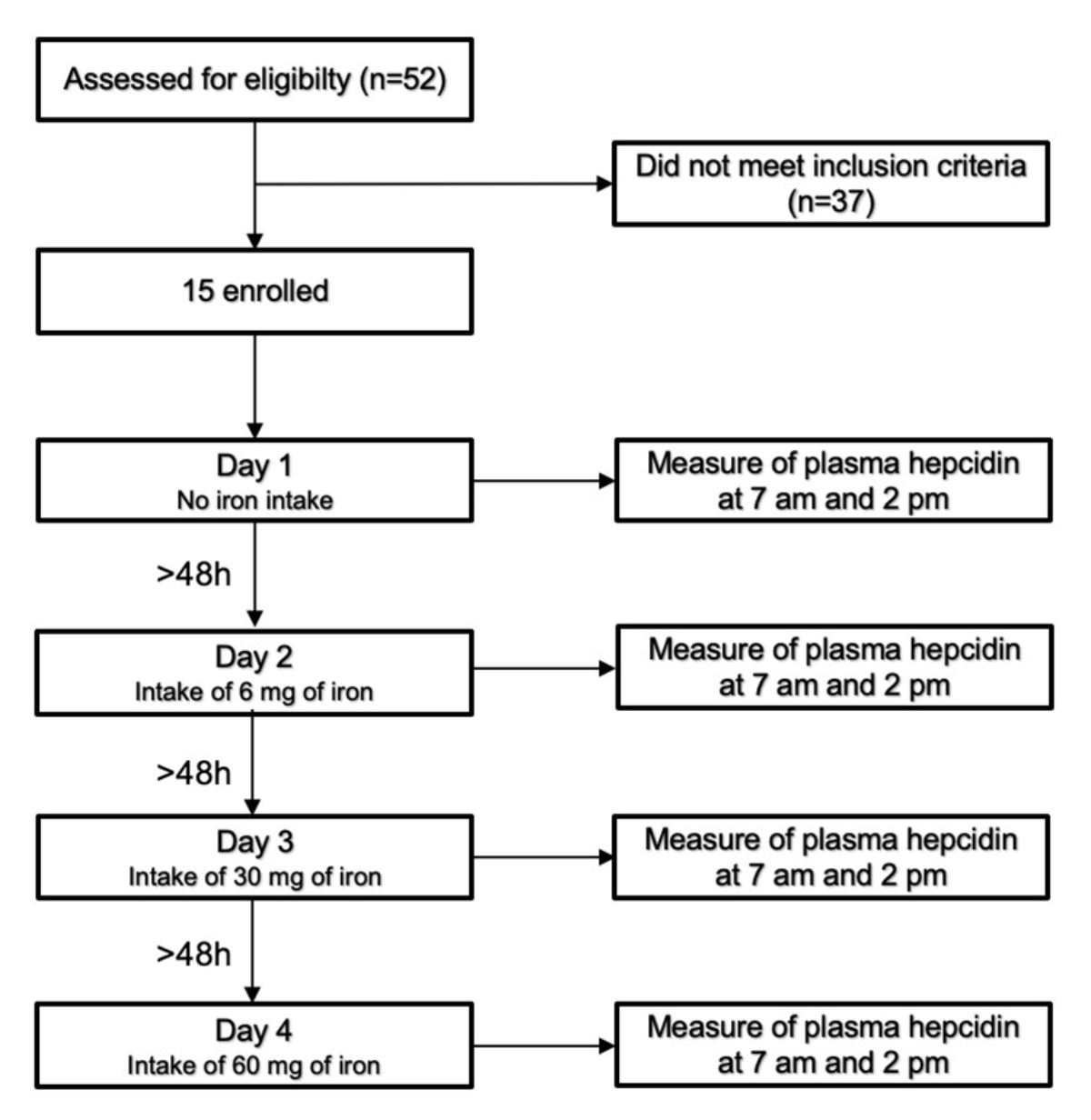
Figure 1Study design.
DOI: https://doi.org/https://doi.org/10.57187/s.3635
Iron deficiency is a global health problem and the leading cause of anaemia [1, 2]. The reasons for iron deficiency include insufficient iron intake and chronic blood loss [2]. Premenopausal women are at particularly high risk of iron deficiency [3]. Individuals with iron deficiency may suffer from symptoms such as fatigue [4], limitations in executive tasks [5], or hair loss [6].
Current guidelines for the oral treatment of iron deficiency recommend a daily supplement of 50–100 mg of elemental iron [7]. However, ingesting high oral iron doses is known to be associated with lower fractional iron absorption in the gut [8]. The reason for the low fractional iron absorption is its strict regulation. In the centre of the regulatory system, hepcidin, a small peptide hormone produced by the liver and secreted into the blood, is the critical player in the complex regulation of iron absorption [9]. The main target of hepcidin is the iron transporter ferroportin localised on the basolateral side of enterocytes in the intestine. As the only iron exporter, ferroportin is essential for exporting iron from the enterocyte and, thus, for iron absorption into the body. Hepcidin binding to its receptor internalises ferroportin into the enterocyte, where it is degraded in lysosomes [9, 10]. Therefore, high hepcidin levels reduce iron absorption from the intestine [11].
Hepcidin production in the liver is regulated by intracellular and extracellular iron, inflammation, and hypoxia [12]. Moretti et al. showed that a sixfold increase in iron intake (60 mg to 240 mg) only caused a threefold increase in absorbed iron and a massive increase in plasma hepcidin, which was still detectable 24 hours after ingesting higher iron doses. Therefore, they recommended ingesting iron supplements of >60 mg only every second day [13]. This physiologic constellation and recommendation fully agree with the “mucosal block theory” formulated by Hahn et al. more than 75 years ago [14] and underscore the importance of hepcidin as a regulator of iron absorption.
It remains unknown at what iron dose the mucosal block of iron absorption becomes clinically relevant. Therefore, this cross-over study investigated the effect of low iron doses (<60 mg) on plasma hepcidin levels in healthy premenopausal women with iron deficiency but without anaemia.
The study was conducted in the Department of Endocrinology at the University Hospital of Zurich according to the Declaration of Helsinki and the Good Clinical Practice Guidelines. Its protocols and amendments were approved by the local ethics committee. There were no deviations from the study protocol after its final approval. All patients included in this study provided written informed consent.
We recruited healthy premenopausal women with iron deficiency but without anaemia, defined as a serum ferritin concentration of ≤30 µg/l and haemoglobin concentration of ≥117 g/l. The other inclusion criteria were: age >18 years, regular menstrual cycle (defined as a cycle with a duration of 23–35 days), body mass index (BMI) of 18–25 kg/m2, no intake of dietary supplements for at least four weeks, no pregnancy, no hypermenorrhea (more than five sanitary pads or five tampons per day or more than 80 ml blood loss during one menstruation), no signs of acute or chronic inflammatory disease, no psychiatric disorders, no hypersensitivity to iron-supplements, no chronic kidney disease (creatinine concentration of ≤80 µmol/l), no hypo/hyperthyroidism (thyroid stimulating hormone [TSH] concentration of 0.16–4.25 mU/l), and no intake of drugs that interact with oral iron supplements. The study participants were recruited via public advertisements on pinboards and social media.
This study enrolled 15 of 52 screened women (Figure 1). It consisted of four test days with two visits per test day, one at 7:00 am after an overnight fast (last meal before 8:00 pm) and one at 2:00 pm, also in the fasting state (only tap water was allowed during the fasting period). The first test day served as the control without any iron ingestion. Iron supplements containing 6, 30, or 60 mg of iron were given on the second, third, and fourth test days, respectively.

Figure 1Study design.
Each morning visit served as the baseline examination, and the afternoon visit served as the follow-up examination. All four test days were separated by at least two days to avoid any carry-over effect of the previous test day. Each participant participated on each test day (i.e. each iron dosage level) and served as their own control. A blood sample was collected at each appointment, and each subject was required to appear after overnight fasting (last meal before 8 pm) and remain fasting until the afternoon blood sample was collected.
All morning vein punctures were performed before administering the iron supplement, and the taking of each supplement was supervised and controlled. The plasma hepcidin concentration was determined at every visit, and the C-reactive protein (CRP) concentration was determined at each morning visit. We repeated the examination on another day if the CRP value was >5 mg/l to minimise any effects of inflammation on the plasma hepcidin concentration.
A low-dose vegan iron supplement (donated by Streuli Pharma AG, Uznach, Switzerland) in the form of coated tablets containing 6 mg of elemental iron (corresponding 18.6 mg ferrous sulfate) was given as a single tablet or multiple tablets to achieve the iron dosage of the corresponding test day.
All other blood values (haemoglobin, iron, serum ferritin, transferrin, CRP, creatinine, alanine aminotransferase, and TSH) were measured in the Institute of Clinical Chemistry at the University Hospital of Zurich directly after the blood samples were collected. The haemoglobin concentration was measured using an ABL-90 blood gas analyser (Radiometer, Copenhagen, Denmark). The other serum analytes were measured using a Cobas 8000 (502c and e801) analyser (Roche Diagnostics, Mannheim, Germany). Samples for hepcidin determination were stored on dry ice in a locked refrigerator at –80°C without freeze-thaw cycles until shipment to the laboratory. Hepcidin was measured in the plasma within two months of the end of the study by the Translational Metabolic Laboratory (TML) in the Department of Laboratory Medicine at the Radboud University Medical Center (Nijmegen, the Netherlands) with a validated but not standardised complement-enzyme linked immunosorbent assay (c-ELISA) method (Hepcidin 25 [bioactive] HS ELISA; cat. #: EIA-5782; lots: 314K111 and 314K051-2; DRG International, New Jersey, USA). The c-ELISA has a low limit of detection (<0.45 nM) and coefficient of variation (<15%).
All data were analysed using SAS 9.4. Normally distributed variables are reported as the mean (± standard deviation [SD]). Non-normally distributed variables are reported as the median (interquartile range). Right-skewed variables were log-transformed and then re-transformed via exponential functions, resulting in a geometric mean and coefficient of variation. Values before and after the intervention were compared using a paired t-test or Wilcoxon signed-rank test. The significance of changes between the morning and afternoon visits (follow-up [FU] – baseline [BL]) by dose was investigated using four paired t-tests. Because of the right-skewed distributions, log-transformed plasma hepcidin values were used in the analysis without correction for multiple testing. The dose effect was explored in an analysis of covariance (ANCOVA) of the log-transformed FU data with baseline values as covariates and patients as random effects. Simple linear regressions were performed between ferritin (iron stores) and mean fasting plasma hepcidin and the mean change in plasma hepcidin after iron intake. All tests were performed at the 5% significance level (p = 0.05). The sample size was calculated based on the study by Moretti et al. [13] with a standard deviation of 5 mM for plasma hepcidin values and 80% power at a two-sided 5% significance level.
Our study cohort had a mean age of 23 years (SD = 3), mean BMI of 21 kg/m2 (SD = 2), median serum ferritin level of 24 ng/ml (interquartile range = 16–27), mean haemoglobin level of 134 g/l (SD = 8), and no signs of inflammation with a median CRP level of 0.7 mg/l (interquartile range = 0.6–1.0). Their other baseline characteristics are summarised in table 1.
Table 1Participants’ characteristics. The results are presented as the mean (standard deviation [SD]) or median (interquartile range).
| Women (n = 15) | |
| Age, years | 23 (3) |
| Body mass index, kg/m2 | 21 (2) |
| Systolic blood pressure, mmHg | 122 (10) |
| Diastolic blood pressure, mmHg | 81 (11) |
| Haemoglobin, g/l | 134 (8) |
| Serum ferritin, ng/ml | 24 (16–27) |
| Serum creatinine, µmol/l | 65 (7) |
| Alanine aminotransferase, U/l | 15 (5) |
| Thyroid-stimulating hormone, mU/l | 1.6 (1.2–1.9) |
| C-reactive protein, mg/l | 0.7 (0.6–1.0) |
Morning and afternoon hepcidin concentrations did not differ significantly on the control day when no iron supplement was administered. The highest mean change in plasma hepcidin concentration occurred after ingesting 60 mg of iron, increasing from 2.1 ng/ml to 4.1 ng/ml (p <0.001; table 2).
Table 2Plasma hepcidin concentrations measured before/after a single iron dose. The results are presented as the geometric mean (confidence interval). The p-values were calculated using a paired t-test. NS: not significant and no trend (p > 0.10).
| Iron dose | Plasma hepcidin concentration (ng/ml) | |||
| 7:00 am | 2:00 pm | Change | p-value | |
| 0 mg | 2.6 (1.6–4.0) | 3.1 (1.7–5.3) | 1.2 (0.9–1.5) | NS |
| 6 mg | 2.4 (1.6–3.7) | 3.5 (2.2–5.6) | 1.5 (1.1–1.9) | 0.005 |
| 30 mg | 2.2 (1.6–2.9) | 3.5 (2.1–5.6) | 1.6 (1.2–2.3) | 0.014 |
| 60 mg | 2.1 (1.5–2.9) | 4.1 (2.5–6.9) | 2.0 (1.5–2.6) | <0.001 |
Furthermore, we observed a significant dose-dependent effect on the absolute change in plasma hepcidin concentration (p = 0.008; figure 2). All results and changes in plasma hepcidin concentration are shown in table 2.
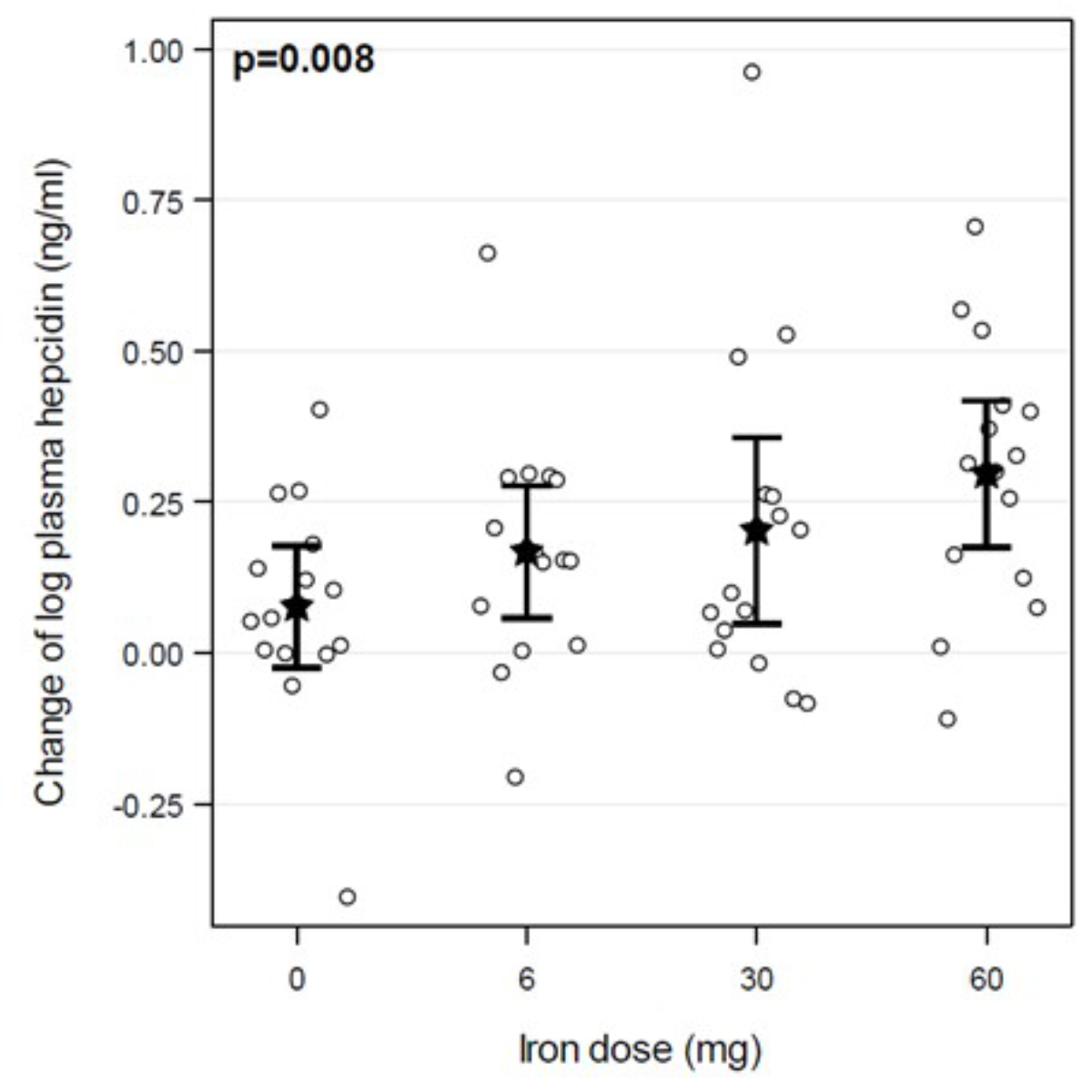
Figure 2The change in plasma hepcidin concentration depends on the iron dosage. Circles are individual values, stars are means, and limit lines are 95% confidence intervals. The p-value was calculated using an ANCOVA jointly considering all doses.
Correlations between serum ferritin and fasting plasma hepcidin concentrations and the change in plasma hepcidin concentration after iron intake
The serum ferritin concentration (at the screening visit) was significantly positively correlated with fasting plasma hepcidin concentrations (figure 3a): the lower the serum ferritin level, the lower the detected plasma hepcidin concentration (R2 = 0.504, p = 0.003). In addition, the serum ferritin concentration (at the screening visit) was significantly positively correlated with the change in plasma hepcidin concentration after iron intake (figure 3b): the higher the serum ferritin concentration, the more pronounced the hepcidin response (R2 = 0.529, p = 0.002).
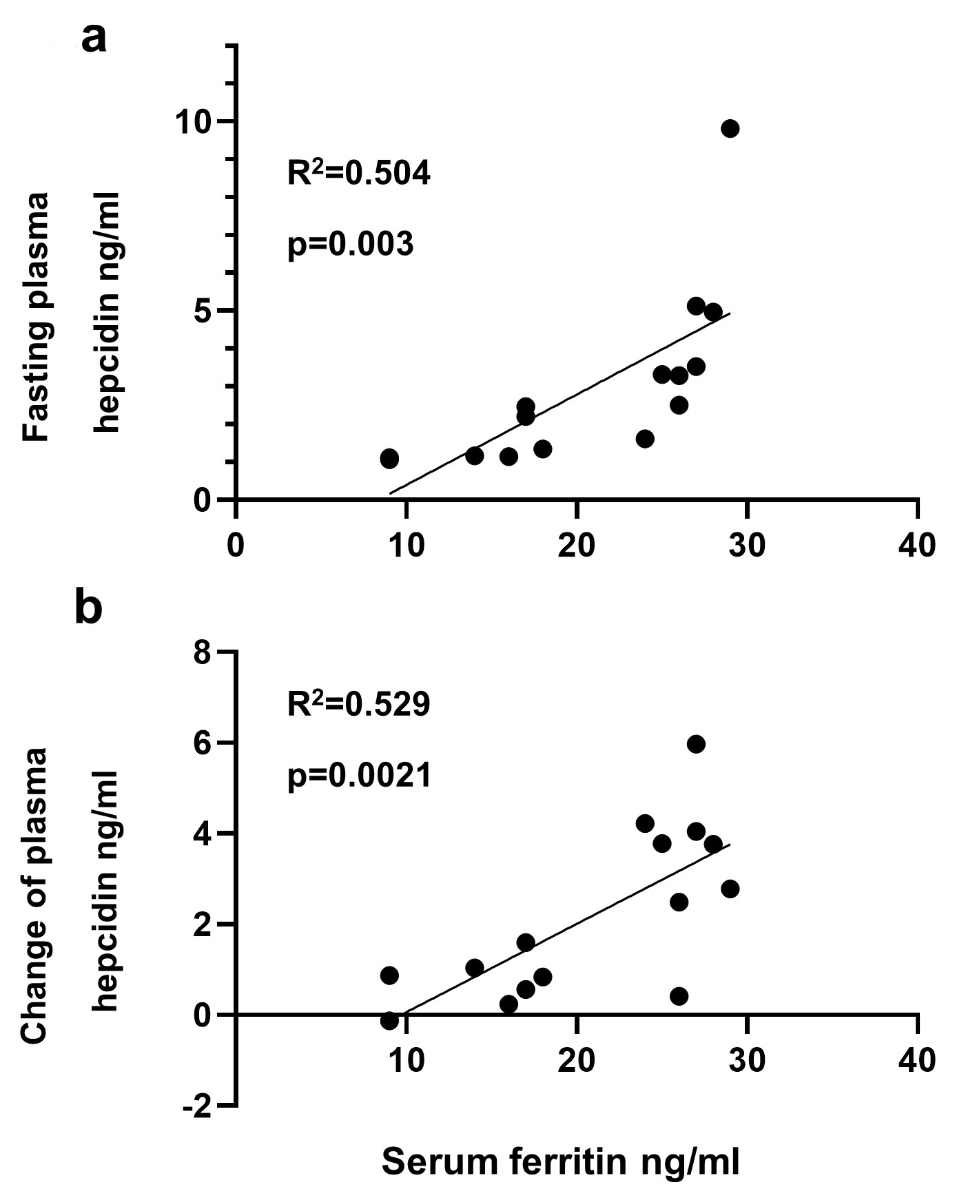
Figure 3The correlations between iron stores (serum ferritin at the screening visit) and (A) fasting plasma hepcidin concentrations and (B) the change in plasma hepcidin concentration after iron intake.
One woman complained of abdominal pain after ingesting 60 mg of iron. No other adverse events were reported.
This study showed for the first time a dose-dependent increase in the plasma hepcidin concentration after ingestion of 6–60 mg iron doses. In addition, the fasting plasma hepcidin concentration and its increase after oral iron intake correlated with serum ferritin levels (i.e. the iron stores): the smaller the iron stores, the smaller the increase in hepcidin. These results underscore the precise regulation of iron absorption, even with low to borderline iron stores (i.e. serum ferritin values of 10–30 ng/ml). These new findings may have highly relevant clinical implications: Earlier studies have shown that iron absorption under standard (higher) iron therapy is comparatively low (around 10%) [15], with large amounts of unabsorbed iron associated with intestinal toxicity such as intestinal epithelial cell damage or altered microbiome composition [16, 17].
Due to the dose-dependent effects of iron on plasma hepcidin concentrations, even at low dosages, it seems physiologically highly probable that low-dose iron therapy at 6–30 mg per daily used in this study is more efficiently absorbed, leading to a better replenishment of the iron stores and lower gastrointestinal iron toxicity. The latter is supported by a recent non-placebo-controlled pilot study involving 36 women with iron deficiency but without anaemia, who showed a significant increase in serum ferritin (from 18 ng/ml to 33 ng/ml; p <0.0001) occurred after eight weeks of treatment with 6 mg iron twice daily [18]. To our knowledge, this is the first study to examine low-dose iron therapy in premenopausal women. However, low-dose iron as a treatment for iron deficiency has been shown to be effective in children and older adults and was associated with fewer side effects [19, 20].
Our results regarding the relationship between existing iron stores and plasma hepcidin concentrations confirmed our clinical experience with iron treatment in daily practice: women with substantial iron deficiency (serum ferritin concentration of <15 ng/ml) and presumably a low hepcidin concentration seem to respond favourably and rapidly to oral iron therapy. Oral iron therapy appears pertinacious and less responsive with higher iron stores (serum ferritin concentration of ~30 ng/ml) and potentially higher hepcidin concentrations.
Our study had some limitations. Firstly, the study population was highly selective, comprising young and healthy women with a normal weight, but nonetheless represents a group at high risk of iron deficiency. Secondly, based on a power calculation, only a few women were included, and all completed the study. Thirdly, plasma hepcidin concentrations were measured after one single iron dose. The hepcidin response with a longer daily low-dose iron therapy might differ. Fourthly, our results cannot be generalised to other patient populations. Fifthly, plasma hepcidin was measured before and seven hours after iron intake in a fasting state, and it cannot be excluded that the hepcidin response is modulated by food intake.
In summary, we found a dose-dependent effect of low-dose oral iron on plasma hepcidinconcentrations. Based on these results, it is conceivable that a low-dose iron therapy may bemore efficient and associated with fewer side effects, leading to better therapy compliance.
Statistical support was provided by Acomed Statistik (Leipzig, Germany).
The study was supported financially by the foundation: Stiftung für klinische Forschung, c/o Universitätsspital Zürich, Rämistrasse 100, CH-8091 Zürich
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. P. A. Krayenbuehl received consulting fee from Streuli Pharma (Uznach, Switzerland). The other authors declare no conflict of interests.
1. Kassebaum NJ, Collaborators GA; GBD 2013 Anemia Collaborators. The Global Burden of Anemia. Hematol Oncol Clin North Am. 2016 Apr;30(2):247–308.
2. C. Camaschella, "Iron-Deficiency Anemia," (in eng), N Engl J Med, vol. 373, no. 5, pp. 485-6, 07 30 2015, doi: . 10.1056/NEJMra1401038
3. DeLoughery TG. Iron Deficiency Anemia. Med Clin North Am. 2017 Mar;101(2):319–32.
4. Krayenbuehl PA, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011 Sep;118(12):3222–7.
5. Scott SP, Murray-Kolb LE. Iron Status Is Associated with Performance on Executive Functioning Tasks in Nonanemic Young Women. J Nutr. 2016 Jan;146(1):30–7.
6. Beatrix J, Piales C, Berland P, Marchiset E, Gerbaud L, Ruivard M. “Non-anemic iron deficiency: correlations between symptoms and iron status parameters,” (in eng). Eur J Clin Nutr. 2021 Nov;
7. Snook J, Bhala N, Beales IL, Cannings D, Kightley C, Logan RP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021 Nov;70(11):2030–51.
8. D. Moretti, "Novel approaches to oral iron treatment," (in eng), Hemasphere, vol. 3, no. Suppl, Jun 2019, doi: .
9. Ganz T, Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol. 2006 Feb;290(2):G199–203.
10. Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004 Dec;306(5704):2090–3.
11. Young MF, Glahn RP, Ariza-Nieto M, Inglis J, Olbina G, Westerman M, et al. Serum hepcidin is significantly associated with iron absorption from food and supplemental sources in healthy young women. Am J Clin Nutr. 2009 Feb;89(2):533–8.
12. V. Sangkhae and E. Nemeth, "Regulation of the Iron Homeostatic Hormone Hepcidin," (in eng), Adv Nutr, vol. 8, no. 1, pp. 126-136, 01 2017, doi: .
13. Moretti D, Goede JS, Zeder C, Jiskra M, Chatzinakou V, Tjalsma H, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015 Oct;126(17):1981–9.
14. Hahn PF, Bale WF, Ross JF, Balfour WM, Whipple GH. “RADIOACTIVE IRON ABSORPTION BY GASTRO-INTESTINAL TRACT : INFLUENCE OF ANEMIA, ANOXIA, AND ANTECEDENT FEEDING DISTRIBUTION IN GROWING DOGS,” (in eng). J Exp Med. 1943 Sep;78(3):169–88.
15. Cook JD, Reddy MB. Efficacy of weekly compared with daily iron supplementation. Am J Clin Nutr. 1995 Jul;62(1):117–20.
16. Richards T, Breymann C, Brookes MJ, Lindgren S, Macdougall IC, McMahon LP, et al. Questions and answers on iron deficiency treatment selection and the use of intravenous iron in routine clinical practice. Ann Med. 2021 Dec;53(1):274–85.
17. Ribeiro M, Fonseca L, Anjos JS, Capo-Chichi JC, Borges NA, Burrowes J, et al. Oral iron supplementation in patients with chronic kidney disease: can it be harmful to the gut microbiota? Nutr Clin Pract. 2022 Feb;37(1):81–93.
18. Simic S, Karczewski M, Klapdor S, Nowak A, Schubert M, Moretti D, et al. Effectiveness of low-dose iron treatment in non-anaemic iron-deficient women: a prospective open-label single-arm trial. Swiss Med Wkly. 2023 May;153(5):40079.
19. Rosado JL, González KE, Caamaño MC, García OP, Preciado R, Odio M. Efficacy of different strategies to treat anemia in children: a randomized clinical trial. Nutr J. 2010 Sep;9(1):40.
20. Rimon E, Kagansky N, Kagansky M, Mechnick L, Mashiah T, Namir M, et al. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med. 2005 Oct;118(10):1142–7.