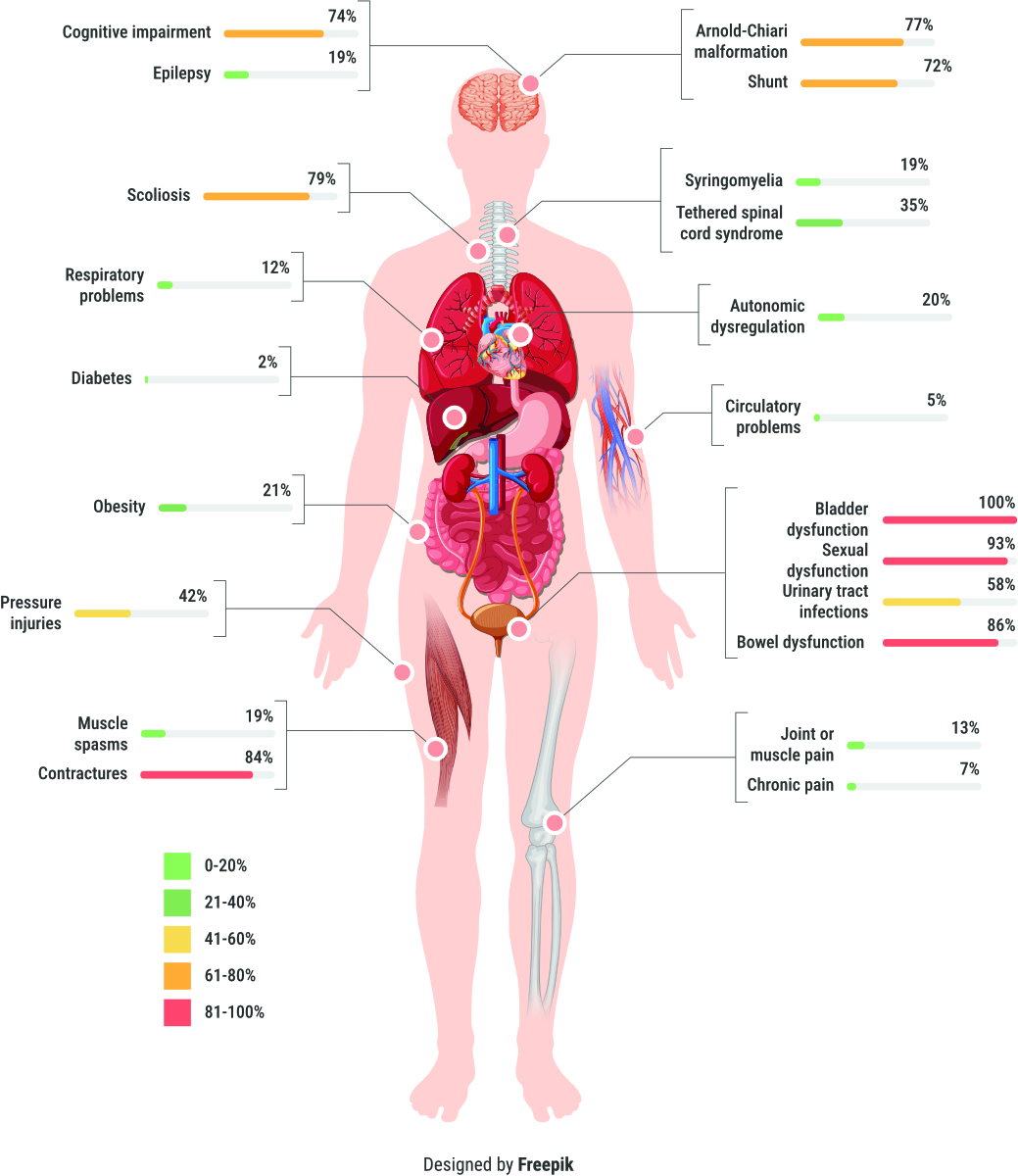
Figure 1
Prevalence of health conditions at time of transition from paediatric to adult care of people with spina bifida (n = 43).
DOI: https://doi.org/https://doi.org/10.57187/s.3836
Spina bifida represents the most common congenital spinal cord anomaly and results from improper fusion of neural folds during embryogenesis [1]. The incidence of spina bifida varies across different cultures, ranging from 0.7 to 5 per 1000 live births [2, 3]. In Switzerland, the incidence was reported at 0.19 per 1000 live births between 2007 and 2012, aligning with the European mean [4]. Advances in prenatal ultrasonography and widespread folate supplementation have contributed to a decrease in the birth prevalence of spina bifida. However, recent studies indicate a stable incidence of spina bifida over the past 12 years, with a rising prevalence attributed to improved access to medical care [5]. The advent of prenatal closure techniques for meningocele correlates with an increase in the number of live births of children with spina bifida, leading over time to a demographic shift where adults living with spina bifida outnumber children with this condition [6, 7]. Genetic predisposition has emerged as a significant risk factor in spina bifida’s aetiology, highlighting the intricate interplay between genetic and environmental determinants. Research has elucidated the influence of genetic variations within folate metabolism pathways, suggesting an elevated risk of spina bifida in offspring of individuals with specific genetic polymorphisms, particularly in the absence of adequate folate supplementation [8].
Population-based studies indicate a 1-year survival rate of approximately 87% for individuals with spina bifida, with roughly 78% surviving to the age of 17 years [9]. Prior to the 1960s, infant survival rates for spina bifida were dismal at 10%, largely due to infections and hydrocephalus complications [10, 11]. The introduction of shunt treatments for hydrocephalus and enhanced medical management led to significantly improved survival rates, reaching up to 90% by the mid 1970s. Despite these advancements, individuals with spina bifida continue to face excess morbidity and mortality into adulthood [12–15]. Depending on the severity of neurological impairment, only 17–61% of affected individuals survive to the age of 40 [16]. Reduced life expectancy in spina bifida is primarily attributed to the development of secondary health conditions, which are prevalent due to congenital nervous system impairment [17]. Neurological deficits may worsen over time due to secondary pathologies such as spinal cord tethering or syrinx formation. Routine follow-up consultations, particularly for bladder and renal function, are crucial, as renal failure has been established as a leading cause of death in adults with spina bifida [18, 19]. Early recognition of secondary health conditions can reduce patient morbidity and healthcare costs [20]. Among the secondary health conditions, bladder and bowel dysfunctions are particularly prevalent, adversely affecting quality of life and leading to frequent hospitalisations. Studies have underscored the extent of these dysfunctions in over 60% of children and adolescents with spina bifida, which lend support for early and sustained management to avert complications such as urinary tract infections and renal damage [21]. While urological and nephrological health problems in people with spina bifida are better documented, data on other secondary health conditions, especially in adults, are limited.
Guidelines recommend annual follow-up appointments for children until the age of eighteen. Paediatric care is typically person-centred, comprehensive and interdisciplinary. However, the transition from paediatric to adult care often fails, with up to 40% of adolescents losing access to specialised adult care [22]. Reasons for this failure include patient and paediatric healthcare system apprehension, lack of financial support for transition by health insurers and absence of interdisciplinary long-term adult care [23]. This results in undertreatment of important health issues such as shunt controls, bladder and bowel management, skin assessments and bone structure evaluations [24]. As seen in the study by Starowicz et al. [25], the most common health concern in adults with spina bifida was care coordination outside transition programmes. Without appropriate adult care, rehabilitation issues such as mobility, relationships, sexuality, family planning and vocational rehabilitation may receive insufficient attention. Transition and adult care programmes have gained recognition in recent years, with the implementation of guidelines and programmes emphasising the importance of a proper transition [23, 26]. Recognising the need for evidence-based clinical practice guidelines, the German-Speaking Society of Spinal Cord Injury (DMGP) commissioned its members to develop a guideline for follow-up care in people with spinal cord injury. During the development process, we found significant gaps in research evidence for long-term follow-up recommendations for individuals with spina bifida.
This observational study sought to delineate the health and functional status of adolescents and young adults with spina bifida during their transition from paediatric to adult care, forming a component of the “Learning Health System for people with spinal cord injury”. This initiative aims to continuously analyse data in order to improve care, promote innovative solutions and optimise individual patient outcomes. The objective of this observational study was to describe the health and functional profiles of young adolescents and young adults with spina bifida during the transition from paediatric to adult care. Specific aims included describing specific health condition clusters and predictive profiles for potentially modifiable health conditions such as urinary tract infections, respiratory problems, obesity and pressure injuries.
Descriptive study of data from all adolescents and young adults with spina bifida (all with meningomyelocele) aged 15–25 years who were referred to a single specialised spinal cord injury centre as part of the transition programme during the period from 1 September 2015 to 31 May 2022. All referred people were included in the study. The selection of people to refer to the centre was made by the paediatricians at the children’s hospitals. Criteria for referral to our centre were: a more severe neurological deficit, prevalence and severity of secondary health conditions and need for interdisciplinary treatment.
Demographic data (age, sex, place of residence, paediatric hospital), neurological and functional profile and information on secondary health conditions were routinely recorded in electronic patient medical records. Secondary health conditions were derived from the structured epicrisis (analytical summing up of the case history), including medical history, which was designed in collaboration with the children’s hospitals, as part of the transition programme. This epicrisis is based on the guideline for follow-up care of people with spinal cord injury [27] and covers all relevant secondary health conditions clustered in the following subcategories: neurological diagnosis (among which spasticity, syringomyelia, tethered cord syndrome and cognitive dysfunction), neuro-orthopaedic diagnosis (for example scoliosis, contractures), urogenital diagnosis (for example urinary tract infections, bowel problems), general internal medicine problems (for example obesity, respiratory problems, pressure injuries).
The neurological level was classified using the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), more commonly referred to as the ASIA Impairment Scale (AIS) [28].
Functional profile was described by assessing the independence in everyday life (by using the Spinal Cord Injury Measurement III [SCIM III]) [29], and mobility (according to the Gross Motor Function Classification System [GMFCS]). Information about secondary health conditions was based on medical diagnoses as structured reporting in medical records. In addition, educational training status and support needed for medical matters (e.g. help with medications, accompaniment to doctor’s appointments and ordering prescriptions) were also recorded.
Data collection was performed in a SecuTrial® database. After data collection, descriptive statistics were calculated for personal and clinical characteristics of included individuals. For continuous variables, normality of distribution was tested using the Shapiro-Wilk test, and data were described using either the mean (and standard deviation) or median (and interquartile range), as applicable. We calculated the frequencies and percentages of congenital conditions and acquired secondary health conditions, including mean and maximum number of conditions co-occurring together. We distinguished potentially modifiable secondary health conditions including urinary tract infections, pressure sores, respiratory problems and obesity and provided the co-occurrence analysis of conditions commonly occurring together. To understand the co-occurring secondary health conditions, we generated a frequency table using the STATA command combination showing the counts of each unique combination of values in the newly created variable. The equality of proportions test was used to assess whether the SHC proportions observed in different comparison groups are significantly different from each other or if they can be considered equal. Comparison groups included men vs women, adults vs minors, individuals with mild/severe cognitive impairment vs those without impairment, obese vs non-obese individuals and those with SCIM III score above (≥72) vs below median population values. For instance, the prtest command for a two-sample proportion test in Stata performs a test of the null hypothesis that the proportion of individuals with respiratory problems is the same between the two sex groups. All statistical analyses were performed using the software Stata 17.0 (StataCorp LLC, College Station, TX, USA) for Windows. All computations were done using two-tailed tests, and a p-value of <0.05 was considered statistically significant.
The study was formally approved by the regional medical ethics committee of northwest and central Switzerland (Project-ID 2022-05). All individuals included in the study consented to anonymised use of their data for research purposes and had the option to opt out.
Between 1 September 2015 and 31 May 2022, 43 adolescents and young adults with spina bifida entered the transition programme. Of these, 19 were males. Mean age was 18 years (SD 2.5 years). Most individuals had lumbar lesions (63%), followed by thoracic (25%) and sacral lesions (12%). Seventy-two percent had motor complete injury (ASIA Impairment Scale A or B). In 77% of the adolescents, a Chiari malformation was diagnosed in addition to spina bifida and 72% of the adolescents had a ventricular shunt (table 1).
Table 1Personal and clinical characteristics of individuals at the time of transition (n = 43).
| Age in years, mean (SD) | 18.39 (2.6) | |
| Sex, n (%) | Male | 19 (44.2%) |
| Female | 24 (55.8%) | |
| Education, n (%) | Regular school | 21 (48.8%) |
| Special school | 18 (41.9%) | |
| Other | 3 (6.9%) | |
| Unknown | 1 (2.3%) | |
| Level of lesion, n (%) | Thoracic | 11 (25.6%) |
| Lumbar | 27 (62.8%) | |
| Sacral | 5 (11.6%) | |
| Completeness of lesion, n (%) | AIS A | 28 (65.1%) |
| AIS B | 3 (6.9%) | |
| AIS C | 4 (9.3%) | |
| AIS D | 6 (13.9%) | |
| Unknown | 2 (4.6%) | |
| Gross Motor Function Classification System level, n (%) | Level I | 8 (18.6%) |
| Level II | 10 (23.3%) | |
| Level III | 2 (4.7%) | |
| Level IV | 8 (18.6%) | |
| Level V | 15 (34.9%) | |
| Total SCIM III score, median (IQR) | 72 (61–89) | |
| SCIM self-care | 20 (15–20) | |
| SCIM sphincter management | 29 (26–34) | |
| SCIM mobility | 22 (18–38) | |
| Body mass index in kg/m2, median (IQR) | 23.74 (21.4–27.3) | |
| Overweight/obese based on BMI cut-off, n (%) | 32 (74.4%) | |
| Overweight/obese based on clinical diagnosis, n (%) | 8 (21.0%) | |
| Cognitive impairment | None | 11 (25.6%) |
| Moderate/severe deficits | 32 (74.4%) | |
| Medical support, n (%) | Parents or family members | 19 (44.2%) |
| None | 17 (39.5%) | |
| Personal assistant | 4 (9.3%) | |
| Home nursing care | 1 (2.3%) | |
| Other | 2 (4.6%) | |
AIS: ASIA Impairment Scale; SCIM: Spinal Cord Injury Measure.
Fifty-eight percent of included individuals were mainly wheelchair-dependent for mobilisation (Gross Motor Function Classification System Levels III, IV and V), 60% of the individuals were dependent on help for medical tasks (like bladder catheterisation, bowel management, making appointments with their healthcare providers or organising/taking medications), which was mostly provided by the parents or family members (44%). The median SCIM score was 72 (IQR 61–89), with the highest independence in self-care domains. Most of the included people with spina bifida attended regular schools (49%) but also a major proportion visited special schools (42%) (table 1).
Figure 1 depicts the prevalence of the most common secondary health conditions among the 43 individuals included in the analysis. The most prevalent secondary health conditions included: lower urinary tract dysfunction (100%), sexual dysfunction (93%), bowel dysfunction (87%) and contractures (84%). The mean number of secondary health conditions per person was 8.8 with the minimum number of conditions per person being 4 (n = 1). Most of the individuals had 7 to 10 co-occurring secondary health conditions (n = 20, 63%).
Among the potentially modifiable secondary health conditions, urinary tract infections were reported in 58% of the individuals, pressure injuries in 42%, and obesity and respiratory problems in 21% and 12%, respectively.

Figure 1
Prevalence of health conditions at time of transition from paediatric to adult care of people with spina bifida (n = 43).
Table 2 displays the potentially modifiable secondary health conditions depicting the proportion of individuals per important demographic and clinical characteristic. This shows that urinary tract infections were more prevalent in males, adults (>18 years), individuals without cognitive impairment, with lower SCIM III scores (<72 points) and obese individuals. Pressure injuries were found to be more prevalent in females, adults, obese individuals and those without cognitive impairments. Obesity was more prevalent in females (30%), those with cognitive impairment and lower SCIM III scores. Also, respiratory problems were more prevalent (24%) among those with SCIM III score below median (72 points) as compared to those equal and above the median score (0%, p = 0.02) and were higher in females (21%) as compared to males (0%, p = 0.03). Since multiple statistical tests were performed, there was an increased likelihood of obtaining significant results purely by random chance. No differences in the prevalence of secondary health conditions were observed after stratification based on sex, age, cognitive impairment, SCIM III score or obesity.
Table 2Prevalence of modifiable secondary health conditions depicting the proportion of individuals per important personal and clinical characteristic.
| Urinary tract infection, n (%) | p value | Pressure sore, n (%) | p value | Respiratory problem, n (%) | p value | Obesity, n (%) | p value | |
| Overall prevalence | 25 (58.1%) | – | 18 (41.9%) | – | 5 (11.6%) | – | 8 (21.1%) | – |
| Males | 12 (63.2%) | 0.5 | 10 (52.6%) | 0.2 | 0 (0%) | 0.03 | 2 (11.1%) | 0.1 |
| Females | 13 (54.2%) | 8 (52.6%) | 5 (20.8%) | 6 (30.0%) | ||||
| Adults | 18 (62.1%) | 0.4 | 13 (44.8%) | 0.6 | 3 (10.3%) | 0.7 | 5 (19.2%) | 0.7 |
| Minors | 7 (50%) | 5 (35.7%) | 2 (14.3%) | 3 (25%) | ||||
| Mild/severe cognitive impairment | 17 (53.1%) | 0.3 | 11 (34.4%) | 0.1 | 5 (16.6%) | 0.2 | 7 (25.9%) | 0.5 |
| No cognitive impairment | 8 (72.7%) | 7 (63.6%) | 0 (0%) | 1 (9.1%) | ||||
| SCIM III ≥72 | 12 (54.5%) | 0.6 | 10 (45.5%) | 0.6 | 0 (0%) | 0.02 | 3 (15.8%) | |
| SCIM III <72 | 13 (61.9%) | 8 (38.1%) | 5 (23.8%) | 5 (26.3%) | 0.4 | |||
| Obese | 4 (50%) | 0.5 | 5 (62.5%) | 0.3 | 1 (12.5%) | 0.9 | – | |
| Not obese | 19 (63.3%) | 12 (40%) | 4 (13.3%) | |||||
SCIM: Spinal Cord Injury Measure.
In the co-occurrence analyses of the four investigated potentially modifiable secondary health conditions (figure 2), the most common pattern included the co-occurrence of pressure injury and urinary tract infection, present in 27% of the individuals.
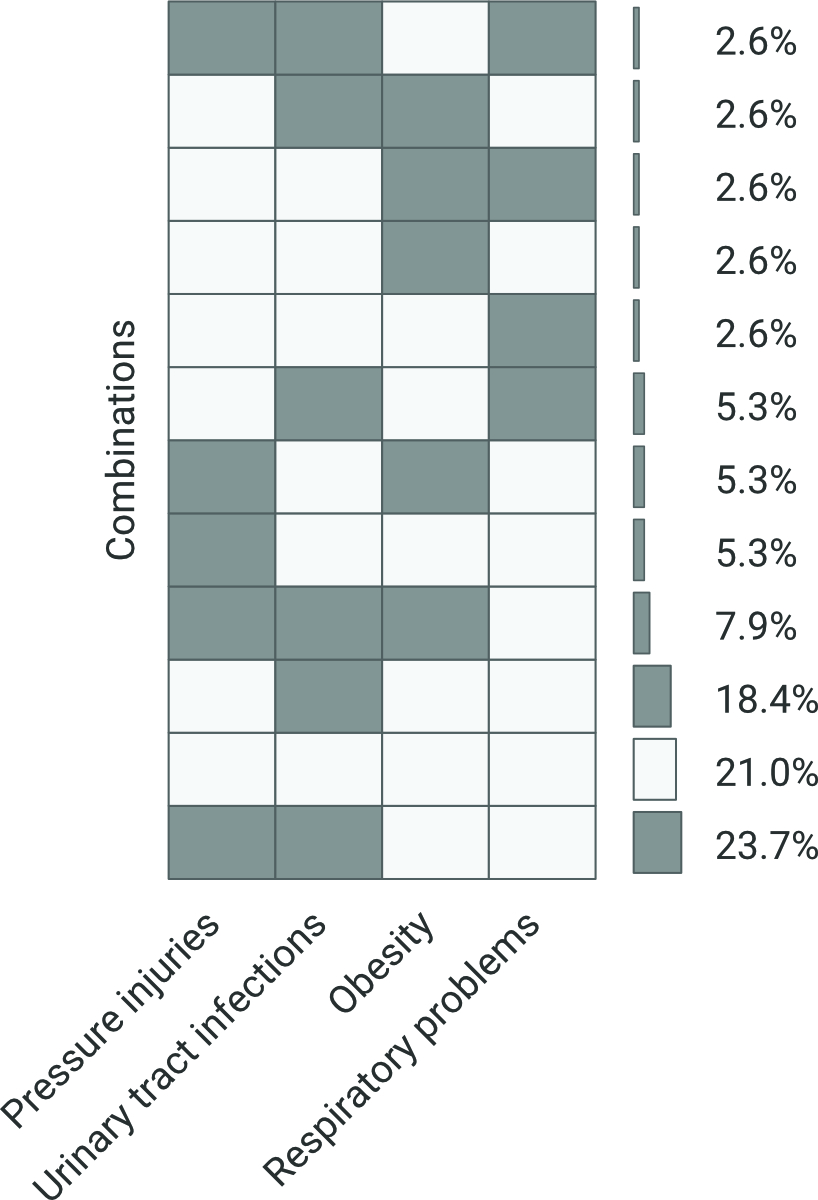
Figure 2
Co-occurrence analysis for modifiable secondary health conditions. This figure shows the co-occurrence of four secondary health conditions in analyses based on individuals who had complete information (n = 38). The dark grey colour indicates presence of secondary health conditions, the light grey colour indicates no SHC. For instance, 23.7% of individuals had pressure injuries and urinary tract infections co-occurring together, whereas 21% of individuals had none of these four secondary health conditions co-occurring together.
This study, describing prevalence and clusters of health problems and functioning at time of transition in people with spina bifida in Switzerland, demonstrates the multifaceted problems this population faces. Not only is the prevalence of secondary health conditions high, but many adolescents and young adults still need help for daily functioning, particularly for bladder and bowel management, but also for taking their medications. Help is mainly provided by parents.
Studies evaluating the prevalence of secondary health conditions in (young) adults with spina bifida are scarce. Although certain details may differ, which may be related to different patient populations and methodology, all studies show a high prevalence of secondary health conditions in adults with spina bifida, reporting a high prevalence of bladder and bowel problems, pain and pressure injuries as main concerns [25, 30, 31]. However, we found some differences in prevalence regarding the above-mentioned secondary health conditions. Compared to the study of Bendt et al. [30], in our population we found a lower prevalence of pain (13% versus 40%) and a higher prevalence of pressure injuries (42% vs 20%). Skin health concerns were mentioned in 31% of the study population of Starowicz et al. [25]; this lower percentage might also be due to the inclusion of people with spina bifida occulta in their study. Bladder and bowel problems in our population were more prevalent than in the study by Starowicz et al. [25], which might be due to the inclusion of people with spina bifida occulta in their study population. Compared to the studies by Heyns et al. [32] and Starowicz et al. [25], we found a much higher presence of scoliosis (44% and 53%, respectively, versus our 79%), which might also be due to inclusion of a different patient population (children, spina bifida occulta). Also, in our study, a BMI >22 was found in 74.4% of adolescents. However, using an adjusted scale for spina bifida, 21% are classified as overweight. Bendt et al. [30] reported that two-thirds of the population had a BMI higher than normal, and about one-third was classified as obese according to a not-for-spina-bifida adjusted scale which might underestimate true obesity in this population [31]. Although it is well known that people with spina bifida may have cognitive impairment, especially those with hydrocephalus [33–35], the prevalence of cognitive impairment in our population was high. Mild or severe cognitive impairment was found in 74% of our population. The cognitive testing of adolescents during transition revealed more frequent cognitive problems as prior diagnosed, especially milder cognitive deficits. In particular, executive dysfunction (e.g. problems with organising, inability to multitask) was found to be more prevalent. Cognitive function, therefore, should be assessed in follow-up care. Although urinary tract infections in teenagers might be common [36, 37], the prevalence of urinary tract infections in our population at time of transition was much higher (58%). Unfortunately, in the study by Bendt et al. [30] prevalence of urinary tract infection was not reported. The diagnosis, treatment and prevention of urinary tract infections in people with spina bifida remain one of the most significant challenges faced by practitioners caring for this patient population. The guideline on diagnosis and treatment of neurogenic bladder dysfunction in spina bifida gives recommendations on this topic [38]. In order to be able to give evidence-based recommendations, research evidence on the prevention of urinary tract infections in the challenging population of (young) adults with spina bifida is necessary [39].
This study also studied the co-occurrence of potentially preventable SCHs. The most common patterns observed were: pressure injuries and urinary tract infection in 27%; no secondary health conditions in 21%; urinary tract infection alone in 18%; urinary tract infection, pressure injuries and obesity in 8%. There are no other studies we could use as comparators, but the results clearly show that urinary tract infection plays a significant role in the health of people with spina bifida. Urinary tract infections in this population are often multifactorial, for example detrusor overactivity, residual urine, the need for catheterisation or presence of bladder stones may contribute to an increased urinary tract infection rate.
Regarding mobility, it was found that 50% of adolescents are predominantly mobile in a wheelchair. Knowing that people with spina bifida tend to lose walking mobility with age, the results of Bendt et al. [30] in adults with spinal cord injury showing a higher percentage might be unsurprising, but we found a lower percentage of people with powered wheelchairs. A possible explanation might be the use of powered tracking devices for manual wheelchairs (for example Swiss Trac®) in Switzerland, which is often prioritised above powered wheelchairs.
A critical dimension that warrants further exploration within the context of transition care for individuals with spina bifida pertains to the indispensable role of family and caregiver support. The findings from this study reveal a pronounced dependency on parents and family members for assistance with daily medical tasks, including bladder catheterisation, bowel management and administration of medications. Medication concerns (requiring or considering new prescriptions; restarting medications; needing to switch or stop medications or adjust their dosage; needing to refill existing medications; compliance) were also reported by Starowicz et al. [25] as a major issue in people with spina bifida. The results of our study show that independence in major life domains (self-care, mobility, sphincter management) is of concern and underscores the imperative to embed family and caregiver education and support as a cornerstone of transition programmes. To be truly effective, transition care must extend its focus beyond the individual with spina bifida to also empower caregivers with the requisite knowledge and competencies needed to support the individual’s progress towards autonomy. The integration of caregiver support mechanisms within transition care models is essential for enhancing the effectiveness of these programmes, ensuring a more seamless transition and improved health outcomes for individuals with spina bifida. Future investigations should aim to delineate the impact of caregiver education and support on the efficacy of transition programmes, with the goal of developing comprehensive care frameworks that cater to the needs of both individuals with spina bifida and their caregivers.
While paediatric care for people with spina bifida is person-centred, interdisciplinary and comprehensive, this approach is often lacking in the long-term medical care of adults [5, 23, 40]. Especially in the challenging period from puberty to adulthood, the accompaniment of self-management of health and socioeconomic issues, such as finding a job, independent living or social security questions are often not included in medical care and might be overlooked. The results of this study show the need for an interdisciplinary and comprehensive approach to follow-up care of adults with spina bifida. Regular, interdisciplinary check-ups in adulthood and a well-planned and structured transition from the paediatric to the adult medical setting are therefore essential, not only to avoid increased morbidity and mortality, but also to optimise functional capacity and autonomy in people with spina bifida along the continuum of life [23, 27, 40].
The complexity of caring for people with spina bifida calls for specialised adult spina bifida teams, ideally at spinal cord injury centres. In Switzerland, to better manage this complex situation and to prevent loss to follow-up care, a collaboration between three major children’s hospitals and a specialised spinal cord injury centre was established a few years ago. At this specialised spinal cord injury centre, an interdisciplinary team was established consisting of a rehabilitation physician, a case manager, a physical and occupational therapist and a neuro-urologist as well as a neuro-orthopaedic specialist [27]. Before entering the adult care system, a transition with a structured epicrisis, a paper transition and an appointment involving the “patient”, paediatrician, adult care physician and case manager and their care providers at the children’s hospital take place. After this appointment, the young adults/adolescents are transferred to adult care, where interdisciplinary annual follow-up appointments are scheduled. Other healthcare professionals (such as vocational therapists, sports medicine specialists, dieticians) are included if necessary and neurosurgical care is organised in the hospital closest to the adolescents/young adults to guarantee timely medical care if needed in case of shunt problems. During follow-up care, the rehabilitation physician acts as a gatekeeper deciding if the inclusion of other specialties e.g. neurology, gynaecology, etc., is needed. They also communicate closely with the general practitioner and outpatient therapy team. The transition programme thus forms a bridge between paediatric and adult medicine. Every year the team evaluates loss to follow-up and actively contacts people that did not show up for their appointment. This active approach led to the result that all 43 adolescents and young adults with spina bifida who were admitted to the transition programme at the centre attend their annual check-ups.
Our results also serve the “Learning Health System” approach to continuously improving medical and rehabilitative services. For example, the study results influenced the selection of International Classification of Functioning, Disability and Health (ICF) categories in the guideline “Life-long follow-up care for people with spinal cord injury” and led to adjustments in the guideline, for example including a cognitive evaluation at time of transition and specific recommendations for patients with shunts [27].
Finally, our results further promote innovative solutions such as the expansion of transition consultations and specific training appointments addressing health management.
Although our study is one of the first addressing health conditions and functioning at the time of transition, the included study population is rather small, thus we were not able to perform adjusted regression analyses and explore potential personal and clinical factors associated with a higher burden of secondary health conditions or SHC co-occurrence. Further, although our findings indicate a high burden of in the transition phase, our results may not be generalisable to all people with spina bifida. It may be that individuals visiting specialised rehabilitation centres experience more severe neurological and functional impairments than those followed up by general practitioners or those without any follow-up at all; thus our findings may overestimate the true SHC burden. On the other hand, studies in other health systems show a high mortality in populations with spina bifida, so we might conclude that the problem of SHC is a generalised problem in this population [17]. During the COVID-19 pandemic no transitions could be done, but the backlog was eliminated as soon as we were allowed to see patients again.
A strength of the study is the data quality owing to the introduction of a structured epicrisis ensuring that data of all included persons were assessed in the same way. The preliminary nature of these results has prompted plans to expand the dataset and establish a prospective cohort of children, adolescents and young adults with spina bifida, aiming to encompass a broader spectrum of this population.
Building upon the findings of this study, which elucidates the multifaceted challenges faced by individuals with spina bifida, we advocate for a more nuanced exploration of contributing factors that could further inform personalised care strategies. Notably, while our study has shed light on the high prevalence of secondary health conditions in people with spina bifida, it concurrently highlights the need for more-tailored prevention strategies. In addition to the longitudinal follow-up of people with spina bifida throughout their lifetime in cooperation with the children’s hospitals, there is a need for better understanding of occurrence of secondary health conditions (especially urinary tract infections and pressure injuries) to develop prevention strategies. One envisioned possibility is to better explore the intersection of the microbiome with spina bifida-related secondary health conditions. Studies of the urinary tract microbiome show the dominance of bacterial genera associated with urinary tract infection [41] and future studies focusing on the role of the microbiome might lead to a better understanding of the dynamics between genetic predisposition and environmental factors specific to people with spina bifida.
In conclusion, our study showed that the prevalence of secondary health conditions among individuals with spina bifida at the time of transition is alarmingly high and functional profiles underscore the need for support of adolescents and young adults for daily medical issues. The study highlights the critical role of continuing interdisciplinary follow-up care beyond childhood, not only to prevent health problems, but also to improve functioning and independence in everyday life. Transition programmes help to prevent loss to follow-up care and therefore might play a role in prevention of secondary health conditions.
The models and findings from this study, particularly those concerning transitions in care and the integration of multidisciplinary approaches, have broader applicability across various chronic health conditions, such as cerebral palsy, diabetes mellitus and cystic fibrosis. By extending our research model to include these conditions, we can explore universal strategies for improving transitional care, thereby making a relevant contribution to stakeholders interested in optimising health outcomes across a spectrum of chronic health issues in young adults transitioning from paediatric to adult care.
Data are available on request.
We thank Beth Padden, Alexandra Wattinger and Prof. Andreas Meyer-Heim (Children’s Hospital Zurich), Prof. Sebastian Grunt and Cordula Scherer and team (Children’s Hospital Bern), Sandra Shavit and team (Children’s Hospital Luzern), Andrea Violka and Rahel Tschopp (Case management transition programme) and the transition team of the Swiss Paraplegic Centre. A special thanks also to the Swiss Spina Bifida und Hydrocephalus Association (SBH) for their help with drawing up the guideline. A sincere thanks also to the private foundation that financially supported us during the developmental phase of the transition programme.
This research received no funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Dicianno BE, Kurowski BG, Yang JM, Chancellor MB, Bejjani GK, Fairman AD, et al. Rehabilitation and medical management of the adult with spina bifida. Am J Phys Med Rehabil. 2008 Dec;87(12):1027–50. doi: https://doi.org/10.1097/PHM.0b013e31818de070
2. Dolk H; EUROCAT Working Group. Prevalence of neural tube defects in 20 regions of Europe and the impact of prenatal diagnosis, 1980-1986. J Epidemiol Community Health. 1991 Mar;45(1):52–8. doi: https://doi.org/10.1136/jech.45.1.52
3. Mandiracioğlu A, Ulman I, Lüleci E, Ulman C. The incidence and risk factors of neural tube defects in Izmir, Turkey: a nested case-control study. Turk J Pediatr. 2004;46(3):214–20.
4. Schweizerisches Gesundheitsobservatorium. Angeborene Erkrankungen und Behinderungen. Nationaler Gesundheitsbericht 2020 2023; Available from: https://www.gesundheitsbericht.ch/de/06-chronische-krankheit-und-behinderung/67-angeborene-erkrankungen-und-behinderungen.html
5. Campbell F, Biggs K, Aldiss SK, O’Neill PM, Clowes M, McDonagh J, et al. Transition of care for adolescents from paediatric services to adult health services. Cochrane Database Syst Rev. 2016 Apr;4(4):CD009794. doi: https://doi.org/10.1002/14651858.CD009794.pub2
6. Atta CA, Fiest KM, Frolkis AD, Jette N, Pringsheim T, St Germaine-Smith C, et al. Global Birth Prevalence of Spina Bifida by Folic Acid Fortification Status: A Systematic Review and Meta-Analysis. Am J Public Health. 2016 Jan;106(1):e24–34. doi: https://doi.org/10.2105/AJPH.2015.302902
7. Davis BE, Daley CM, Shurtleff DB, Duguay S, Seidel K, Loeser JD, et al. Long-term survival of individuals with myelomeningocele. Pediatr Neurosurg. 2005;41(4):186–91. doi: https://doi.org/10.1159/000086559
8. Au KS, Ashley-Koch A, Northrup H. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev Disabil Res Rev. 2010;16(1):6–15. doi: https://doi.org/10.1002/ddrr.93
9. Wong LY, Paulozzi LJ. Survival of infants with spina bifida: a population study, 1979-94. Paediatr Perinat Epidemiol. 2001 Oct;15(4):374–8. doi: https://doi.org/10.1046/j.1365-3016.2001.00371.x
10. Shurtleff DB, Hayden PW, Chapman WH, Broy AB, Hill ML. Myelodysplasia. Problems of long-term survival and social function. West J Med. 1975 Mar;122(3):199–205.
11. Pruitt LJ. Living with spina bifida: a historical perspective. Pediatrics. 2012 Aug;130(2):181–3. doi: https://doi.org/10.1542/peds.2011-2935
12. Hunt, G.M., Non-Selective Intervention in Newborn Babies with Open Spina Bifida: The Outcome 30 Years on for the Complete Cohort. Eur J Pediatr Surg, 1999. 9(S 1): p. 5-8. doi: https://doi.org/10.1055/s-2008-1072302
13. Singhal B, Mathew KM. Factors affecting mortality and morbidity in adult spina bifida. Eur J Pediatr Surg. 1999 Dec;9(S 1 Suppl 1):31–2. doi: https://doi.org/10.1055/s-2008-1072310
14. McDonnell GV, McCann JP. Why do adults with spina bifida and hydrocephalus die? A clinic-based study. Eur J Pediatr Surg. 2000 Dec;10(S 1 Suppl 1):31–2. doi: https://doi.org/10.1055/s-2008-1072411
15. Bowman RM, McLone DG, Grant JA, Tomita T, Ito JA. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg. 2001 Mar;34(3):114–20. doi: https://doi.org/10.1159/000056005
16. Oakeshott P, Hunt GM, Poulton A, Reid F. Expectation of life and unexpected death in open spina bifida: a 40-year complete, non-selective, longitudinal cohort study. Dev Med Child Neurol. 2010 Aug;52(8):749–53. doi: https://doi.org/10.1111/j.1469-8749.2009.03543.x
17. Buzzell A, Chamberlain JD, Eriks-Hoogland I, Hug K, Jordan X, Schubert M, et al.; for the SwiSCI study group and the Swiss National Cohort. All-cause and cause-specific mortality following non-traumatic spinal cord injury: evidence from a population-based cohort study in Switzerland. Spinal Cord. 2020 Feb;58(2):157–64. doi: https://doi.org/10.1038/s41393-019-0361-6
18. Ahmad I, Granitsiotis P. Urological follow-up of adult spina bifida patients. Neurourol Urodyn. 2007;26(7):978–80. doi: https://doi.org/10.1002/nau.20447
19. Müller T, Arbeiter K, Aufricht C. Renal function in meningomyelocele: risk factors, chronic renal failure, renal replacement therapy and transplantation. Curr Opin Urol. 2002 Nov;12(6):479–84. doi: https://doi.org/10.1097/00042307-200211000-00006
20. Kinsman SL, Doehring MC. The cost of preventable conditions in adults with spina bifida. Eur J Pediatr Surg. 1996 Dec;6(S 1 Suppl 1):17–20. doi: https://doi.org/10.1055/s-2008-1071031
21. Sawin KJ, Bellin MH, Roux G, Buran CF, Brei TJ. The experience of self-management in adolescent women with spina bifida. Rehabil Nurs. 2009;34(1):26–38. doi: https://doi.org/10.1002/j.2048-7940.2009.tb00245.x
22. Van Walleghem N, Macdonald CA, Dean HJ. Evaluation of a systems navigator model for transition from pediatric to adult care for young adults with type 1 diabetes. Diabetes Care. 2008 Aug;31(8):1529–30. doi: https://doi.org/10.2337/dc07-2247
23. Gesellschaft für Transitionsmedizin. S3-Leitlinie: Transition von der Pädiatrie in die Erwachsenenmedizin. 2021. https://register.awmf.org/assets/guidelines/186-001l_S3_Transition_Paediatrie_Erwachsenenmedizin_2021-04-verlaengert.pdf
24. Ito JA, Stevenson E, Nehring W, Alpeter A, Grant J. A qualitative examination of adolescents and adults with myelomeningocele: their perspective. Eur J Pediatr Surg. 1997 Dec;7 Suppl 1:53–4.
25. Starowicz J, Cassidy C, Brunton L. Health Concerns of Adolescents and Adults With Spina Bifida. Front Neurol. 2021 Nov;12:745814. doi: https://doi.org/10.3389/fneur.2021.745814
26. Berliner Transitions Programm. Berliner TransitionsProgramm e.V. 2009 19.05.20]; Available from: https://www.btp-ev.de/berliner-transitionsprogramm-e-v/
27. Eriks-Hoogland I, et al. S2k-Leitlinie Lebenslange Nachsorge für Menschen mit Querschnittlähmung. 2022. https://register.awmf.org/assets/guidelines/179-014l_S2k_Lebenslange-Nachsorge-fuer-Menschen-mit-Querschnittlaehmung_2022-12.pdf
28. Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011 Nov;34(6):535–46. doi: https://doi.org/10.1179/204577211X13207446293695
29. Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC, et al. The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil Rehabil. 2007 Dec;29(24):1926–33. doi: https://doi.org/10.1080/09638280601046302
30. Bendt M, Gabrielsson H, Riedel D, Hagman G, Hultling C, Franzén E, et al. Adults with spina bifida: A cross-sectional study of health issues and living conditions. Brain Behav. 2020 Aug;10(8):e01736. doi: https://doi.org/10.1002/brb3.1736
31. Liu JS, Dong C, Vo AX, Dickmeyer LJ, Leung CL, Huang RA, et al. Obesity and anthropometry in spina bifida: what is the best measure. J Spinal Cord Med. 2018 Jan;41(1):55–62. doi: https://doi.org/10.1080/10790268.2016.1195071
32. Heyns A, Negrini S, Jansen K, Moens P, Schelfaut S, Peers K, et al. The Prevalence of Scoliosis in Spina Bifida Subpopulations: A Systematic Review. Am J Phys Med Rehabil. 2018 Nov;97(11):848–54. doi: https://doi.org/10.1097/PHM.0000000000000966
33. Dennis M, Landry SH, Barnes M, Fletcher JM. A model of neurocognitive function in spina bifida over the life span. J Int Neuropsychol Soc. 2006 Mar;12(2):285–96. doi: https://doi.org/10.1017/S1355617706060371
34. Hampton LE, Fletcher JM, Cirino PT, Blaser S, Kramer LA, Drake J, et al. Hydrocephalus status in spina bifida: an evaluation of variations in neuropsychological outcomes. J Neurosurg Pediatr. 2011 Sep;8(3):289–98. doi: https://doi.org/10.3171/2011.6.PEDS10584
35. Wetzel JS, Heaner DP, Gabel BC, Tubbs RS, Chern JJ. Clinical evaluation and surveillance imaging of children with myelomeningocele and shunted hydrocephalus: a follow-up study. J Neurosurg Pediatr. 2018 Oct;23(2):153–8. doi: https://doi.org/10.3171/2018.7.PEDS1826
36. Hanna-Wakim RH, Ghanem ST, El Helou MW, Khafaja SA, Shaker RA, Hassan SA, et al. Epidemiology and characteristics of urinary tract infections in children and adolescents. Front Cell Infect Microbiol. 2015 May;5:45. doi: https://doi.org/10.3389/fcimb.2015.00045
37. Zincir H, Kaya Erten Z, Ozkan F, Seviğ U, Başer M, Elmalı F. Prevalence of urinary tract infections and its risk factors in elementary school students. Urol Int. 2012;88(2):194–7. doi: https://doi.org/10.1159/000335554
38. Stein, R., et al., Diagnostik und Therapie der neurogenen Blasenfunktionsstörungen bei Kindern und Jugendlichen mit spinaler Dysraphie. S2k Leitlinie 043-047. 2013.
39. Tradewell M, Pariser JJ, Nimeh T, Elliott SP; Neurogenic Bladder Research Group. Systematic review and practice policy statements on urinary tract infection prevention in adults with spina bifida. Transl Androl Urol. 2018 May;7(S2 Suppl 2):S205–19. doi: https://doi.org/10.21037/tau.2018.04.21
40. Spina Bifida Association. Guidelines for the Care of People with Spina Bifida. 2020 10.06.20]; Available from: https://www.spinabifidaassociation.org/guidelines/
41. Valido E, Bertolo A, Fränkl GP, Itodo OA, Pinheiro T, Pannek J, et al. Systematic review of the changes in the microbiome following spinal cord injury: animal and human evidence. Spinal Cord. 2022 Apr;60(4):288–300. doi: https://doi.org/10.1038/s41393-021-00737-y