, Chang JY, Chetty IJ, Ginsburg ME, Kestin LL, Kong FM, et al.; Expert Panel on Radiation OncologyLung. ACR appropriateness Criteria® early-stage non-small-cell lung cancer. Am J Clin Oncol. 2014 Apr;37(2):201–7. doi: https://doi.org/10.1097/COC.0000000000000013
50. Vansteenkiste J, De Ruysscher D, Eberhardt WE, Lim E, Senan S, Felip E, et al.; ESMO Guidelines Working Group. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013 Oct;24 Suppl 6:vi89–98. doi: https://doi.org/10.1093/annonc/mdt241
51. Iguchi T, Hiraki T, Matsui Y, Mitsuhashi T, Katayama N, Katsui K, et al. Survival Outcomes of Treatment with Radiofrequency Ablation, Stereotactic Body Radiotherapy, or Sublobar Resection for Patients with Clinical Stage I Non-Small-Cell Lung Cancer: A Single-Center Evaluation. J Vasc Interv Radiol. 2020 Jul;31(7):1044–51. doi: https://doi.org/10.1016/j.jvir.2019.11.035
52. Lam A, Yoshida EJ, Bui K, Fernando D, Nelson K, Abi-Jaoudeh N. A National Cancer Database Analysis of Radiofrequency Ablation versus Stereotactic Body Radiotherapy in Early-Stage Non-Small Cell Lung Cancer. J Vasc Interv Radiol. 2018 Sep;29(9):1211–1217.e1. doi: https://doi.org/10.1016/j.jvir.2018.04.029
53. Bi N, Shedden K, Zheng X, Kong FS. Comparison of the Effectiveness of Radiofrequency Ablation With Stereotactic Body Radiation Therapy in Inoperable Stage I Non-Small Cell Lung Cancer: A Systemic Review and Pooled Analysis. Int J Radiat Oncol Biol Phys. 2016 Aug;95(5):1378–90. doi: https://doi.org/10.1016/j.ijrobp.2016.04.016
54. Uhlig J, Ludwig JM, Goldberg SB, Chiang A, Blasberg JD, Kim HS. Survival Rates after Thermal Ablation versus Stereotactic Radiation Therapy for Stage 1 Non-Small Cell Lung Cancer: A National Cancer Database Study. Radiology. 2018 Dec;289(3):862–70. doi: https://doi.org/10.1148/radiol.2018180979
55. Uhlig J, Mehta S, Case MD, Dhanasopon A, Blasberg J, Homer RJ, et al. Effectiveness of Thermal Ablation and Stereotactic Radiotherapy Based on Stage I Lung Cancer Histology. J Vasc Interv Radiol. 2021 Jul;32(7):1022–1028.e4. doi: https://doi.org/10.1016/j.jvir.2021.02.025
56. S. J. Genshaft et al., Society of Interventional Radiology Quality Improvement Standards on Percutaneous Ablation of Non-Small Cell Lung Cancer and Metastatic Disease to the Lungs, J Vasc Interv Radiol, vol. 32, no. 8, pp. 1242 e1-1242 e10, Aug 2021.
57. Venturini M, Cariati M, Marra P, Masala S, Pereira PL, Carrafiello G. CIRSE Standards of Practice on Thermal Ablation of Primary and Secondary Lung Tumours. Cardiovasc Intervent Radiol. 2020 May;43(5):667–83. doi: https://doi.org/10.1007/s00270-020-02432-6
58. de Baère T, Palussière J, Aupérin A, Hakime A, Abdel-Rehim M, Kind M, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology. 2006 Aug;240(2):587–96. doi: https://doi.org/10.1148/radiol.2402050807
59. Palussière J, Chomy F, Savina M, Deschamps F, Gaubert JY, Renault A, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in patients ineligible for surgery: results of a prospective multicenter phase II trial. J Cardiothorac Surg. 2018 Aug;13(1):91. doi: https://doi.org/10.1186/s13019-018-0773-y
60. Abtin F, De Baere T, Dupuy DE, Genshaft S, Healey T, Khan S, et al. Updates on Current Role and Practice of Lung Ablation. J Thorac Imaging. 2019 Jul;34(4):266–77. doi: https://doi.org/10.1097/RTI.0000000000000417
61. de Baère T, Woodrum D, Tselikas L, Abtin F, Littrup P, Deschamps F, et al. The ECLIPSE Study: Efficacy of Cryoablation on Metastatic Lung Tumors With a 5-Year Follow-Up. J Thorac Oncol. 2021 Nov;16(11):1840–9. doi: https://doi.org/10.1016/j.jtho.2021.07.021
62. Callstrom MR, Woodrum DA, Nichols FC, Palussiere J, Buy X, Suh RD, et al. Multicenter Study of Metastatic Lung Tumors Targeted by Interventional Cryoablation Evaluation (SOLSTICE). J Thorac Oncol. 2020 Jul;15(7):1200–9. doi: https://doi.org/10.1016/j.jtho.2020.02.022
63. Gahide G, Pavic M, Sirois C, Sirois M, Poon J, Grondin-Beaudoin B, et al. Outpatient Approach for the Treatment of Lung Tumors with Cryoablation under Moderate Sedation. J Vasc Interv Radiol. 2021 Dec;32(12):1701–3. doi: https://doi.org/10.1016/j.jvir.2021.09.003
64. Kauczor HU, Baird AM, Blum TG, Bonomo L, Bostantzoglou C, Burghuber O, et al.; European Society of Radiology (ESR) and the European Respiratory Society (ERS). ESR/ERS statement paper on lung cancer screening. Eur Radiol. 2020 Jun;30(6):3277–94. doi: https://doi.org/10.1007/s00330-020-06727-7
65. McKee BJ, McKee AB, Kitts AB, Regis SM, Wald C. Low-dose computed tomography screening for lung cancer in a clinical setting: essential elements of a screening program. J Thorac Imaging. 2015 Mar;30(2):115–29. doi: https://doi.org/10.1097/RTI.0000000000000139
66. Mazzone P, Powell CA, Arenberg D, Bach P, Detterbeck F, Gould MK, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest. 2015 Feb;147(2):295–303. doi: https://doi.org/10.1378/chest.14-2500
67. Kazerooni EA, Austin JH, Black WC, Dyer DS, Hazelton TR, Leung AN, et al.; American College of Radiology; Society of Thoracic Radiology. ACR-STR practice parameter for the performance and reporting of lung cancer screening thoracic computed tomography (CT): 2014 (Resolution 4). J Thorac Imaging. 2014 Sep;29(5):310–6. doi: https://doi.org/10.1097/RTI.0000000000000097
68. Eberth JM, Qiu R, Linder SK, Gallant NR, Munden RF. Computed tomography screening for lung cancer: a survey of society of thoracic radiology members. J Thorac Imaging. 2014 Sep;29(5):289–92. doi: https://doi.org/10.1097/RTI.0000000000000105
69. Chelala L, Hossain R, Kazerooni EA, Christensen JD, Dyer DS, White CS. Lung-RADS Version 1.1: Challenges and a Look Ahead, From the AJR Special Series on Radiology Reporting and Data Systems. AJR Am J Roentgenol. 2021 Jun;216(6):1411–22. doi: https://doi.org/10.2214/AJR.20.24807
70. Radiology AC. Lung CT Screening Reporting & Data System (Lung-RADS). Available at: https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf
71. Munden RF, Black WC, Hartman TE, MacMahon H, Ko JP, Dyer DS, et al. Managing Incidental Findings on Thoracic CT: Lung Findings. A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2021 Sep;18(9):1267–79. doi: https://doi.org/10.1016/j.jacr.2021.04.014
72. Mino-Kenudson M, Schalper K, Cooper W, Dacic S, Hirsch FR, Jain D, et al.; IASLC Pathology Committee. Predictive Biomarkers for Immunotherapy in Lung Cancer: Perspective From the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol. 2022 Dec;17(12):1335–54. doi: https://doi.org/10.1016/j.jtho.2022.09.109
73. Petrella F, Rizzo S, Attili I, Passaro A, Zilli T, Martucci F, et al. Stage III Non-Small-Cell Lung Cancer: An Overview of Treatment Options. Curr Oncol. 2023 Mar;30(3):3160–75. doi: https://doi.org/10.3390/curroncol30030239
74. Neppl C, Keller MD, Scherz A, Dorn P, Schmid RA, Zlobec I, et al. Comparison of the 7th and 8th Edition of the UICC/AJCC TNM Staging System in Primary Resected Squamous Cell Carcinomas of the Lung-A Single Center Analysis of 354 Cases. Front Med (Lausanne). 2019 Sep;6:196.
75. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009 Jan;45(2):228–47. doi: https://doi.org/10.1016/j.ejca.2008.10.026
76. Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol. 2018 Nov;4(11):1543–52. doi: https://doi.org/10.1001/jamaoncol.2018.3676
77. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al.; RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017 Mar;18(3):e143–52. doi: https://doi.org/10.1016/S1470-2045(17)30074-8
78. Horeweg N, van Rosmalen J, Heuvelmans MA, van der Aalst CM, Vliegenthart R, Scholten ET, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol. 2014 Nov;15(12):1332–41. doi: https://doi.org/10.1016/S1470-2045(14)70389-4
79. Larici AR, Farchione A, Franchi P, Ciliberto M, Cicchetti G, Calandriello L, et al. Lung nodules: size still matters. Eur Respir Rev. 2017 Dec;26(146):170025. doi: https://doi.org/10.1183/16000617.0025-2017
80. Chen CY, Chen CH, Shen TC, Cheng WC, Hsu CN, Liao CH, et al. Lung cancer screening with low-dose computed tomography: experiences from a tertiary hospital in Taiwan. J Formos Med Assoc. 2016 Mar;115(3):163–70. doi: https://doi.org/10.1016/j.jfma.2015.11.007
81. Heuvelmans MA, Vliegenthart R, de Koning HJ, Groen HJ, van Putten MJ, Yousaf-Khan U, et al. Quantification of growth patterns of screen-detected lung cancers: the NELSON study. Lung Cancer. 2017 Jun;108:48–54. doi: https://doi.org/10.1016/j.lungcan.2017.02.021
82. Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Tazelaar HD, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology. 2007 Feb;242(2):555–62. doi: https://doi.org/10.1148/radiol.2422052090
83. Christe A, Brönnimann A, Vock P. Volumetric analysis of lung nodules in computed tomography (CT): comparison of two different segmentation algorithm softwares and two different reconstruction filters on automated volume calculation. Acta Radiol. 2014 Feb;55(1):54–61. doi: https://doi.org/10.1177/0284185113492454
84. National Lung Screening Trial Research Team. Lung Cancer Incidence and Mortality with Extended Follow-up in the National Lung Screening Trial. J Thorac Oncol. 2019 Oct;14(10):1732–42. doi: https://doi.org/10.1016/j.jtho.2019.05.044
85. Pastorino U, Sverzellati N, Sestini S, Silva M, Sabia F, Boeri M, et al. Ten-year results of the Multicentric Italian Lung Detection trial demonstrate the safety and efficacy of biennial lung cancer screening. Eur J Cancer. 2019 Sep;118:142–8. doi: https://doi.org/10.1016/j.ejca.2019.06.009
86. Silva M, Milanese G, Sestini S, Sabia F, Jacobs C, van Ginneken B, et al. Lung cancer screening by nodule volume in Lung-RADS v1.1: negative baseline CT yields potential for increased screening interval. Eur Radiol. 2021 Apr;31(4):1956–68. doi: https://doi.org/10.1007/s00330-020-07275-w
87. Oudkerk M, Liu S, Heuvelmans MA, Walter JE, Field JK. Lung cancer LDCT screening and mortality reduction - evidence, pitfalls and future perspectives. Nat Rev Clin Oncol. 2021 Mar;18(3):135–51. doi: https://doi.org/10.1038/s41571-020-00432-6
88. Nishino M, Hatabu H, Sholl LM, Ramaiya NH. Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care. Radiographics. 2017;37(5):1371–87. doi: https://doi.org/10.1148/rg.2017170015
89. Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016 Feb;278(2):563–77. doi: https://doi.org/10.1148/radiol.2015151169
90. Rizzo S, Petrella F, Buscarino V, De Maria F, Raimondi S, Barberis M, et al. CT Radiogenomic Characterization of EGFR, K-RAS, and ALK Mutations in Non-Small Cell Lung Cancer. Eur Radiol. 2016 Jan;26(1):32–42. doi: https://doi.org/10.1007/s00330-015-3814-0
91. de Jong EE, van Elmpt W, Rizzo S, Colarieti A, Spitaleri G, Leijenaar RT, et al. Applicability of a prognostic CT-based radiomic signature model trained on stage I-III non-small cell lung cancer in stage IV non-small cell lung cancer. Lung Cancer. 2018 Oct;124:6–11. doi: https://doi.org/10.1016/j.lungcan.2018.07.023
92. Botta F, Raimondi S, Rinaldi L, Bellerba F, Corso F, Bagnardi V, et al. Association of a CT-Based Clinical and Radiomics Score of Non-Small Cell Lung Cancer (NSCLC) with Lymph Node Status and Overall Survival. Cancers (Basel). 2020 May;12(6):1432. doi: https://doi.org/10.3390/cancers12061432
93. Chaudhuri AA, Chabon JJ, Lovejoy AF, Newman AM, Stehr H, Azad TD, et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017 Dec;7(12):1394–403. doi: https://doi.org/10.1158/2159-8290.CD-17-0716
94. Nielsen LR, Stensgaard S, Meldgaard P, Sorensen BS. ctDNA-based minimal residual disease detection in lung cancer patients treated with curative intended chemoradiotherapy using a clinically transferable approach. Cancer Treat Res Commun. 2024;39:100802. doi: https://doi.org/10.1016/j.ctarc.2024.100802
95. Deterding K, Spinner CD, Schott E, Welzel TM, Gerken G, Klinker H, et al.; HepNet Acute HCV IV Study Group. Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect Dis. 2017 Feb;17(2):215–22. doi: https://doi.org/10.1016/S1473-3099(16)30408-X
96. Klang E. Deep learning and medical imaging. J Thorac Dis. 2018 Mar;10(3):1325–8. doi: https://doi.org/10.21037/jtd.2018.02.76
97. Xu Y, Hosny A, Zeleznik R, Parmar C, Coroller T, Franco I, et al. Deep Learning Predicts Lung Cancer Treatment Response from Serial Medical Imaging. Clin Cancer Res. 2019 Jun;25(11):3266–75. doi: https://doi.org/10.1158/1078-0432.CCR-18-2495
98. Sesen MB, Nicholson AE, Banares-Alcantara R, Kadir T, Brady M. Bayesian networks for clinical decision support in lung cancer care. PLoS One. 2013 Dec;8(12):e82349. doi: https://doi.org/10.1371/journal.pone.0082349
99. Cunliffe A, Armato SG 3rd, Castillo R, Pham N, Guerrero T, Al-Hallaq HA. Lung texture in serial thoracic computed tomography scans: correlation of radiomics-based features with radiation therapy dose and radiation pneumonitis development. Int J Radiat Oncol Biol Phys. 2015 Apr;91(5):1048–56. doi: https://doi.org/10.1016/j.ijrobp.2014.11.030
100. Krafft SP, Rao A, Stingo F, Briere TM, Court LE, Liao Z, et al. Erratum: “The utility of quantitative CT radiomics features for improved prediction of radiation pneumonitis” [Med. Phys. Vol. 45(11):5317-5324 (2018)] [Med. Phys. Vol. 45(11):5317-5324 (2018)]. Med Phys. 2019 Feb;46(2):1079.
101. Traverso A, Wee L, Dekker A, Gillies R. Repeatability and Reproducibility of Radiomic Features: A Systematic Review. Int J Radiat Oncol Biol Phys. 2018 Nov;102(4):1143–58. doi: https://doi.org/10.1016/j.ijrobp.2018.05.053
102. Zwanenburg A, Vallières M, Abdalah MA, Aerts HJ, Andrearczyk V, Apte A, et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology. 2020 May;295(2):328–38. doi: https://doi.org/10.1148/radiol.2020191145
103. Ligero M, Jordi-Ollero O, Bernatowicz K, Garcia-Ruiz A, Delgado-Muñoz E, Leiva D, et al. Minimizing acquisition-related radiomics variability by image resampling and batch effect correction to allow for large-scale data analysis. Eur Radiol. 2021 Mar;31(3):1460–70. doi: https://doi.org/10.1007/s00330-020-07174-0
104. Setio AA, Traverso A, de Bel T, Berens MS, Bogaard CV, Cerello P, et al. Validation, comparison, and combination of algorithms for automatic detection of pulmonary nodules in computed tomography images: the LUNA16 challenge. Med Image Anal. 2017 Dec;42:1–13. doi: https://doi.org/10.1016/j.media.2017.06.015
105. Liang M, Tang W, Xu DM, Jirapatnakul AC, Reeves AP, Henschke CI, et al. Low-Dose CT Screening for Lung Cancer: Computer-aided Detection of Missed Lung Cancers. Radiology. 2016 Oct;281(1):279–88. doi: https://doi.org/10.1148/radiol.2016150063
106. Grossmann P, Stringfield O, El-Hachem N, Bui MM, Rios Velazquez E, Parmar C, et al. Defining the biological basis of radiomic phenotypes in lung cancer. eLife. 2017 Jul;6:e23421. doi: https://doi.org/10.7554/eLife.23421
107. Wu G, Jochems A, Refaee T, Ibrahim A, Yan C, Sanduleanu S, et al. Structural and functional radiomics for lung cancer. Eur J Nucl Med Mol Imaging. 2021 Nov;48(12):3961–74. doi: https://doi.org/10.1007/s00259-021-05242-1
108. Balagurunathan Y, Beers A, Mcnitt-Gray M, Hadjiiski L, Napel S, Goldgof D, et al. Lung Nodule Malignancy Prediction in Sequential CT Scans: summary of ISBI 2018 Challenge. IEEE Trans Med Imaging. 2021 Dec;40(12):3748–61. doi: https://doi.org/10.1109/TMI.2021.3097665
109. Xiao N, Qiang Y, Zia MB, Wang S, Lian J. Ensemble classification for predicting the malignancy level of pulmonary nodules on chest computed tomography images. Oncol Lett. 2020 Jul;20(1):401–8. doi: https://doi.org/10.3892/ol.2020.11576
110. Liu S, Liu S, Zhang C, Yu H, Liu X, Hu Y, et al. Exploratory Study of a CT Radiomics Model for the Classification of Small Cell Lung Cancer and Non-small-Cell Lung Cancer. Front Oncol. 2020 Sep;10:1268. doi: https://doi.org/10.3389/fonc.2020.01268
111. Zhu X, Dong D, Chen Z, Fang M, Zhang L, Song J, et al. Radiomic signature as a diagnostic factor for histologic subtype classification of non-small cell lung cancer. Eur Radiol. 2018 Jul;28(7):2772–8. doi: https://doi.org/10.1007/s00330-017-5221-1
112. Song L, Zhu Z, Mao L, Li X, Han W, Du H, et al. Clinical, Conventional CT and Radiomic Feature-Based Machine Learning Models for Predicting ALK Rearrangement Status in Lung Adenocarcinoma Patients. Front Oncol. 2020 Mar;10:369. doi: https://doi.org/10.3389/fonc.2020.00369
113. Gaga M, Powell CA, Schraufnagel DE, Schönfeld N, Rabe K, Hill NS, et al.; ATS/ERS Task Force on the Role of the Pulmonologist in the Management of Lung Cancer. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013 Aug;188(4):503–7. doi: https://doi.org/10.1164/rccm.201307-1269ST
114. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021 Aug;398(10299):535–54. doi: https://doi.org/10.1016/S0140-6736(21)00312-3
115. Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013 May;143(5 Suppl):e211S–50S. doi: https://doi.org/10.1378/chest.12-2355
116. Vilmann P, Frost Clementsen P, Colella S, Siemsen M, De Leyn P, Dumonceau JM, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2015 Jul;48(1):1–15. doi: https://doi.org/10.1093/ejcts/ezv194
117. Navani N, Butler R, Ibrahimo S, Verma A, Evans M, Doherty GJ, et al. Optimising tissue acquisition and the molecular testing pathway for patients with non-small cell lung cancer: A UK expert consensus statement. Lung Cancer. 2022 Oct;172:142–53. doi: https://doi.org/10.1016/j.lungcan.2022.08.003
118. Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, et al.; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members, and Participating Institutions. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2015 Dec;10(12):1675–84. doi: https://doi.org/10.1097/JTO.0000000000000678
119. Eberhardt WE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A 3rd, et al.; International Association for Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members, and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2015 Nov;10(11):1515–22. doi: https://doi.org/10.1097/JTO.0000000000000673
120. Yang L, Wang S, Zhou Y, Lai S, Xiao G, Gazdar A, et al. Evaluation of the 7th and 8th editions of the AJCC/UICC TNM staging systems for lung cancer in a large North American cohort. Oncotarget. 2017 May;8(40):66784–95. doi: https://doi.org/10.18632/oncotarget.18158
121. Robinson LA, Ruckdeschel JC, Wagner H Jr, Stevens CW; American College of Chest Physicians. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007 Sep;132(3 Suppl):243S–65S.
122. De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014 May;45(5):787–98. doi: https://doi.org/10.1093/ejcts/ezu028
123. Turna A, Melek H, Kara HV, Kılıç B, Erşen E, Kaynak K. Validity of the updated European Society of Thoracic Surgeons staging guideline in lung cancer patients. J Thorac Cardiovasc Surg. 2018 Feb;155(2):789–95. doi: https://doi.org/10.1016/j.jtcvs.2017.09.090
124. Travis WD. Pathology of lung cancer. Clin Chest Med. 2011 Dec;32(4):669–92. doi: https://doi.org/10.1016/j.ccm.2011.08.005
125. Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al.; European Respiratory Society; European Society of Thoracic Surgeons Joint Task Force on Fitness For Radical Therapy. The European Respiratory Society and European Society of Thoracic Surgeons clinical guidelines for evaluating fitness for radical treatment (surgery and chemoradiotherapy) in patients with lung cancer. Eur J Cardiothorac Surg. 2009 Jul;36(1):181–4. doi: https://doi.org/10.1016/j.ejcts.2009.04.022
126. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN Guidelines® Insights: Non-Small Cell Lung Cancer, Version 2.2023. J Natl Compr Canc Netw. 2023 Apr;21(4):340–50. doi: https://doi.org/10.6004/jnccn.2023.0020
127. Ezer N, Kale M, Sigel K, Lakha S, Mhango G, Goodman E, et al. Outcomes after Video-assisted Thoracoscopic Lobectomy versus Open Lobectomy for Early-Stage Lung Cancer in Older Adults. Ann Am Thorac Soc. 2018 Jan;15(1):76–82. doi: https://doi.org/10.1513/AnnalsATS.201612-980OC
128. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al.; West Japan Oncology Group and Japan Clinical Oncology Group. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022 Apr;399(10335):1607–17. doi: https://doi.org/10.1016/S0140-6736(21)02333-3
129. Zhu L, Wang T, Wu J, Zhai X, Wu Q, Deng H, et al. [Updated Interpretation of the NCCN Clinical Practice Guidelines (Version 3. 2023) for Non-small Cell Lung Cancer]. Zhongguo Fei Ai Za Zhi. 2023 Jun;26(6):407–15.