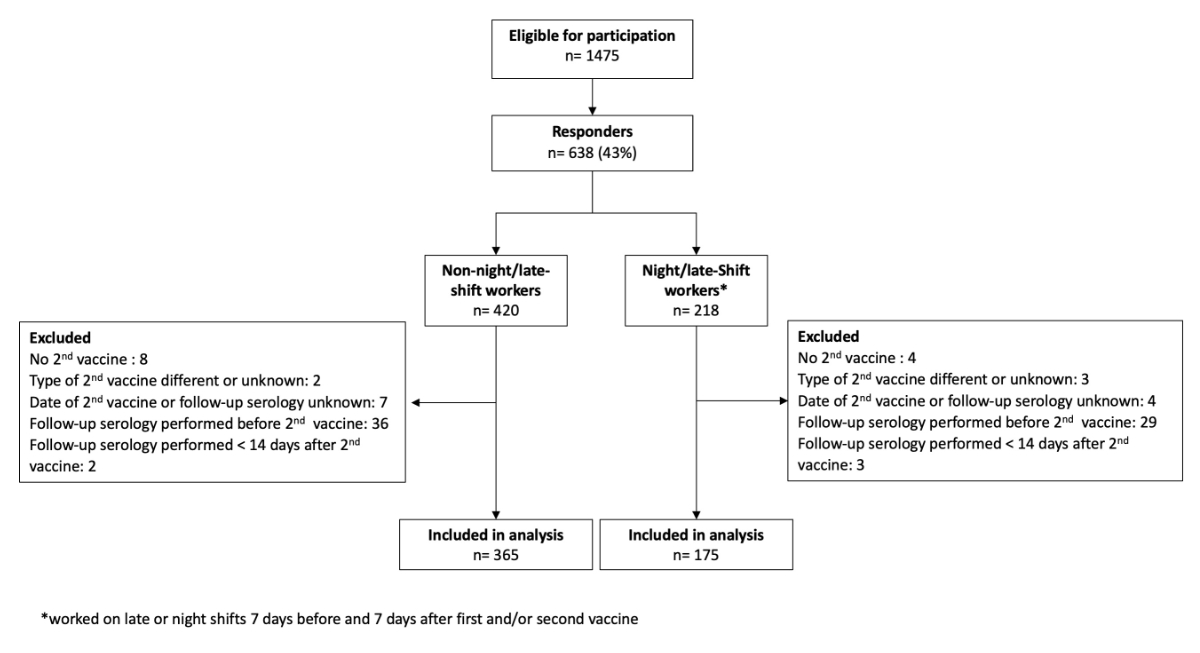
Figure 1Flowchart of the study population. * Worked on late or night shifts 7 days before first and/or second vaccine.
DOI: https://doi.org/https://doi.org/10.57187/s.3708
The development of vaccines against COVID-19 has fundamentally changed the course of the pandemic [1]. The effectiveness of mRNA vaccines has been repeatedly proven [2–4]. Healthcare workers are at increased risk of infection [5] and require robust protection via vaccination to maintain a well-functioning healthcare system. Offering vaccinations to healthcare workers on-site and at convenient times has been shown to increase readiness to vaccinate [6, 7].
In addition to well-known factors influencing the antibody response to vaccines, such as being immunocompromised [8, 9], increased age [10], sex [11], obesity [12], and chronic diseases [13], other “soft factors” are also discussed frequently. Some data suggest that sleep time and deprivation [14, 15] and when the vaccine is administered during the day [16] also impact vaccine efficacy. Avoiding any factors that could weaken the immune response is critical to maximise vaccine efficacy.
The immune system is strongly influenced by the circadian rhythm [17]. Shift work affects the innate and adaptive immune system [18]. Day-night rhythm and regular sleep seemed to influence the strength of the immune response after meningococcal conjugate vaccination in shift workers. Decreased slow-wave and rapid eye movement sleep was associated with a reduced humoral response, lower CD4 T-lymphocyte counts, and other hormonal and immunological effects after vaccination [19].
Little is known about the impact of shift work on antibody response to the SARS-CoV-2 mRNA vaccine. To our knowledge, only a single study has been conducted, which showed nonsignificantly lower SARS-CoV-2 antibody titers in healthcare workers on regular night shifts [20]. However, it did not examine the temporal correlation between night shift status and vaccination date. Nonetheless, Loef et al. revealed that recent exposure to night shifts particularly influences the immune status of healthcare workers [21]. Therefore, we aimed to investigate the influence of shift work and other work-related factors around the two primary vaccination dates on the humoral immune response to COVID-19 mRNA vaccines, measured by the serum anti-SARS-CoV-2 spike protein immunoglobulin G (IgG; anti-S) antibody levels, which have been shown to be a surrogate for neutralising antibodies [22]. We conducted this study using existing data and antibody levels from the prospective, longitudinal SURPRISE cohort.
Nested within the prospective longitudinal SURPRISE (SURveillance of infectious diseases among health PRofessionals In SwitzErland) cohort study [23], we performed an online survey to collect additional data on shift work around the first two immunisation dates. The SURPRISE cohort study has been previously described [24]. Briefly, between June 2020 and October 2020, 17 healthcare institutions in Northern and Eastern Switzerland were originally included, including acute care hospitals, rehabilitation clinics, and geriatric and psychiatric clinics. All healthcare workers aged ≥16 years were eligible and asked to participate via their institution’s website. Healthcare workers completed weekly to monthly web-based questionnaires and underwent SARS-CoV-2 serology measurements in August 2020, January 2021, and September/October 2021. Blood samples were obtained at local sites and were centrally analysed except for those from one study site. The two laboratories used the same diagnostic test (see the Sample Processing section below).
At the time we planned our nested cross-sectional survey, two institutions had already withdrawn from the SURPRISE cohort study. Six of the remaining 15 institutions agreed to participate, including the main and all larger study centres. A link for the additional electronic questionnaire (available for download as a separate file at https://doi.org/10.57187/s.3708) on shift work was sent to all participants at these six institutions. For those who completed the survey, we used serology and survey data from the original SURPRISE cohort, collected at registration, around vaccination in March/April 2021, and at follow-up serology in September/October 2021. We retrieved detailed information on participants’ demographics, professions, full- or part-time employment, comorbidities, medication, vaccination dates, vaccine manufacturers, and sleep quality as measured by the Patient Health Questionnaire-9 (PHQ-9) [25]. The ethics committees of Eastern Switzerland (EKOS 2022-01605) approved this study as a quality improvement project without additional requirements. Therefore, they waived the requirement for written consent. However, participants were informed that by participating in the online survey, they consented to the use of their anonymised data.
Because the SURPRISE data did not include information on work schedules around the vaccination dates, we created an online survey to obtain detailed information on healthcare workers’ exact work history around their primary immunisation dates. We collected information on the type and lengths of shifts healthcare workers worked within seven days before and after their first and second vaccinations. We also recorded data on their time of vaccine administration, use of analgesics or antipyretics within 24 hours before and after the vaccination, and the effective number of working hours per week around the vaccination dates (Supplement). The online survey was pilot-tested in early September 2022 and sent out in late September 2022. Two reminders were sent after two and four weeks.
It was originally planned to include all participants who had received at least one vaccination and had their anti-S antibody levels measured in autumn 2021. However, given the growing evidence that the number of vaccinations and manufacturer choice impacts vaccination response [26], we decided to alter our plan to create a more homogeneous study population. Since the standard regimen for primary mRNA vaccination comprised two doses, we excluded those participants with only one vaccination before collection of their follow-up serum sample. The earliest time point of the follow-up serum sample was no earlier than 14 days after the second vaccination to allow for an adequate immune response. Then, we excluded participants with a mix of mRNA vaccinations (BNT162b2/Pfizer and mRNA-1273/Moderna) since data suggested humoral responses differed by the vaccine type [26].
There remains no uniform definition of shift work [27–30]. Assuming a certain degree of disturbance to the circadian rhythm, we categorised shift work as follows: Late shift was defined as working at least six hours starting after 12 pm and ending between 7 pm and 1 am. Night shift was defined as a work period of at least six hours between 10 pm and 7 am. All other participants were considered to be working day shifts.
Serum anti-S and serum SARS-CoV-2 nucleocapsid protein immunoglobulin (anti-N) antibody levels were detected using the electro-chemiluminescence immunoassay (ECLIA) Elecsys® (Roche Diagnostics, Rotkreuz, Switzerland) [31] on a COBAS 6000 instrument. Following the manufacturer’s recommendations, seropositivity was defined using a cut-off of ≥0.80 binding antibody units (BAU)/ml for serum anti-S and a cut-off index (COI) of ≥1.0 for serum anti-N.
Regarding baseline characteristics, categorical variables are described using numbers and percentages and continuous variables are described using means and standard deviations or medians and interquartile ranges (IQRs) as appropriate. We compared proportions between groups using the Chi-square test and compared means and medians between groups using Student’s t-test or the Mann–Whitney U test, respectively. A two-sided p-value of <0.05 was considered statistically significant.
Our primary endpoint was serum anti-S antibody levels measured in September/October 2021. Due to right skewness, we log10 transformed the dependent variable and used multivariate linear regression to examine the association between shift work and serum anti-S antibody levels. We checked for potential violations of the linear regression assumptions by inspecting the normal P-P plot and performing the Breusch-Pagan and White tests. We also used robust standard errors in all models to overcome issues with biased estimates of standard errors. Regarding potential confounders, the model adjusted for age, sex, body mass index (BMI), vaccine type, prior infection (determined by positive serum anti-N antibody), work-related factors (profession and degree of employment), and all other significant variables (alpha <0.05) in the univariate analyses. In our primary model, shift work was defined as a binary variable: whether or not respondents worked late or night shifts within seven days before and after the first and/or second vaccination. Variables were entered in blocks of 3–4 in the model. The shift work variable was entered last. We inspected the change in R2 to determine how much variation in the model could be explained by the shift work variable. We addressed multicollinearity by examining the variance inflation factor (VIF) and removed highly correlated (VIF >3) variables. Immunosuppression was not considered in the multivariate model due to low numbers in the cohort. Only complete cases with no missing data were considered in the multivariate analysis.
The results are summarised by coefficients, standard errors, 95% confidence intervals (CIs), corresponding p-values, and the percentage increase or decrease in the back-transformed response variable.
To examine a potential dose-response effect, we categorised shift work according to the number of late or night shift duties performed (1–3 late or night shifts, 4–6 late or night shifts, or >6 late or night shifts versus none) around each vaccination date (±7 days of vaccination) and repeated the regression model with all other parameters.
All statistical analyses were conducted using Microsoft Excel and SPSS® (version 29.0.0) [32].
All 1475 eligible healthcare workers with documented vaccination and available anti-S antibody levels at follow-up received the additional questionnaire. After removing duplicates (n = 85), blank questionnaires (n = 55), and those with non-matchable identifying study numbers (n = 15), 638 (43%) evaluable responses were available, of which 98 were excluded mainly because of unknown vaccination dates or administration of the second dose after or close to (within 14 days) the follow-up serology. Ultimately, our analysis included 540 healthcare workers, of which 365 had not worked late or night shifts seven days before or after their primary vaccinations, while 175 (32.4%) had worked at least one late or night shift during this period (figure 1).

Figure 1Flowchart of the study population. * Worked on late or night shifts 7 days before first and/or second vaccine.
Most healthcare workers were female in both the non-night/late-shift worker (77.6%) and the night/late-shift worker (80.6%) groups. There were significantly more nurses (69.9% vs 37.8%) and fewer administrative personnel (10.4% vs 45.2%) in the night/late-shift worker group (p <0.001). While most healthcare workers received their primary vaccination with the BNT162b2 vaccine (Pfizer), significantly more non-night/late-shift workers were double vaccinated with the mRNA-1273 vaccine (Moderna; 9.0% vs 2.9%, p = 0.009). Seropositivity for serum anti-N antibodies at the follow-up was twice as high among shift workers (22.2% vs 9.8%, p <0.001). Most healthcare workers were scheduled for vaccination in the afternoon, with no significant difference between groups. Many healthcare workers in the non-night/late-shift worker (77.8%) and night/late-shift worker (82.9%) groups indicated having moderate sleeping difficulties as assessed via the PHQ-9 questionnaire (p = 0.379). The time between the second vaccination and the follow-up serology differed significantly between non-night/late-shift workers (median = 156 days, IQR = 106-183) and night/late-shift workers (median = 170 days, IQR = 130–188; p <0.001) (table 1).
Table 1Healthcare workers’ baseline characteristics (n = 540).
| Late or night shift work (±7 days of primary vaccination) | ||||||
| No (n = 365) | Yes (n = 175) | p-value | ||||
| Females, n (%) | 281 | (77.6) | 141 | (80.6) | 0.435 | |
| Age (years), median (IQR) | 43 | (36–53) | 44 | (33–53) | 0.294 | |
| BMI in kg/m2, median (IQR) | 23.4 | (21.3–26.2) | 23.7 | (21.5–26.7) | 0.167 | |
| At least one comorbidity, n (%) | 324 | (94.5) | 161 | (97.0) | 0.207 | |
| Immunodeficiency, n (%) | 11 | (3.2) | 9 | (5.4) | 0.234 | |
| Baseline anti-N antibody positive, n (%) | 4 | (1.2) | 3 | (1.9) | 0.687 | |
| Profession | Physicians, n (%) | 58 | (17.0) | 32 | (19.6) | <0.001 |
| Nurses, n (%) | 129 | (37.8) | 114 | (69.9) | ||
| Administrative personnel, n (%) | 154 | (45.2) | 17 | (10.4) | ||
| Employment degree | Working more than 80%, n (%) | 193 | (52.9) | 101 | (57.7) | 0.519 |
| Working 60%–80%, n (%) | 123 | (33.7) | 55 | (31.4) | ||
| Working ≤50%, n (%) | 49 | (13.4) | 19 | (10.9) | ||
| Level of shift work within seven days before and/or after baseline immunisation | Never worked late shifts, n (%) | 365 | (100.0) | 20 | (11.4) | n.a. |
| Worked 1–3 late shifts, n (%) | 0 | 39 | (22.3) | |||
| Worked 4–6 late shifts, n (%) | 0 | 55 | (31.4) | |||
| Worked ≥7 late shifts, n (%) | 0 | 61 | (34.9) | |||
| Never worked night shifts, n (%) | 365 | (100.0) | 70 | (40.2) | n.a. | |
| Worked 1–3 night shifts, n (%) | 0 | 34 | (19.4) | |||
| Worked 4–6 night shifts, n (%) | 0 | 39 | (22.3) | |||
| Worked ≥7 night shifts, n (%) | 0 | 32 | (18.3) | |||
| Baseline immunisation | mRNA vaccine BNT162b2 from Pfizer, n (%) | 332 | (91.0) | 169 | (97.1) | 0.009 |
| mRNA vaccine 1273 from Moderna, n (%) | 33 | (9.0) | 5 | (2.9) | ||
| NSAIDs used within 24 hours before or after the first and/or second vaccine dose, n (%) | 21 | (6.5) | 15 | (9.2) | 0.292 | |
| Vaccination time | Twice in the morning (07:00 am–11:50 am), n (%) | 42 | (13.2) | 22 | (13.0) | 0.835 |
| Twice in the afternoon (12:00 pm–5:50 pm), n (%) | 175 | (55.0) | 94 | (55.6) | ||
| Once in the morning and once at night (6:00 pm–10:00 pm), n (%) | 63 | (19.8) | 29 | (17.2) | ||
| Twice at night or once in the afternoon and once at night, n (%) | 38 | (11.9) | 24 | (14.2) | ||
| Sleeping difficulties | None, n (%) | 72 | (19.7) | 26 | (14.9) | 0.379 |
| Moderate, n (%) | 284 | (77.8) | 145 | (82.9) | ||
| Severe, n (%) | 9 | (2.5) | 4 | (2.3) | ||
| Smoking status | Never smoked, n (%) | 249 | (68.2) | 110 | (62.9) | 0.429 |
| Ex-smoker, n (%) | 66 | (18.1) | 35 | (20.0) | ||
| Current smoker, n (%) | 50 | (13.7) | 30 | (17.1) | ||
| Follow-up anti-N antibody positive, n (%) | 35 | (9.8) | 38 | (22.2) | <0.001 | |
| Follow-up anti-S antibody quant., median (IQR) | 1271 | (731–2375) | 1169 | (669–2081) | 0.284 | |
| No. days between the first and second vaccine dose, median (IQR) | 31 | (28–39) | 31 | (28–38) | 0.528 | |
| No. days between the second vaccine dose and the follow-up serology, median (IQR) | 156 | (106–183) | 170 | (130–188) | <0.001 | |
| Time elapsed between the second vaccine dose and the follow-up serology | 14–84 days (2–12 weeks after the second vaccine) | 63 | (17.3) | 14 | (8.0) | <0.001 |
| 85–168 days (3–6 months after the second vaccine) | 165 | (45.2) | 68 | (38.9) | ||
| 169–265 days (6–8 months after the second vaccine) | 137 | (37.5) | 93 | (53.1) | ||
BMI: body mass index; NSAID: non-steroidal anti-inflammatory drug; n.a.: not applicable.
Missing data: comorbidities = 31 (5.7%), baseline anti-N antibody = 44 (8.1%), immunodeficiency = 27 (5.0%), profession = 36 (6.7%), NSAID use = 56 (10.4%), time of vaccination = 53 (9.8%), follow-up anti-N antibody = 13 (2.4%).
Serum anti-S antibody levels were lower in night/late-shift workers (median = 1169 BAU/ml, IQR = 669–2081) than in non-night/late-shift workers (median = 1271 BAU/ml, IQR = 731–2375). However, the difference was not statistically significant (p = 0.284). Serum anti-S antibody levels were markedly increased in night/late-shift workers and non-night/late-shift workers with a previous SARS-CoV-2 infection, as determined by a positive serum anti-N antibody level of ≥1.0 COI/ml at follow-up (figure 2).
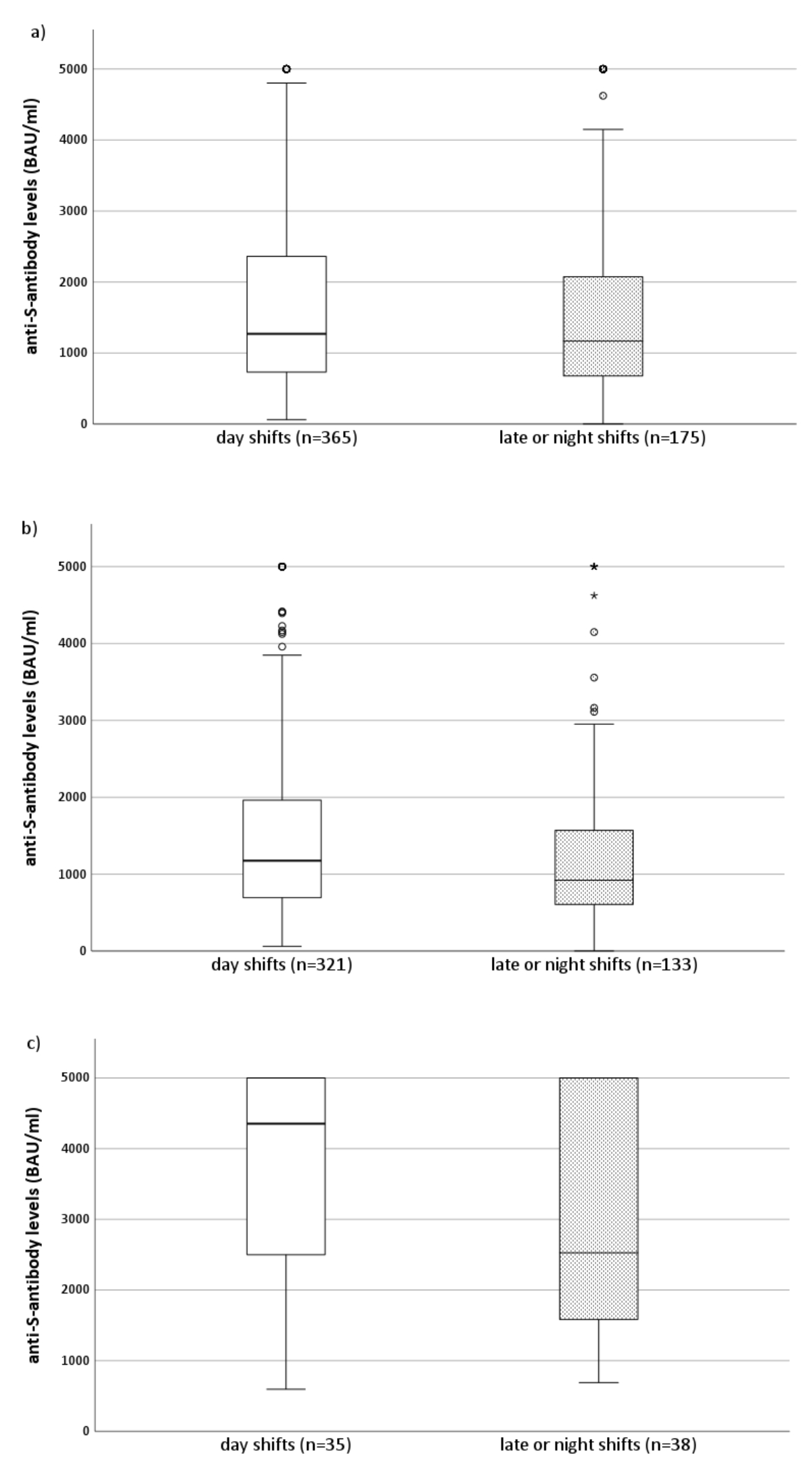
Figure 2Simple boxplots of serum anti-S antibody levels in night/late-shift workers and non-night/late-shift workers after baseline vaccination (two mRNA vaccine doses) and depending on the presence or absence of anti-N antibodies at follow-up. The horizontal line in the box represents the median of the variable on the y-axis. The box represents the interquartile range, with Q1 on the lower end and Q3 on the upper end of the box. The whisker (vertical line) spans from the value that is 1.5× the IQR below Q1 (bottom) to the value that is 1.5× the IQR above Q3 (top). Outliers (blank circles) are set with a default of >1.5× the IQR below Q1 or above Q3. Extreme cases (asterisks) are defined as values >3.0× the IQR below Q1 or above Q3. (a) All healthcare workers (anti-N antibody sero-negative and -positive), (b) seronegative (anti-N antibody COI of <0.1) healthcare workers only, and (c) seropositive (anti-N antibody of ≥0.1) healthcare workers only.
Vaccinated but uninfected physicians and nurses had lower median serum anti-S antibody levels than vaccinated uninfected administrative personnel. In contrast, serum anti-S antibody levels were elevated among those with previous SARS-CoV-2 infections in all professional groups (figure 3).
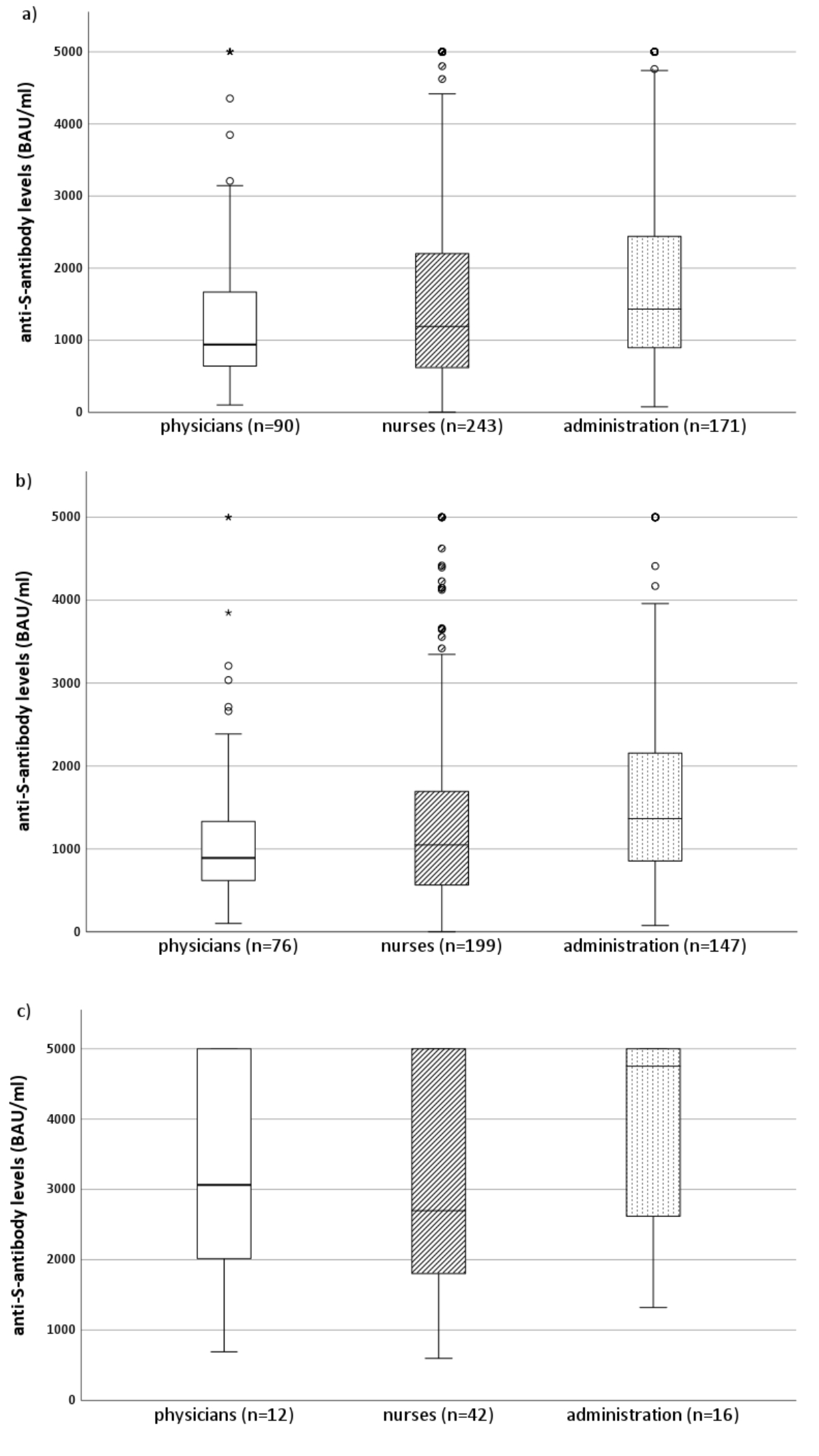
Figure 3Simple boxplots of serum anti-S antibody levels in physicians, nurses, and administrative personnel after baseline vaccination (two mRNA vaccine doses) and depending on the presence or absence of anti-N antibodies at follow-up. The horizontal line in the box represents the median of the variable on the y-axis. The box represents the interquartile range, with Q1 on the lower end and Q3 on the upper end of the box. The whisker (vertical line) spans from the value that is 1.5× the IQR below Q1 (bottom) to the value that is 1.5× the IQR above Q3 (top). Outliers (blank circles) are set with a default of >1.5× the IQR below Q1 or above Q3. Extreme cases (asterisks) are defined as values >3.0× the IQR below Q1 or above Q3. (a) All healthcare workers (anti-N antibody sero-negative and -positive), (b) seronegative (anti-N antibody COI of <0.1) healthcare workers only, and (c) seropositive (anti-N antibody of ≥0.1) healthcare workers only.
In the univariate analyses, night/late-shift work caused a nonsignificant −15.1% decrease in serum anti-S antibody levels (p = 0.090; table S1 in the appendix). After considering all other variables, working late or night shifts within seven days before or after vaccination was associated with a nonsignificant 13.5% reduction in anti-S antibody levels (p = 0.108; table 2). In addition, the change in R2 was only 0.004, indicating that this variable does not contribute appreciably to the model. However, prior infection was associated with a 197.2% increase in serum anti-S antibody levels (p <0.001). Moreover, the mRNA-1273 vaccine from Moderna was associated with a significantly greater increase in serum anti-S antibody levels than the BNT162b2 vaccine from Pfizer (+63.7%; p <0.001). In contrast, antibody levels decreased significantly with each additional decade of age (−11.1%, p <0.001) and week elapsed between the second vaccination and follow-up blood sampling (−4.5%, p <0.001).
Table 2Multivariate linear regression model to determine the impact of shift work and other work-related factors on anti-S antibody levels after baseline vaccination (two mRNA vaccine doses).
| Coefficient | 95% confidence interval | p-value | % increase or decrease | ||
| Lower bound | Upper bound | ||||
| (Constant) | 3.610 | 3.369 | 3.851 | <0.001 | |
| Age (in 10 years, starting from 20 years) | −0.051 | −0.081 | −0.021 | <0.001 | −11.1 |
| Female sex | 0.021 | −0.058 | 0.101 | 0.567 | 5.0 |
| BMI | 0.003 | −0.003 | 0.009 | 0.241 | 0.7 |
| Nurse (vs administrative personnel) | −0.071 | −0.145 | 0.002 | 0.031 | −15.1 |
| Physician (vs administrative personnel) | 0.018 | −0.076 | 0.112 | 0.65 | 4.2 |
| Employment degree of 60%–80% (vs ≤50%) | −0.015 | −0.115 | 0.086 | 0.721 | −3.4 |
| Employment degree of >80% (vs ≤50%) | −0.010 | −0.108 | 0.087 | 0.791 | −2.3 |
| Baseline vaccination with the mRNA-1273 vaccine (Moderna) | 0.214 | 0.086 | 0.341 | <0.001 | 63.7 |
| Previous infection (positive anti-N antibody at follow-up) | 0.473 | 0.386 | 0.559 | <0.001 | 197.2 |
| Interval (in weeks) between the second vaccination and follow-up serology | −0.020 | −0.025 | −0.016 | <0.001 | −4.5 |
| Worked late or night shifts seven days before and after the first and/or second vaccine dose | −0.063 | −0.133 | 0.006 | 0.108 | −13.5 |
BMI: body mass index.
Like in our primary analysis, no statistically significant impact of shift work was found. Compared to working day shifts, working 1–3 or 4–6 late shifts was associated with decreased serum anti-S antibody levels, while working >6 late shifts was associated with increased serum anti-S antibody levels. However, these associations were not statistically significant. Similar results were obtained in the separate evaluation of the association of night shift work with anti-S antibody levels. Working for 1–3, 4–6, or >6 nights did not significantly affect serum anti-S antibody levels compared to working day shifts (tables S2 and S3 in the appendix).
In our cohort of 540 healthcare workers, we demonstrated that working late or night shifts does not affect the humoral response to primary mRNA vaccination (two doses) against COVID-19. Instead, our results are consistent with previous findings that serum anti-S antibody levels are influenced most by well-described factors, such as age, vaccine type, and previous infection. Unexpectedly, more night/late-shift workers than non-night/late-shift workers were infected with SARS-CoV-2.
Our results are consistent with the findings of Coppeta et al., who did not find an association between serum levels of anti-S IgG against SARS-CoV-2 and regular night shifts among healthcare workers after controlling for previous SARS-CoV-2 infection and time elapsed between the second vaccination and serological evaluation [20]. However, our findings are of additional value because neither working late or night shifts nor the frequency of shift work immediately before and after vaccination appointments seems to negatively affect the antibody response. This observation is particularly important for healthcare workers because they usually cannot completely disengage from shift work and can benefit from the convenience of getting vaccinated on-site while working a shift.
In contrast, our multivariate analysis results suggest that increasing age at vaccination was independently associated with lower serum anti-S antibody levels, which others have also shown [26, 33].
However, night/late-shift workers in our cohort had a higher rate of COVID-19 infection. This phenomenon has also been previously reported [34, 35]. Whether it is due to increased susceptibility or exposure to SARS-CoV-2 cannot be concluded from this study. One possible explanation could be that disruption of the circadian rhythm affects the innate and cellular immune system more than the humoral response [21]. In addition, shift work seems to impact cytokine and inflammatory markers [36]. Worse health behaviour [37] and cardiovascular diseases [38] among shift workers may also increase their susceptibility to infections. However, since the mRNA of the vaccine remains in the body for several days [39], there may be sufficient time for the immune system to elicit an appropriate humoral response to the vaccine. Furthermore, exposure time and the type of and adherence to personal protective equipment may also impact the risk of SARS-CoV-2 infection [5, 40].
Our results are also consistent with previous studies showing that baseline vaccination with the mRNA-1273 vaccine (Moderna) elicits a higher SARS-CoV-2 antibody response than the BNT162b2 vaccine (Pfizer) [26]. Given these findings, even the choice of vaccine manufacturer seems important to ensure the highest possible effectiveness of vaccination. Our findings showed that antibody levels progressively decreased between the second vaccination and follow-up serology, supporting the current understanding that a vaccine booster dose is needed for sufficient vaccine protection.
This study had certain limitations. Firstly, its design did not allow us to draw causal conclusions, and residual confounders may be present despite multivariate adjustment. Secondly, its overall sample size and that of healthcare workers who reported working late or night shifts within one week before and after vaccination was relatively small and may preclude a smaller but potentially clinically significant association between shift work and immune response. Thirdly, the time between sending the questionnaire about shift work around the vaccination dates and the vaccination dates themselves was relatively large. Nonetheless, we asked participants to match their responses to working shifts around vaccination dates with their work schedules to reduce this information bias. Fourthly, the roles of binding and neutralising antibodies in conferring protection with mRNA vaccines against SARS-CoV-2 are still incompletely characterised. It has been shown that neutralising antibody levels have a very high predictive value for immune protection [41]. Unfortunately, we were unable to correlate anti-S antibody levels with neutralising serum antibody levels. However, a recent study has demonstrated a strong positive correlation between anti-S IgG levels and neutralising antibody levels [42].
The major strengths of this study are its collection of real-world data and its use of a multicentre cohort with diverse settings from acute care to rehabilitation and geriatric clinics. The confirmation of previously documented effects on immune response, such as age, vaccine type, and time elapsed since vaccination, from a highly differentiated online survey contributed to the credibility of our main finding.
Our data showed no significant impact of shift work on the humoral response to primary mRNA vaccination. These findings support flexible vaccination schedules and on-site vaccination for healthcare workers, including those working night/late shifts, for increased vaccination readiness. Increasing age, time elapsed since the last vaccination, and vaccine manufacturer are significant predictors of lower antibody levels after vaccination and seem to remain critical for vaccine selection and targeted booster vaccination.
The deidentified data reported in this article are available from the last author upon reasonable request.
We thank all the participants of the SURPRISE cohort study and all SURPRISE contributors for kindly providing their data.
This study was supported by the Swiss National Sciences Foundation (grant numbers 31CA30_196544 and PZ00P3_179919 to PK), the Federal Office of Public Health (grant number: 20.008218/421-28/1), and the Health Department of the Canton of St. Gallen.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1.Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022 Sep;22(9):1293–302. 10.1016/S1473-3099(22)00320-6
2.Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022 Jan;114:252–60. 10.1016/j.ijid.2021.11.009
3.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al.; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec;383(27):2603–15. 10.1056/NEJMoa2034577
4.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al.; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021 Feb;384(5):403–16. 10.1056/NEJMoa2035389
5.Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, et al.; COronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020 Sep;5(9):e475–83. 10.1016/S2468-2667(20)30164-X
6.Norton SP, Scheifele DW, Bettinger JA, West RM. Influenza vaccination in paediatric nurses: cross-sectional study of coverage, refusal, and factors in acceptance. Vaccine. 2008 Jun;26(23):2942–8. 10.1016/j.vaccine.2008.03.033
7.Black CL, Yue X, Ball SW, Fink RV, de Perio MA, Laney AS, et al. Influenza Vaccination Coverage Among Health Care Personnel - United States, 2017-18 Influenza Season. MMWR Morb Mortal Wkly Rep. 2018 Sep;67(38):1050–4. 10.15585/mmwr.mm6738a2
8.Rahav G, Lustig Y, Lavee J, Ohad Benjamini, Magen H, Hod T, et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: A prospective cohort study. EClinicalMedicine. 2021 Nov;41:101158. 10.1016/j.eclinm.2021.101158
9.Bertram S, Blazquez-Navarro A, Seidel M, Hölzer B, Seibert FS, Doevelaar A, et al. Predictors of impaired SARS-CoV-2 immunity in healthcare workers after vaccination with BNT162b2. Sci Rep. 2022 Apr;12(1):6243. 10.1038/s41598-022-10307-8
10.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006 Feb;24(8):1159–69. 10.1016/j.vaccine.2005.08.105
11.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010 May;10(5):338–49. 10.1016/S1473-3099(10)70049-9
12.Park HL, Shim SH, Lee EY, Cho W, Park S, Jeon HJ, et al. Obesity-induced chronic inflammation is associated with the reduced efficacy of influenza vaccine. Hum Vaccin Immunother. 2014;10(5):1181–6. 10.4161/hv.28332
13.Nath KD, Burel JG, Shankar V, Pritchard AL, Towers M, Looke D, et al. Clinical factors associated with the humoral immune response to influenza vaccination in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:51–6.
14.Taylor DJ, Kelly K, Kohut ML, Song KS. Is Insomnia a Risk Factor for Decreased Influenza Vaccine Response? Behav Sleep Med. 2017;15(4):270–87. 10.1080/15402002.2015.1126596
15.Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65(5):831–5. 10.1097/01.PSY.0000091382.61178.F1
16.Rayatdoost E, Rahmanian M, Sanie MS, Rahmanian J, Matin S, Kalani N, et al. Sufficient Sleep, Time of Vaccination, and Vaccine Efficacy: A Systematic Review of the Current Evidence and a Proposal for COVID-19 Vaccination. Yale J Biol Med. 2022 Jun;95(2):221–35.
17.Wang C, Lutes LK, Barnoud C, Scheiermann C. The circadian immune system. Sci Immunol. 2022 Jun;7(72):eabm2465. 10.1126/sciimmunol.abm2465
18.Cermakian N, Stegeman SK, Tekade K, Labrecque N. Circadian rhythms in adaptive immunity and vaccination. Semin Immunopathol. 2022 Mar;44(2):193–207. 10.1007/s00281-021-00903-7
19.Ruiz FS, Rosa DS, Zimberg IZ, Dos Santos Quaresma MV, Nunes JO, Apostolico JS, et al. Night shift work and immune response to the meningococcal conjugate vaccine in healthy workers: a proof of concept study. Sleep Med. 2020 Nov;75:263–75. 10.1016/j.sleep.2020.05.032
20.Coppeta L, Ferrari C, Somma G, Mazza A, D’Ancona U, Marcuccilli F, et al. Reduced Titers of Circulating Anti-SARS-CoV-2 Antibodies and Risk of COVID-19 Infection in Healthcare Workers during the Nine Months after Immunization with the BNT162b2 mRNA Vaccine. Vaccines (Basel). 2022 Jan;10(2):141. 10.3390/vaccines10020141
21.Loef B, Nanlohy NM, Jacobi RH, van de Ven C, Mariman R, van der Beek AJ, et al. Immunological effects of shift work in healthcare workers. Sci Rep. 2019 Dec;9(1):18220. 10.1038/s41598-019-54816-5
22.Rubio-Acero R, Castelletti N, Fingerle V, Olbrich L, Bakuli A, Wölfel R, et al.; KoCo19 study team. In Search of the SARS-CoV-2 Protection Correlate: Head-to-Head Comparison of Two Quantitative S1 Assays in Pre-characterized Oligo-/Asymptomatic Patients. Infect Dis Ther. 2021 Jun;10(3):1–14. 10.1007/s40121-021-00475-x
23.Kohler PP, Kahlert CR, Sumer J, Flury D, Güsewell S, Leal-Neto OB, et al. Prevalence of SARS-CoV-2 antibodies among Swiss hospital workers: results of a prospective cohort study. Infect Control Hosp Epidemiol. 2021 May;42(5):604–8. 10.1017/ice.2020.1244
24.Kahlert CR, Persi R, Güsewell S, Egger T, Leal-Neto OB, Sumer J, et al. Non-occupational and occupational factors associated with specific SARS-CoV-2 antibodies among hospital workers - A multicentre cross-sectional study. Clin Microbiol Infect. 2021 Sep;27(9):1336–44. 10.1016/j.cmi.2021.05.014
25.MacGregor KL, Funderburk JS, Pigeon W, Maisto SA. Evaluation of the PHQ-9 Item 3 as a screen for sleep disturbance in primary care. J Gen Intern Med. 2012 Mar;27(3):339–44. 10.1007/s11606-011-1884-5
26.Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA. 2021 Oct;326(15):1533–5. 10.1001/jama.2021.15125
27.Garde AH, Hansen J, Kolstad HA, Larsen AD, Hansen ÅM. How do different definitions of night shift affect the exposure assessment of night work? Chronobiol Int. 2016;33(6):595–8. 10.3109/07420528.2016.1167729
28.Garde AH, Albertsen K, Nabe-Nielsen K, Carneiro IG, Skotte J, Hansen SM, et al. Implementation of self-rostering (the PRIO-project): effects on working hours, recovery, and health. Scand J Work Environ Health. 2012 Jul;38(4):314–26. 10.5271/sjweh.3306
29.Härmä M, Ropponen A, Hakola T, Koskinen A, Vanttola P, Puttonen S, et al. Developing register-based measures for assessment of working time patterns for epidemiologic studies. Scand J Work Environ Health. 2015 May;41(3):268–79. 10.5271/sjweh.3492
30.Vistisen HT, Garde AH, Frydenberg M, Christiansen P, Hansen ÅM, Hansen J, et al. Short-term effects of night shift work on breast cancer risk: a cohort study of payroll data. Scand J Work Environ Health. 2017 Jan;43(1):59–67. 10.5271/sjweh.3603
31.Baron RC, Risch L, Weber M, Thiel S, Grossmann K, Wohlwend N, et al. Frequency of serological non-responders and false-negative RT-PCR results in SARS-CoV-2 testing: a population-based study. Clin Chem Lab Med. 2020 Aug;58(12):2131–40. 10.1515/cclm-2020-0978
32.Corp IB. Released 2022. IBM SPSS Statistics for Windows, Version 29.0. Armonk, NY: IBM Corp.
33.Jolliffe DA, Faustini SE, Holt H, Perdek N, Maltby S, Talaei M, et al. Determinants of Antibody Responses to SARS-CoV-2 Vaccines: Population-Based Longitudinal Study (COVIDENCE UK). Vaccines (Basel). 2022 Sep;10(10):1601. 10.3390/vaccines10101601
34.Maidstone R, Anderson SG, Ray DW, Rutter MK, Durrington HJ, Blaikley JF. Shift work is associated with positive COVID-19 status in hospitalised patients. Thorax. 2021 Jun;76(6):601–6. 10.1136/thoraxjnl-2020-216651
35.Coppeta L, Ferrari C, Mazza A, Trabucco Aurilio M, Rizza S. Factors Associated with Pre-Vaccination SARS-CoV-2 Infection Risk among Hospital Nurses Facing COVID-19 Outbreak. Int J Environ Res Public Health. 2021 Dec;18(24):13053. 10.3390/ijerph182413053
36.Wright KP Jr, Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015 Jul;47:24–34. 10.1016/j.bbi.2015.01.004
37.Bae MJ, Song YM, Shin JY, Choi BY, Keum JH, Lee EA. The Association Between Shift Work and Health Behavior: Findings from the Korean National Health and Nutrition Examination Survey. Korean J Fam Med. 2017 Mar;38(2):86–92. 10.4082/kjfm.2017.38.2.86
38.Torquati L, Mielke GI, Brown WJ, Kolbe-Alexander T. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand J Work Environ Health. 2018 May;44(3):229–38. 10.5271/sjweh.3700
39.Anand P, Stahel VP. Review the safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg. 2021 May;15(1):20. 10.1186/s13037-021-00291-9
40.Dörr T, Haller S, Müller MF, Friedl A, Vuichard D, Kahlert CR, et al. Risk of SARS-CoV-2 Acquisition in Health Care Workers According to Cumulative Patient Exposure and Preferred Mask Type. JAMA Netw Open. 2022 Aug;5(8):e2226816. 10.1001/jamanetworkopen.2022.26816
41.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021 Jul;27(7):1205–11. 10.1038/s41591-021-01377-8
42.Dolscheid-Pommerich R, Bartok E, Renn M, Kümmerer BM, Schulte B, Schmithausen RM, et al. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J Med Virol. 2022 Jan;94(1):388–92. 10.1002/jmv.27287
Table S1Univariate linear regression models to determine the impact of shift work and other factors on the level of anti-S antibody concentrations after baseline vaccination.
| Coefficient | p-value | % increase or decrease | |
| (Constant) | 3.459 | ||
| Age (decades) | −0.088 | <0.001 | −18.3 |
| (Constant) | 3.047 | ||
| Female (vs male) | 0.035 | 0.410 | 8.4 |
| (Constant) | 3.159 | ||
| BMI | −0.003 | 0.317 | −0.7 |
| (Constant) | 3.037 | ||
| Any comorbidity (vs no comorbidity) | 0.029 | 0.344 | 6.9 |
| (Constant) | 3.158 | ||
| Nurse (vs administrative personnel) | −0.12 | 0.003 | −24.1 |
| Physician (vs administrative personnel) | −0.13 | 0.004 | −25.9 |
| (Constant) | 3.082 | ||
| Employment degree of 60%–80% (vs ≤50%) | −0.025 | 0.627 | −5.6 |
| Employment degree of >80% (vs ≤50%) | 0.005 | 0.917 | 1.2 |
| (Constant) | 3.019 | ||
| Prior infection (seropositive for N antibodies; vs seronegative) | 0.415 | <0.001 | 160.0 |
| (Constant) | 3.047 | ||
| Baseline vaccination with mRNA-1273 from Moderna (vs BNT162b2 from Pfizer) | 0.419 | <0.001 | 162.4 |
| (Constant) | 3.590 | ||
| Follow-up time after the second vaccine (weeks) | −0.024 | <0.001 | −5.4 |
| (Constant) | 3.100 | ||
| Late or night shifts within seven days of vaccination (vs day shifts only) | −0.071 | 0.090 | −15.1 |
BMI: body mass index.
Table S2Multivariate linear regression model to determine the impact of the number of late shifts worked around the primary vaccination date on anti-S antibody levels after baseline vaccination (two mRNA vaccine doses).
| Coefficient | 95% confidence interval | p-value | % increase or decrease | ||
| Lower bound | Upper bound | ||||
| (Constant) | 3.632 | 3.417 | 3.847 | <0.001 | |
| Age (in 10 years, starting from 20 years) | −0.052 | −0.080 | −0.023 | <0.001 | −11.3 |
| Female sex | 0.02 | −0.053 | 0.093 | 0.586 | 4.7 |
| BMI | 0.003 | −0.003 | 0.009 | 0.308 | 0.7 |
| Nurse (vs administrative personnel) | −0.082 | −0.072 | 0.083 | 0.027 | −17.2 |
| Physician (vs administrative personnel) | 0.005 | −0.102 | 0.060 | 0.893 | 1.2 |
| Employment degree of 60%–80% (vs ≤50%) | −0.021 | −0.092 | 0.065 | 0.613 | −4.7 |
| Employment degree of >80% (vs ≤50%) | −0.014 | −0.003 | 0.009 | 0.734 | −3.2 |
| Baseline vaccination with mRNA-1273 (Moderna) | 0.225 | 0.132 | 0.319 | <0.001 | 67.9 |
| Previous infection (seropositive for anti-N antibodies at follow-up) | 0.469 | 0.393 | 0.545 | <0.001 | 194.4 |
| Interval (in weeks) between the second vaccination and follow-up serology | −0.003 | −0.004 | −0.002 | <0.001 | −0.7 |
| Worked 1–3 late shifts within seven days before and/or after vaccinations one and two | −0.144 | −0.325 | 0.036 | 0.117 | −28.2 |
| Worked 4–6 late shifts within seven days before and/or after vaccinations one and two | −0.005 | −0.090 | 0.080 | 0.907 | −1.1 |
| Worked more than six late shifts within seven days before and/or after vaccinations one and two | 0.037 | −0.061 | 0.136 | 0.457 | 8.9 |
BMI: body mass index.
Table S3Multivariate linear regression model to determine the impact of the number of night shifts worked around the primary vaccination date on anti-S antibody levels after baseline vaccination (two mRNA vaccine doses).
| Coefficient | 95% confidence interval | p-value | % increase or decrease | ||
| Lower bound | Upper bound | ||||
| (Constant) | 3.623 | 3.382 | 3.864 | <0.001 | |
| Age (in 10 years, starting from 20 years) | −0.053 | −0.083 | −0.023 | <0.001 | −11.5 |
| Female sex | 0.027 | −0.053 | 0.106 | 0.473 | 6.4 |
| BMI | 0.003 | −0.003 | 0.009 | 0.337 | 0.7 |
| Nurse (vs administrative personnel) | −0.081 | −0.153 | −0.01 | 0.020 | −17.0 |
| Physician (vs administrative personnel) | 0.017 | −0.077 | 0.11 | 0.660 | 4.0 |
| Employment degree of 60%–80% (vs ≤50%) | −0.013 | −0.113 | 0.088 | 0.756 | −2.9 |
| Employment degree of >80% (vs ≤50%) | −0.006 | −0.104 | 0.092 | 0.885 | −1.4 |
| Baseline vaccination with mRNA-1273 (Moderna) | 0.220 | 0.093 | 0.348 | <0.001 | 66.0 |
| Previous infection (seropositive for anti-N antibodies at follow-up) | 0.471 | 0.385 | 0.558 | <0.001 | 195.8 |
| Interval (in weeks) between the second vaccination and follow-up serology | −0.003 | −0.004 | −0.002 | <0.001 | −0.7 |
| Worked 1–3 night shifts within seven days before and/or after vaccinations one and two | −0.078 | −0.205 | 0.05 | 0.360 | −16.4 |
| Worked 4–6 night shifts within seven days before and/or after vaccinations one and two | −0.14 | −0.257 | −0.023 | 0.146 | −27.6 |
| Worked more than six night shifts within seven days before and/or after vaccinations one and two | 0.005 | −0.125 | 0.135 | 0.933 | 1.2 |
BMI: body mass index.