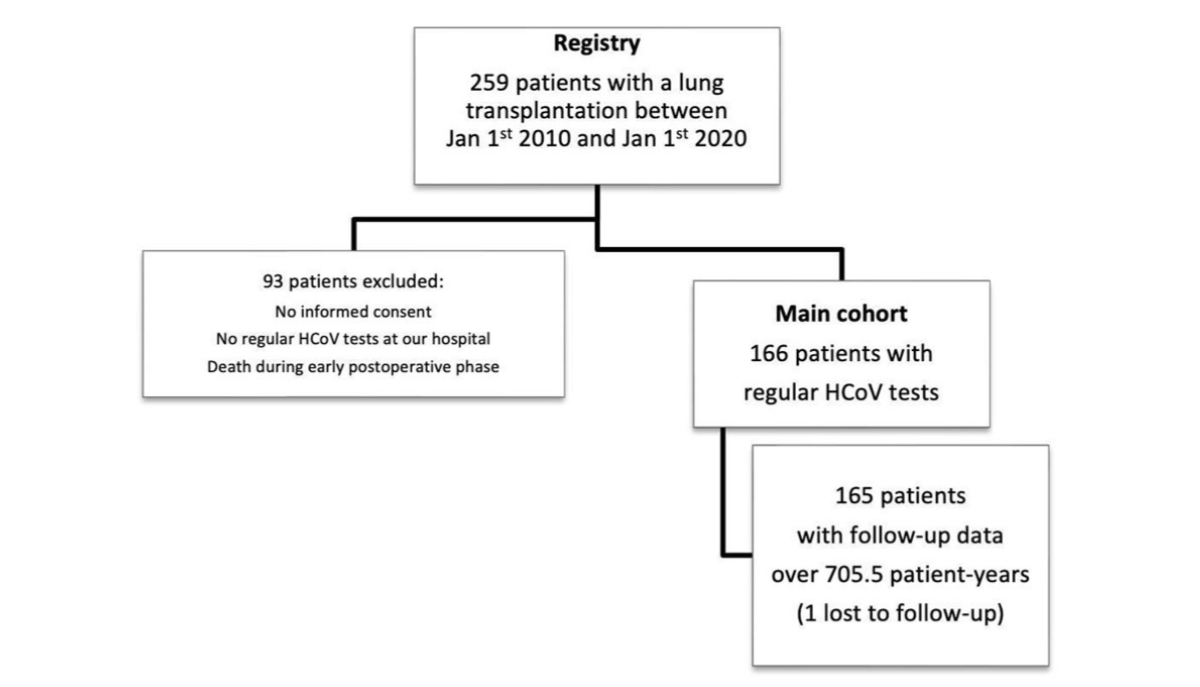
Figure 1Study flow. Overall, 64% (n = 166) of lung transplant recipients in the observation period were included in this study. The last follow-up day was November 30th, 2020, and one patient was lost to follow-up. HCoV: human coronavirus.
DOI: https://doi.org/https://doi.org/10.57187/s.3568
Lung transplantation is a life-saving procedure for patients suffering from end-stage lung diseases. Unfortunately, the survival of transplanted organs is limited by acute and chronic allograft rejection, resulting in a median survival rate of slightly more than 6 years [1].
Improvements in managing patients in the early postoperative phase has led to reduced 1-year mortality. Regrettably, the mortality rate remains high beyond the first year, with roughly half of all patients developing chronic lung allograft dysfunction (CLAD) within 5 years, and thus allograft rejection is a significant obstacle to long-term success [2].
Lung transplant recipients require extensive immunosuppressive therapy and thus are more prone to infections in contrast to other solid organ transplant recipients [3]. Therefore, respiratory tract infections potentially have a larger impact on long-term outcomes [3]. Major underlying factors include the graft’s continuous contact with the environment, impaired cough reflex, and reduced mucociliary clearance [4, 5]. In addition to causing direct illness, a relationship between respiratory viral infection and the subsequent development of bronchiolitis obliterans syndrome has been established by several studies [6]. Bronchiolitis obliterans syndrome (BOS) is a major factor limiting long-term survival.
Infections can also result in restrictive allograft syndrome (RAS). Both are part of the umbrella term “chronic lung allograft dysfunction” (CLAD) [7].
The causes of chronic lung allograft dysfunction are still unknown, but it is believed that a combination of internal and external factors, immune processes, and adaptive immunity contribute to the development of alloimmune responses against the lung’s structural proteins [8]. Thus, identifying modifiable epidemiological risk factors that lead to chronic lung allograft dysfunction in lung transplant recipients is crucial for efforts to extend their life expectancy.
Human coronavirus (HCoV) is one of the most frequent causes of respiratory tract infections in humans [9]. HCoV, including the subtypes 229E, NL63, OC43, and HKU1, usually cause mild upper respiratory infections but may develop into lower respiratory tract infections in lung transplant recipients because of their compromised immune reaction [9]. A nasopharyngeal swab is taken to diagnose an infection, and a polymerase chain reaction (PCR) analysis is performed [10, 11]. Currently, no specific treatments are available to alter the course of HCoV infections in humans. Therefore, it is crucial to focus on preventive measures. HCoV infections are predominantly transmitted from humans to humans but may be able to cross the genetic barrier between animal reservoirs and humans because of the genetic variability of the viruses [23]. Therefore, the risk of infection can potentially be reduced through behavioural interventions.
Previous studies suggest that there is a biological link between respiratory tract infections and the development of chronic lung allograft dysfunction in lung transplant recipients [12]. A recent cohort study indicates that there may be a time-dependent connection between the development of HCoV infection and chronic lung allograft dysfunction [13]. Preventing respiratory tract infections may reduce the risk of chronic lung allograft dysfunction in this population.
Additionally, since HCoV infections tend to occur during certain seasons in the general population, it may be possible to implement more specific interventions. This 10-year retrospective cohort study examines the association between HCoV infections and the development of chronic lung allograft dysfunction in our single-centre cohort and describes the typical symptoms, seasonal patterns, laboratory findings, and the link between HCoV infections and their effect on respiratory function.
The study population comprised adults who underwent lung transplantation between January 1st, 2010, and January 1st, 2020 (follow-up until November 30th, 2020) at the University Hospital in Zurich (USZ), Switzerland, and survived the initial postoperative period in the intensive care unit. The USZ lung transplantation centre provides lung transplantations for selected patients with advanced lung disease due to cystic fibrosis, interstitial lung diseases, chronic obstructive pulmonary disease, pulmonary arterial hypertension, and terminal lung diseases of other causes.
We conducted a retrospective cohort study by gathering data from the clinical information system up until November 30th, 2020. The data were extracted by a sorting program and manually checked for plausibility from the medical records of the USZ transplant centre by the first author. The data were then entered into an encrypted study database. The observation period was set at 10 years to encompass the average postoperative survival of lung transplant recipients, which is typically 5–6 years. This extended duration allows for a larger patient cohort and data collection from those exceeding the average survival time.
Clinical and laboratory data were recorded during both outpatient and inpatient visits. We collected information on symptoms, infectious parameters (CRP), lung function tests, and viral sampling results from nasopharyngeal swabs.
To date, seven HCoVs have been identified, namely HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and the novel coronavirus (2019-nCoV, a.k.a. SARS-CoV-2) [9]. However, in this study, we did not investigate SARS-CoV, MERS-CoV, or SARS-CoV-2 because very few infections were detected at our centre during the observation period. Therefore, we refer to “HCoVs” in the context of the four viruses, namely HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1. The primary choice of post-transplantation immunosuppression consisted of cyclosporine A, mycophenolate mofetil, and prednisone after induction with basiliximab [14] Generally, lung transplant recipients were treated according to the USZ transplantation centre guidelines. Specialists in infectious diseases or microbiology were consulted for treatment, if deemed necessary.
There was no specific time scheme for respiratory testing in lung transplant recipients. Because they were undergoing immunosuppressive therapy, they were encouraged to present even with very mild symptoms. At each visit, the treating clinician conducted viral sampling via nasopharyngeal swabs following the internal standard operating procedures. A viral test was also performed in cases of emergency room visits at the discretion of the treating physician. A set of symptoms was assessed: joint and muscle pain, cough and shivering, malaise, shortness of breath, running nose, throat pain, and increased sputum. Some patients did not clearly show the aforementioned symptoms and were labelled asymptomatic. Every patient in our study was tested for HCoV as well as co-infections (respiratory viruses, bacteria, or fungi), including the patients of the control group. If there was a co-infection detected, those periods were excluded from the analysis. Thus, the clinical diagnosis at the time of our investigation was upper respiratory tract infection by HCoV. Nasopharyngeal swab samples yield the highest virus concentrations [25] and, during this study, mainly nasopharyngeal swabs were used. If a person tested positive for a virus, we followed internal guidelines and performed nasopharyngeal swabs every 5–8 days (usually every 7 days) as long as genetic viral material was detectable. The repeated testing of all patients within short intervals helped mitigate both false-positive and false-negative results. A new HCoV infection was detected if a new viral strain was found. Persistent infection was defined by continuous positive PCR tests.
Medical records on bronchoalveolar lavages (BAL) during bronchoscopy were not within the scope of this study. Bronchoalveolar lavages may be performed if there is clinical concern about a lower respiratory tract infection, but it is not routinely employed since it was not expected to change the management. Consequently, bronchoalveolar lavage results were available only at irregular intervals for a few patients compared to the numerous HCoV swab samples. Positive PCR tests (even without symptoms) were recorded. With current molecular diagnostic tests, it is possible to rapidly detect various viruses through multiplex PCR, which was performed by the Institute of Medical Virology at the University of Zurich. A viral swab analysis named “PCR respiratory block analysis” consisted of Influenza, respiratory syncytial virus, adenovirus, coronavirus (four types, namely N63, 229E, OC43, and HKU1), entero/rhinovirus, metapneumovirus, parainfluenza virus (1-4). The aforementioned laboratory tests did not routinely include cycle threshold (CT) or procalcitonin values. All patients who did not provide signed written consent for research (general informed consent) were excluded from the registry of this study.
The Ethics Committee of Zurich, Switzerland, approved the study (BASEC-ID 2020-02797).
The primary outcome was the association between any HCoV infection subtype and subsequent chronic lung allograft dysfunction occurrence in lung transplant recipients. The early postoperative period (first 4 weeks posttransplant) was not investigated because lung transplant recipients typically experience acute rejection reactions and other postoperative complications during this time. According to Thabut et al. [2], “Improvements (…) in the early postoperative period led to a reduction (…) in 1-year mortality (…). However, the attrition rate after the first year (…) is mainly attributable to chronic lung allograft dysfunction (CLAD).” Additionally, neither the type nor the duration of hospital stay was analysed in our study, as these factors are not strictly relevant to chronic lung allograft dysfunction development; mild infections can still lead to chronic lung allograft dysfunction over time.
The secondary outcome comprised changes in forced expiratory volume in 1 second (FEV1) before and after HCoV infection, seasonal patterns, and laboratory and clinical parameters associated with HCoV infections in lung transplant recipients, as well as all-cause mortality. Our findings cannot be interpreted as confirmatory evidence; rather, they support a hypothesis that requires further research.
Chronic lung allograft dysfunction was defined as a significant and constant decline (≥20%) in the measured FEV1 value from the baseline value, following the current International Society for Heart and Lung Transplantation (ISHLT) recommendations. The baseline value is defined as the mean of the best two postoperative FEV1 measurements (taken >3 weeks apart) [7]. This reference value was calculated based on the mean of the two best post-transplant FEV1 values within the first two post-transplant years [7]. Chronic lung allograft dysfunction may be subcategorised into an obstructive ventilatory pattern, a restrictive pattern, or a mixed obstructive and restrictive pattern. Other potential pathologies may also cause a chronic loss of allograft function, including mechanical factors such as airway stenosis, persistent pleural effusion, or weight gain, as well as persistent oedema due to heart failure or infections. These factors are not components of chronic lung allograft dysfunction and were ruled out prior to a chronic lung allograft dysfunction diagnosis [8].
To detect heart failure, clinicians evaluated echocardiographic results and serum brain natriuretic peptide levels, monitored weight gain, and assessed lung infections by analysing weight data and C-reactive protein levels in the blood 3 months before and after the suspected onset of chronic lung allograft dysfunction. Viral infections were identified through laboratory results compiled by the medical virology department, while bacterial infections were diagnosed based on bacteriology results, including blood and urine cultures. Available lung histology data from transbronchial biopsies, bronchoscopy data, and CT scan changes were checked over a period of 3 months before and after the onset of chronic lung allograft dysfunction. The biopsies were evaluated by a skilled pulmonary pathologist, whereas chest CT scans were assessed by a qualified chest radiologist to classify chronic lung allograft dysfunction changes.
Descriptive statistics include the mean and standard deviation (SD) for continuous variables, median and interquartile range [IQR] for ordinal and non-normal variables, and number and percentage of total for categorical variables. A cause-specific Cox proportional hazards regression model was applied to assess the association between HCoV infection (exposure variable) and chronic lung allograft dysfunction (outcome variable) using the number of days since lung transplantation as the timescale. The model was adjusted for sex, age, initial diagnosis, and HCoV infection, which was treated as a binary variable (yes/no), irrespective of the number of infections. Patients were censored at the time of death, regardless of the cause. Furthermore, a secondary crude Cox regression analysis was conducted with death as the outcome variable to further investigate its relationship with chronic lung allograft dysfunction. A log-rank test was applied to assess the association between the exposure and the binary outcome. Given that only one patient was lost to follow-up, we assumed all censoring events to be non-informative and, therefore, did not make adjustments for any potential biases in this particular cohort. In a sensitivity analysis, the choice of statistical test did not alter the observed outcome, as evidenced by consistent results when employing a Wilcoxon log-rank test.
The assessment of the proportional-hazards assumption using the global Schoenfeld residual test yielded a p-value of 0.375, indicating no significant violation of the assumption.
The observation period was from January 1st, 2010 to January 1st, 2020. Time zero was the date of lung transplantation for each patient. The observation ended either with the development of chronic lung allograft dysfunction (irrespective of its severity or subcategory), the death of the patient, or the end of the follow-up period (November 30th, 2020). Reasons for censoring over the 10-year observation period are provided for all patients. No missing data were imputed. If any variable was missing, we report it in the analysis. To analyse how outcomes change over time, we used a difference-in-difference estimation method. Specifically, we used lung function (FEV1 in ml and %predicted) and laboratory values (CRP) as the outcome and analysed it in a 21-day period before and after the HCoV infection without any further adjustments.
Independent hypothesis tests (t-test) and 95% confidence intervals were calculated for each day, with day 0 (time of infection) as the reference. The selection of a 21-day timeframe was carefully considered by the distribution of lung function tests centred on the time of infection with the majority of data clustered within this 21-day window and it effectively captured the critical phase following infection and encompassed over 95% of the corresponding laboratory values.
To account for multiple infections in some patients, we divided the longitudinal analysis into distinct observation periods, allowing a single individual to contribute multiple observations. These repeated observations within the same patient were modelled as random effects using the STATA “mixed” command to appropriately capture within-subject variability. An observation was limited by the study-observation time or another observation in the same patient. We made sure that there were no overlapping days between observations. If there was a conflict, we divided the lung function tests at the median point between the two HCoV infections. Day zero was assigned to all observations, denoting the first day of a recorded HCoV infection (day of positive PCR result).
An epidemiologist conducted all analyses using either R v4.3 (R Core Team, Austria) or STATATM v18 (StataCorp LP, College Station, TX, USA), along with dynamic reporting. A two-tailed p-value of less than 0.05 was deemed statistically significant for all the reported tests. The results were reported according to the STROBE guideline [15].
Between January 1st, 2010, and January 1st, 2020, 259 patients underwent lung transplantation at the USZ. The main cohort consisted of 166 lung transplant recipients who survived the early postoperative period, provided general consent, and performed regular follow-up visits at our centre (USZ). The median time to the first HCoV infection was 381 days after transplantation [IQR 178–933], with a median of three HCoV infections [IQR 2–5] per patient (figure 1).

Figure 1Study flow. Overall, 64% (n = 166) of lung transplant recipients in the observation period were included in this study. The last follow-up day was November 30th, 2020, and one patient was lost to follow-up. HCoV: human coronavirus.
One patient (always HCoV negative results) was lost to follow-up. The mean age of all patients at lung transplantation was 46 years and 72 (43.4%) patients were female and 94 (56.6%) were male. The underlying disease was COPD in 36% (n = 60) of cases, followed by cystic fibrosis in 33.7% (n = 56), and pulmonary fibrosis in 16.9% (n = 28). The total number of patient days, including multiple observation periods within the same patient due to multiple infections, was 259,833 patient days and medical records from 29,726 days (including inpatient days) were analysed.
Throughout the 10-year observation period, which included 259,833 patient days (711.4 patient-years), 76 patients (45.8%) developed chronic lung allograft dysfunction (all severities combined). A total of 41 patients (24.7%) died during the observation period after a median of 1255 days [IQR 761–1692] after the transplantation. In an unadjusted Cox regression analysis, patients with at least one documented HCoV infection experienced a higher likelihood of chronic lung allograft dysfunction (hazard ratio [HR] = 2.59, 95% CI 1.43–4.72, p = 0.001, figure 2). This hazard ratio remained stable after adjusting for multiple variables in a multivariable analysis (HR adjusted = 2.52, 95% CI 1.32–4.80, p = 0.005, n = 166) (table 1).
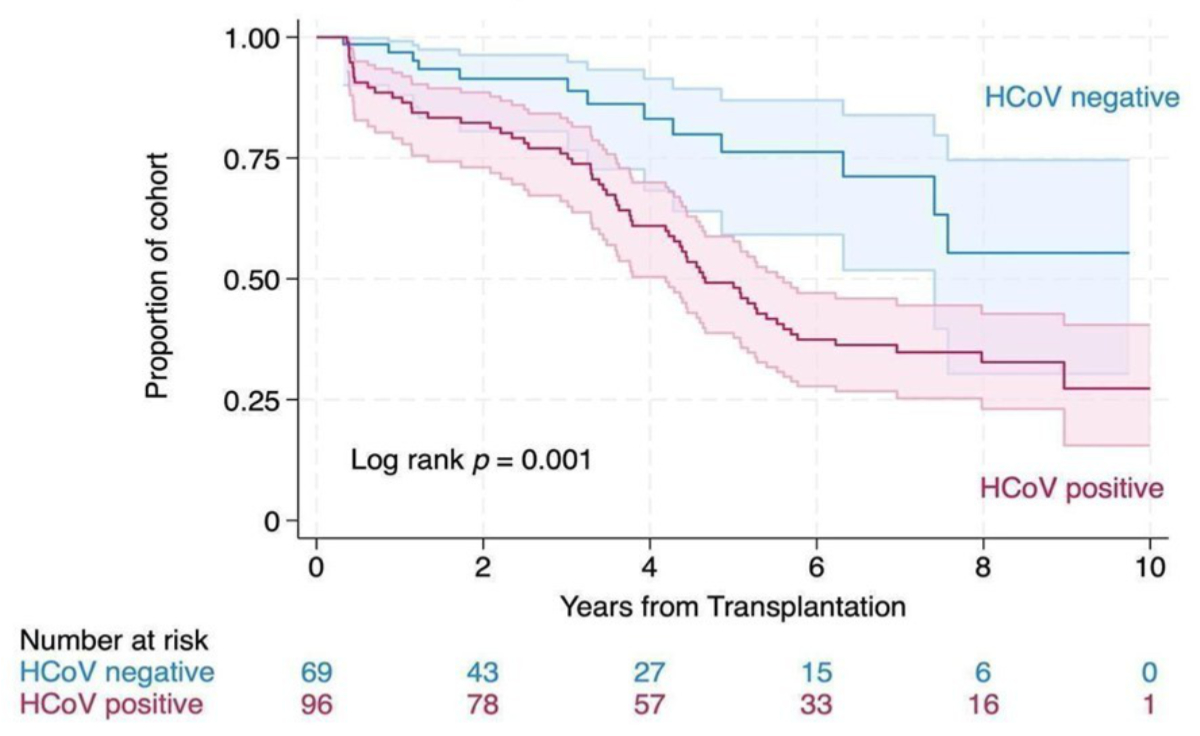
Figure 2Kaplan-Meier failure estimate for chronic lung allograft dysfunction with 95% confidence intervals by human coronavirus (HCoV) infection status (irrespective of the total number of HCoV infections) over 10 years. No data were available for one patient without HCoV infection (lost to follow-up).
Table 1Multivariable Cox proportional hazards model for time to chronic lung allograft dysfunction (CLAD). Continuous variables: sex (male/female); age (per one year increase); initial diagnosis (before lung transplantation): COPD, cystic fibrosis, idiopathic pulmonary fibrosis, pulmonary arterial hypertension, lymphangioleiomyomatosis, bronchiectasis, and other; number of HCoV infections (increase binary: yes/no). Total individuals: n = 166. Patients with chronic lung allograft dysfunction also experienced higher all-cause mortality (hazard ratio crude 2.25, 95% CI 1.29–3.89, p = 0.001).
| Static variable | Hazard ratio (adjusted) | 95% confidence interval | p-value | |
| ≥1 infection with HCoV (reference = no HCoV detection at any time) | 2.52 | 1.32–4.80 | 0.005 | |
| Diagnosis (COPD = reference) | Cystic fibrosis | 0.40 | 0.13–1.20 | 0.103 |
| Idiopathic pulmonary fibrosis | 0.92 | 0.43–1.96 | 0.834 | |
| Pulmonary arterial hypertension | 0.81 | 0.27–2.46 | 0.713 | |
| Lymphangioleiomyomatosis | 0.92 | 0.21–3.98 | 0.909 | |
| Bronchiectasis | 2.25 | 0.28–18.05 | 0.446 | |
| Other | 1.02 | 0.38–2.74 | 0.974 | |
| Male sex (reference = female; n = 165) | 0.71 | 0.17–1.33 | 0.156 | |
| Age at lung transplant (years; n = 165) | 0.98 | 0.95–1.01 | 0.230 | |
| Multiple HCoV infections (reference = 1 HCoV Infection; n = 96) | 1.25 | 0.61–5.27 | 0.549 | |
COPD: Chronic obstructive pulmonary disease; HCoV: Human Coronavirus.
During the observation period, 6,738 lung function tests were analysed for 96 patients with at least one HCoV infection. Each patient had a median of 69 lung function tests [IQR 46–90] during the observation period. In total, 121 observation periods in 96 patients were recorded (due to multiple infections in some patients). The 121 observations with corresponding unique HCoV infections had their median lung function 29 days after infection [IQR −564–661] with a range from −2954 days to 2917 days before and after infection, respectively. To conduct a more precise analysis, the lung function tests performed within a range of ±21 days before or after the infection were analysed on a day-to-day basis. In a difference-in-difference estimation at ±21 days, there was no change in lung function after HCoV infection (Figure 3) and regression analysis indicated no changes in the slopes of lung function before and after the HCoV infection (p = 0.863 for FEV1 and p = 0.688 for FEV1 pred.). Only a small elevation in CRP could be identified when compared to baseline on days 4 and 5 after diagnosis of HCoV infection (figure 3).
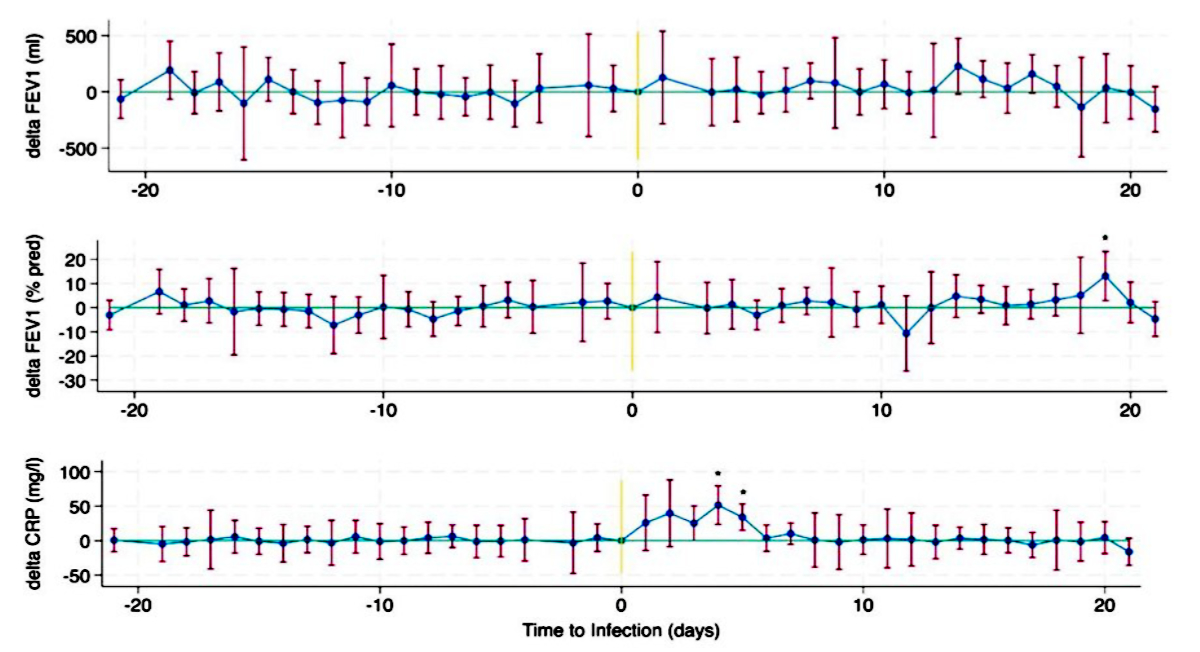
Figure 3Difference-in-difference estimation for FEV1, FEV1% pred., and C-reactive protein (CRP) levels at ±21 days with the day of the infection as the baseline in 128 observation periods from 96 patients. 95% confidence intervals are shown. Significant deviations from baseline are marked with an asterisk. For some days, no data were available.
Throughout the observation period, 4,150 multiplex PCR tests for viral pathogens were conducted. Of all the tests, 380 (9.2%) showed a positive result for HCoV, and 96 individual lung transplant recipients (57.8%) were infected at least once with HCoV virus during the observation period. Twenty-one lung transplant recipients (12.7%) were infected twice, 14 lung transplant recipients (8.4%) were infected thrice, and two lung transplant recipients (1.2%) and one lung transplant recipient (0.6%) were infected on four and five different occasions, respectively. For multiple HCoV infections, the median time between infections was 5 months [IQR 2–12], and unique HCoV infection lasted for a median of 9 days [IQR 3–26]. The average HCoV infection rate during the observation period was 0.53 ± 0.33 infections per patient-year. Patient characteristics were similar among patients with and without documented HCoV infections (table 2).
Table 2Baseline characteristics of the study cohort.
| Patients without HCoV infection | Patients with ≥1 HCoV infection | All patients | p-value | ||
| N | 70 (42.2%) | 96 (57.8%) | 166 (100.0%) | ||
| Age at lung transplantation | 49.0 (14.5) | 45.4 (14.5) | 46.9 (14.6) | 0.111 | |
| Sex | 0.044 | ||||
| Female | 24 (34.3%) | 48 (50.0%) | 72 (43.4%) | ||
| Male | 46 (65.7%) | 48 (50.0%) | 94 (56.6%) | ||
| Reason for lung transplantation | 0.188 | ||||
| COPD (including alpha-1 antitrypsin deficiency) | 27 (38.6%) | 33 (34.4%) | 60 (36.1%) | ||
| Cystic fibrosis | 20 (28.6%) | 36 (37.5%) | 56 (33.7%) | ||
| Pulmonary fibrosis | 13 (18.6%) | 15 (15.6%) | 28 (16.9%) | ||
| Pulmonary hypertension | 0 (0.0%) | 6 (6.2%) | 6 (3.6%) | ||
| Lymphangioleiomyomatosis | 1 (1.4%) | 2 (2.1%) | 3 (1.8%) | ||
| Bronchiectasis | 1 (1.4%) | 0 (0.0%) | 1 (0.6%) | ||
| Other | 8 (11.4%) | 4 (4.2%) | 12 (7.2%) | ||
| Lung transplantation method | 0.096 | ||||
| Unilateral | 2 (2.9%) | 0 (0.0%) | 2 (1.2%) | ||
| Bilateral | 68 (97.1%) | 96 (100.0%) | 164 (98.8%) | ||
COPD = Chronic obstructive pulmonary disease, HCoV = Human coronavirus.
Of the positive HCoV tests (n = 380), 292 (76.8%) were positive for coronavirus RNA (not specific for any HCoV subtype). Initially, the subtypes of HCoV (N63, 229E, OC43, and HKU1) were not included in the PCR analysis; however, they were integrated from December 27th, 2017. Therefore, a more detailed analysis of the individual subtypes was not possible because of the smaller sample size. The sensitivity of the PCR test did not change throughout the years. Twenty-nine (7.6%) tests were positive for HCoV NL63, 25 (6.6%) were positive for HCoV HKU1, 22 (5.8%) were positive for HCoV 229E, and 12 (3.1%) were positive for HCoV OC43. Seasonal patterns were observed with a peak of HCoV infection during February (figure 4).
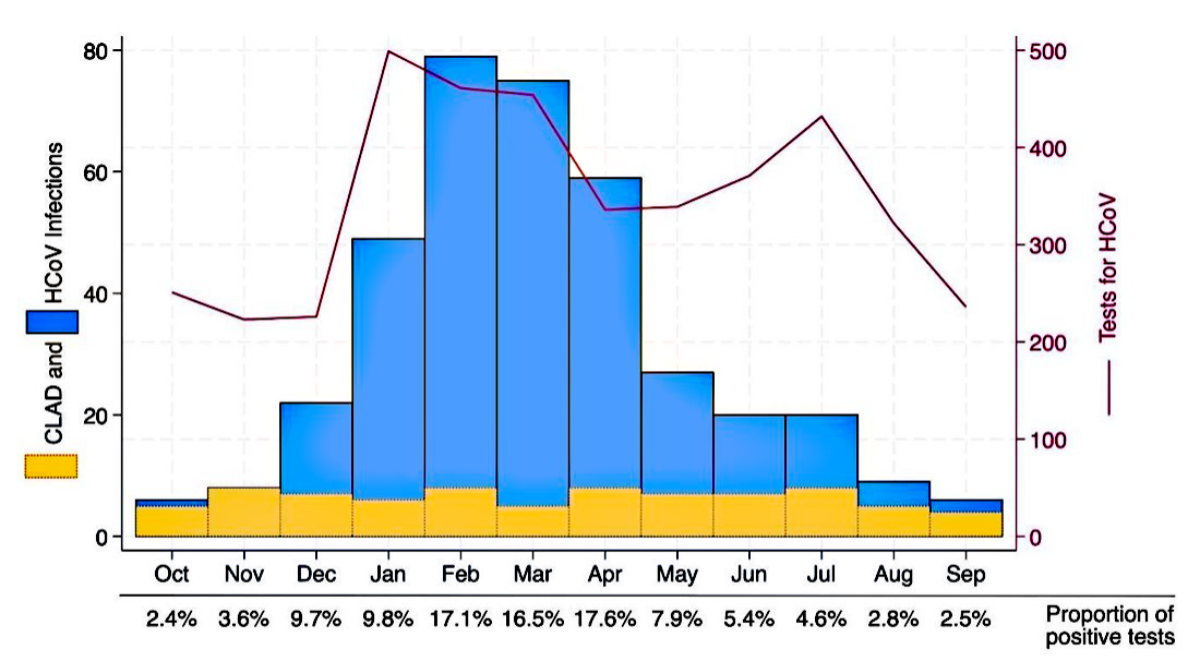
Figure 4Seasonal pattern of human coronavirus (HCoV) infections (n = 380) in lung transplant recipients over 10 years with an overlaid monthly pattern of chronic lung allograft dysfunction (CLAD).
Of all HCoV infections (n = 380), the following symptoms were reported at day zero (multiple symptoms possible): 194 (51.1%) cough or shivering, 129 (33.9%) shortness of breath, 110 (28.9%) increased sputum, 86 (22.6%) malaise, 28 (7.4%) throat pain, 20 (5.3%) running nose, and 16 (4.2%) joint and muscle pain. In 125 (32.9%) patients, an asymptomatic infection was documented at the time of the viral sampling.
According to the data from this 10-year cohort, viral respiratory tract infections caused by HCoV were associated with a relevant increase in developing chronic lung allograft dysfunction during follow-up. We observed that lung transplant recipients are symptomless in around one-third of HCoV infections. Our study shows a clear seasonal HCoV infection pattern, peaking in February.
In a recent study, Allyn et al. [16] highlighted that viral pneumonia, which is characterised by a symptomatic viral infection and a radiographic infiltrate without an apparent alternative cause, can accelerate the development of chronic lung allograft dysfunction (p = 0.001). This aligns with our findings from the Kaplan-Meier failure estimate analysis, which shows that chronic lung allograft dysfunction was more likely to develop among those who had suffered from an HCoV infection. In 2018, Magnusson et al. [13] presented similar data. In addition to nasopharyngeal swabs, bronchoalveolar lavage samples were included, which was not within the scope of the present study. Therefore, we cannot comment on the discovery made by Magnusson et al. [13] regarding the detection of HCoV in the lower respiratory tract. Magnusson et al. detected CoV infections mostly during winter and spring [13], corresponding to our findings of a seasonal pattern with a peak of infection in February (figure 4).
In our study, we investigated HCoV solely using nasopharyngeal swabs. In line with this, Peghinet et al. [18] used PCR testing of nasopharyngeal swabs to diagnose respiratory tract infection in lung transplant recipients [18]. Their results are very similar (HR adjusted 3.00 [95% CI 1.52–5.91]; however, their study investigated HCoV within a larger group of common community-acquired respiratory infections. Therefore, we cannot assess whether HCoV in association with other respiratory viruses has a different effect on the development of chronic lung allograft dysfunction.
Similarly, Fisher et al. [17] published a retrospective study in 2015 with a 1-year follow-up, in which tests for viral respiratory tract infections by PCR, fluorescence microscopy, and cultures were carried out, but only when there were clinical signs of infection and without surveillance testing. In contrast, in our study, asymptomatic patients were retested. Nevertheless, Fisher et al. could correlate the development of chronic lung allograft dysfunction and the length of the post-transplantation period, similar to our study. However, the presence of HCoV was examined only within the group of viral respiratory tract infections and not as a standalone factor and, therefore, the results of our study differ from the aforementioned results. There are already several detailed studies on other respiratory viruses, e.g. respiratory syncytial virus, human metapneumovirus, and parainfluenza virus, by de Zwart [24]. To our knowledge, the current study is the only one to investigate HCoV infections in lung transplant recipients exclusively and to identify it as a potential independent risk factor for chronic lung allograft dysfunction development.
Based on the retrospective design, our findings can only report on this association and generate the hypothesis that HCoV may trigger chronic lung allograft dysfunction. Prospective studies would be required to prove this hypothesis.
We conducted an analysis to track the development of lung function measurements before, during, and after infections. Based on our regression analysis, lung function did not change immediately after infection. In line with our findings, Khalifah et al. [19] presented data in 2004 that suggested respiratory tract infections pose a risk for bronchiolitis obliterans syndrome, which is the most frequent cause of chronic lung allograft dysfunction. For patients in his study with CARV infections, the average time from transplant to viral infection was 304 days and the time from CARV infection to bronchiolitis obliterans syndrome stage 1 was 479 (median 378) days. In our study, the median time to the first HCoV infection was 381 days and the median time to chronic lung allograft dysfunction was 2045 days. The median time from transplantation to infection was similar. Although the time to chronic lung allograft dysfunction was longer in our study, Khalifah et al. state that the development of advanced bronchiolitis obliterans syndrome stages and death were greater in the CARV group, which supports our hypothesis.
A considerable portion of lung transplant recipients (32.9%) did not display any symptoms at the time of HCoV infection. Viral respiratory tract infections caused by HCoV were associated with a statistically relevant increase in developing chronic lung allograft dysfunction in comparison with patients who did not have HCoV infection. That means that lung functions changed over time (as part of the definition of chronic lung allograft dysfunction), even though the lung function decline was not detected shortly after the initial HCoV infection. Therefore, in our opinion, it may be valuable to sample patients more often for HCoVs both for infection control reasons and as a detection method for a possible chronic lung allograft dysfunction development risk factor, which may be amenable to preventative strategies.
There seems to be no correlation between the number of HCoV infections and an increased risk of developing chronic lung allograft dysfunction, which suggests that there is no relationship between the two events, i.e. we did not detect a pattern of increasing risk with an increase in the number of HCoV infections (table 1).
The process by which HCoV causes chronic lung allograft dysfunction is not clear. Studies show, for example, that the strain HCoV-HKU1 has an affinity for type 2 pneumocytes [20] and strain 229E for alveolar macrophages [21]. Both can activate the innate immune system and a T-cell-mediated adaptive immune response [20, 21], which may lead to chronic lung allograft dysfunction without directly affecting FEV1 measurements at the time of infection. This may explain why our study did not observe a significant decline in FEV1 during or immediately after infection.
Another study [12] supports our hypothesis that HCoV infections can eventually lead to chronic lung allograft dysfunction development even if there is no immediate effect on FEV1. Patients with HCoV had significantly higher levels of HCoV antigens and antibodies to non-HLA lung-associated self-antigens detected in their circulating exosomes compared with negative lung transplant recipients. These antigens and antibodies increase humoral and cellular immune responses, which ultimately increase the risk of chronic lung allograft dysfunction over time [12]. Studies have found that the human respiratory virome, which includes various viral species, is present in both healthy and diseased lungs. Coronavirus has been observed to be common in this virome [22] and may be a vital constituent in the immunosuppressed host, but the exact significance is poorly understood.
Despite the epidemiological evidence and the described mechanisms at a molecular level, the specific way in which HCoV infections may lead to chronic lung allograft dysfunction in lung transplant recipients remains unclear. Further prospective studies need to be carried out to elucidate the mechanisms by which HCoV infections potentially trigger or promote chronic lung allograft dysfunction development.
It is important to note that our study has limitations. We used nasopharyngeal swabs for HCoV diagnosis and did not perform bronchoalveolar lavage routinely. Our data also lack follow-up information on clinical symptoms after the initial infection. Additionally, we did not differentiate between single lung transplantation and bilateral transplant recipients in our analysis, although in the observational period at our centre, we almost exclusively performed bilateral transplantations. The sample size was slightly reduced as patients who did not sign general consent were excluded. Owing to our retrospective study design, a detailed analysis of symptomatic versus asymptomatic HCoV infections was not possible. A prospective clinical study with pre-planned testing intervals would be necessary to further clarify this subject.
Due to the complex nature of chronic lung allograft dysfunction development, there were a large number of chronic lung allograft dysfunction cases in the HCoV-negative group. During the observation period, our statistical model assumes that all patients were at a steady risk of developing chronic lung allograft dysfunction, without considering the recorded variables. However, we examined the “proportional hazards assumption” with known risk factors for chronic lung allograft dysfunction and found it to be valid (the global Schoenfeld residual test suggested a global p = 0.204). Future studies may require a larger sample size or more subtype-specific tests, as the number of each HCoV subtype was too small for subgroup analysis regarding chronic lung allograft dysfunction. The strength of our study is that patients were followed up and treated according to predefined and published guidelines of the USZ, and guideline adherence is monitored on a regular basis [3, 14].
Due to our study design, we may not have recorded all of the HCoV infections in the HCoV-negative group, because some patients may have been asymptomatic and may not have been tested, although our threshold to obtain nasopharyngeal swabs for viral pathogens was very low. Therefore, the actual difference between groups regarding chronic lung allograft dysfunction development may have been underestimated, which is a potential bias.
In this 10-year cohort of lung transplant recipients, HCoV infections were associated with a relevantly higher incidence of subsequent chronic lung allograft dysfunction. We conclude that infections with HCoV strains, including 229E, HKU1, NL63, and OC43, may potentially be considered as an independent risk factor for chronic lung allograft dysfunction and thus may limit long-term survival in lung transplant recipients.
The source data and the analytical STATA code that support the findings are available upon reasonable request from the corresponding author. Because this is a retrospective study, no study protocol or registration is available.
Author contribution: L. Canham, and M. Schuurmans had full access to all data in the study and took responsibility for the data integrity and the accuracy of the data analysis. Study concept and design: L. Canham, M. Schuurmans, and T. Gaisl. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: L. Canham and T. Gaisl. Tables and figures: T. Gaisl. Critical revision of the manuscript for intellectual content: all authors. Statistical analysis: T. Gaisl. Administrative, technical, or material support: M. Schuurmans. Study supervision: M. Schuurmans, T. Gaisl.
There was no external financial support for this study.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. RH received fees for lectures, presentations, speakers bureaus, manuscript writing or educational events from Vertex, OM Pharma. TG received consulting fees from Bayer AG. All are not related to this work. No other potential conflict of interest related to the content of this manuscript was disclosed.
1. Scheffert JL, Raza K. Immunosuppression in lung transplantation. J Thorac Dis. 2014 Aug;6(8):1039–53.
2. Thabut G, Mal H. Outcomes after lung transplantation. J Thorac Dis. 2017 Aug;9(8):2684–91. doi: https://doi.org/10.21037/jtd.2017.07.85
3. Schuurmans MM, Benden C, Huber LC. Managing viral respiratory tract infections in lung transplant recipients. Eurasian J Pulmonol. 2015;16(3):144–9. doi: https://doi.org/10.5152/ejp.2014.87597
4. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007 Dec;357(25):2601–14. doi: https://doi.org/10.1056/NEJMra064928
5. Shalhoub S, Husain S. Community-acquired respiratory viral infections in lung transplant recipients. Curr Opin Infect Dis. 2013 Aug;26(4):302–8. doi: https://doi.org/10.1097/QCO.0b013e3283630e85
6. Vu DL, Bridevaux PO, Aubert JD, Soccal PM, Kaiser L. Respiratory viruses in lung transplant recipients: a critical review and pooled analysis of clinical studies. Am J Transplant. 2011 May;11(5):1071–8. doi: https://doi.org/10.1111/j.1600-6143.2011.03490.x
7. Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019 May;38(5):493–503. doi: https://doi.org/10.1016/j.healun.2019.03.009
8. Byrne D, Nador RG, English JC, Yee J, Levy R, Bergeron C, et al. Chronic lung allograft dysfunction: review of CT and pathologic findings. Radiol Cardiothorac Imaging. 2021 Feb;3(1):e200314. doi: https://doi.org/10.1148/ryct.2021200314
9. Liu DX, Liang JQ, Fung TS. Human Coronavirus-229E, -OC43, -NL63, and - HKU1 (Coronaviridae). Encycl Virol. 2020;1-5:428–40. doi: https://doi.org/10.1016/B978-0-12-809633-8.21501-X
10. Munting A, Manuel O. Viral infections in lung transplantation. J Thorac Dis. 2021 Nov;13(11):6673–94. doi: https://doi.org/10.21037/jtd-2021-24
11. Zumla A, Al-Tawfiq JA, Enne VI, Kidd M, Drosten C, Breuer J, et al. Rapid point of care diagnostic tests for viral and bacterial respiratory tract infections—needs, advances, and future prospects. Lancet Infect Dis. 2014 Nov;14(11):1123–35. doi: https://doi.org/10.1016/S1473-3099(14)70827-8
12. Gunasekaran M, Bansal S, Ravichandran R, Sharma M, Perincheri S, Rodriguez F, et al. Respiratory viral infection in lung transplantation induces exosomes that trigger chronic rejection. J Heart Lung Transplant. 2020 Apr;39(4):379–88. doi: https://doi.org/10.1016/j.healun.2019.12.009
13. Magnusson J, Westin J, Andersson LM, Lindh M, Brittain-Long R, Nordén R, et al. Viral respiratory tract infection during the first postoperative year is a risk factor for chronic rejection after lung transplantation. Transplant Direct. 2018 Jul;4(8):e370. doi: https://doi.org/10.1097/TXD.0000000000000808
14. Schuurmans MM, Benden C, Inci I. Practical approach to early postoperative management of lung transplant recipients. Swiss Med Wkly. 2013 Apr;143(4):w13773. doi: https://doi.org/10.4414/smw.2013.13773
15. Elm E Von, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Published online 2007:1453-1457.
16. Allyn PR, Duffy EL, Humphries RM, Injean P, Weigt SS, Saggar R, et al. Graft loss and CLAD onset hastened by viral pneumonia after lung transplantation. Transplantation. 2016 Nov;100(11):2424–31. doi: https://doi.org/10.1097/TP.0000000000001346
17. Fisher CE, Preiksaitis CM, Lease ED, Edelman J, Kirby KA, Leisenring WM, et al. Symptomatic respiratory virus infection and chronic lung allograft dysfunction. Clin Infect Dis. 2016 Feb;62(3):313–9. doi: https://doi.org/10.1093/cid/civ871
18. Peghin M, Los-Arcos I, Hirsch HH, Codina G, Monforte V, Bravo C, et al. Community-acquired respiratory viruses as a risk factor for chronic lung allograft dysfunction. Clin Infect Dis. 2019 Sep;69(7):1192–7. doi: https://doi.org/10.1093/cid/ciy1047
19. Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004 Jul;170(2):181–7. doi: https://doi.org/10.1164/rccm.200310-1359OC
20. Dominguez SR, Travanty EA, Qian Z, Mason RJ. Human coronavirus HKU1 infection of primary human type II alveolar epithelial cells: cytopathic effects and innate immune response. PLoS One. 2013 Jul;8(7):e70129. doi: https://doi.org/10.1371/journal.pone.0070129
21. Funk CJ, Wang J, Ito Y, Travanty EA, Voelker DR, Holmes KV, et al. Infection of human alveolar macrophages by human coronavirus strain 229E. J Gen Virol. 2012 Mar;93(Pt 3):494–503. doi: https://doi.org/10.1099/vir.0.038414-0
22. Mitchell AB, Oliver BG, Glanville AR. Translational aspects of the human respiratory virome. Am J Respir Crit Care Med. 2016 Dec;194(12):1458–64. doi: https://doi.org/10.1164/rccm.201606-1278CI
23. Li X, Luk KH. Human coronaviruses: general features. Reference Module in Biomedical Sciences. 2019 Mar 11:B978-0-12-801238-3.95704-0. doi: https://doi.org/10.1016/B978-0-12-801238-3.95704-0
24. de Zwart A, Riezebos-Brilman A, Lunter G, Vonk J, Glanville AR, Gottlieb J, et al. Respiratory syncytial virus, human metapneumovirus, and parainfluenza virus infections in lung transplant recipients: a systematic review of outcomes and treatment strategies. Clin Infect Dis. 2022 Jul;74(12):2252–60. doi: https://doi.org/10.1093/cid/ciab969
25. Jung EJ, Lee SK, Shin SH, Kim JS, Woo H, Cho EJ, et al. Comparison of nasal swabs, nasopharyngeal swabs, and saliva samples for the detection of SARS-CoV-2 and other respiratory virus infections. Ann Lab Med. 2023 Sep;43(5):434–42. doi: https://doi.org/10.3343/alm.2023.43.5.434