Rational use of immunoglobulins (IVIgs and SCIgs) in secondary antibody deficiencies
DOI: https://doi.org/https://doi.org/10.57187/s.3559
Jeroen S.
Goedea,
Christa K.
Baumannb,
Richard
Cathomasc,
Nina
Khannad,
Jean-François
Lamberte,
Thomas
Lehmannf,
Ulrich J. M.
Meyc,
Jörg
Seebachg,
Urs C.
Steinerh,
Astrid
Tschan-Plessli,
Frank
Stennerj
a Clinic
for Medical Oncology and Haematology, Winterthur Cantonal Hospital, Winterthur,
Switzerland
b Department
of Oncology and Haematology, Lindenhofgruppe (Prolindo), Berne, Switzerland
c Department of Internal Medicine, Medical Oncology and Haematology, Graubünden
Cantonal Hospital, Chur, Switzerland
d Division
of Infectious Diseases and Hospital Epidemiology, Basel University Hospital,
Basel, Switzerland
e Department
of Oncology and Haematology, Nyon Hospital, Nyon, Switzerland
f Clinic
for Medical Oncology and Haematology, St. Gallen Cantonal Hospital, St. Gallen,
Switzerland
g Immunology and Allergology Division, Geneva University Hospital, Geneva,
Switzerland
h Department of Immunology, Zurich University Hospital, Zurich, Switzerland
i Department
of Hematology, Basel University Hospital, Basel, Switzerland
j Department
of Oncology, Basel University Hospital, Basel, Switzerland
Summary
Immunoglobulins for intravenous
use (IVIgs) and subcutaneous use (SCIgs) can prevent recurrent and severe infections
in patients with secondary antibody deficiencies that are frequently linked to haematological/oncological
malignancies as well as other clinical conditions and their respective treatments.
Even so, as IVIgs and SCIgs are costly and their supply is limited, their clinical
use must be optimised. The aim of this position paper is to provide structured practical
guidance on the optimal use of IVIgs and SCIgs in secondary
antibody deficiencies, particularly in haematological and oncological practice.
The authors agree that the occurrence
of severe infections is a prerequisite for the use of IVIgs. Serum IgG levels in
general as well as IgG subclass levels can be additional indicators of whether a
patient could benefit from IVIgs. Responsiveness to vaccines can help to identify
immunodeficiency. Patients with chronic lymphocytic leukaemia or multiple myeloma
who are receiving respective treatment, especially B-cell depletion therapy, but
also some patients with autoimmune diseases are prone to antibody deficiencies and
need IVIgs. For the optimal use of IVIgs and to maximise their potential benefit,
the indication must be individually assessed for each patient. As a primary treatment
goal, the authors define a sufficient prophylaxis of severe infections, which can
be supported by normalising IgG levels. If the initiated treatment is insufficient
or linked to intolerable adverse reactions, switching the product within the class
of IVIgs or changing to a different batch of the same product can be considered.
Pausing treatment can also be considered if there are no infections, which happens
more frequently in summer, but treatment needs to be resumed once infections return.
These structured recommendations
for IVIg treatment in patients with secondary antibody deficiency may provide guidance
for clinical practice and therefore help to allocate IVIgs to those who will benefit
the most, without overusing valuable resources.
Introduction
Immunodeficiencies are characterised by malfunctioning
of the innate and/or adaptive immune system. They are classified into primary immunodeficiency
diseases and secondary immunodeficiency diseases, and are associated with complications
such as infections, autoimmunity and a variety of malignancies. Whereas primary
immunodeficiency diseases are of mono- or polygenetic origin, secondary
immunodeficiency diseases are acquired and may have a variety of causes, including
haematological malignancies, metabolic disorders, infections and medical treatments
[1–3].
Secondary antibody deficiency, a type of secondary
immunodeficiency disease, is often multifactorial in aetiology, related to both
the underlying condition and its treatment. Secondary antibody deficiencies are
estimated to be 30 times more common than primary antibody deficiencies. Moreover,
their prevalence is increasing, not least due to the growing number of novel therapies,
especially the B-cell- and plasma cell-targeting drugs used to treat haematological
malignancies [3]. Secondary antibody deficiencies are most commonly caused by haematological
malignancies, such as chronic lymphocytic leukaemia (CLL), lymphoma and multiple
myeloma, but they can also be associated with other conditions, such as inflammatory
and autoimmune diseases. As severe infections due to secondary antibody
deficiencies can be life-threatening, especially for patients with haematological
malignancies and those on chemotherapy or immunotherapy, optimising the treatment
and management of secondary antibody deficiencies is of broad interest among clinicians.
The diagnosis and the decision on appropriate treatment should always be based on
careful clinical and laboratory risk assessment and must be individualised for each
patient. Current treatment of symptomatic secondary antibody deficiencies includes
a range of interventions and preventive measures, including antibiotic prophylaxis,
non-live vaccines and immunoglobulins for intravenous or subcutaneous use [3].
Immunoglobulin substitution has evolved to become
an important treatment option for secondary antibody deficiencies in the last few
decades, especially for patients with myeloma or chronic lymphocytic leukaemia,
with increasing clinical experience suggesting that many of these patients receiving
immunoglobulin therapy experience fewer and less-severe infections [4–7].
However, the supply of immunoglobulins is limited,
as they are derived from healthy plasma donations and their production is time-consuming,
with multiple steps of fractionation, purification and strict quality control [8].
Until recently, the indication for IVIgs had
formally been restricted to patients with myeloma or chronic lymphocytic
leukaemia with secondary antibody deficiency and recurrent infections, excluding
their wider use in the treatment of immune defects from other causes, especially
drug-induced conditions due to long-term immunosuppressive medication. In 2018,
the European Medicines Agency (EMA) extended the therapeutic indication of IVIgs
to include “SID [secondary immunodeficiency disease] in patients who suffer from
severe or recurrent infections, ineffective antimicrobial treatment and either serum
IgG level of <4 g/l or proven specific antibody failure (PSAF = failure to mount
at least a 2-fold rise in IgG antibody titre to pneumococcal polysaccharide and
polypeptide antigen vaccines)” [9] This label extension has been endorsed by
Swissmedic for various IVIgs, meaning that today many IVIgs are approved and can
be reimbursed for the treatment of secondary antibody deficiencies irrespective
of the underlying cause [10]. The extension of the label is mainly based on evidence
and clinical studies including patients with primary antibody deficiencies [5].
Currently, prospective clinical data from controlled trials regarding the use of
IVIgs in secondary antibody deficiencies are lacking. A blueprint for an intergroup
cooperative effort to address this important clinical shortcoming could be the investigator-initiated
UK trial TEAMM [11].
As clinical experience shows, the use of IVIgs
represents a major opportunity for patients with secondary antibody deficiencies
but the potential side effects, such as infusion reactions, as well as the relatively
high costs and the limited availability must be considered when allocating treatment
and optimising resources. Even so, straightforward guidance on the use of IVIgs
in secondary antibody deficiencies is difficult to find. Experts in immunology,
haematology and oncology from Switzerland therefore met to discuss the diagnosis,
treatment and management of secondary antibody deficiencies, and the appropriate
role of IVIgs. The resulting position paper aims to provide practical guidance to
clinicians from different specialties to optimise the use of IVIgs in secondary
immunodeficiency disease and to suggest clinical situations in which IVIgs can be
paused or stopped. Subcutaneous use of Ig is not explicitly discussed in this position
paper but can be considered within the respective indications listed in table 1.
Table 1Authorised Ig products for immunoglobulin
replacement therapy in Switzerland [32–35]. Note regarding the marketing authorisation
status in Switzerland for
the subcutaneous administration of immunoglobulins: While Hizentra® is approved
for the treatment of secondary immunodeficiency diseases irrespective of the underlying
cause, subcutaneous Cutaquig® and Cuvitru® for secondary
immunodeficiency disease are only indicated in the context of myeloma and chronic
lymphocytic leukaemia [32–35] and subcutaneous Hyqvia® is not indicated for secondary antibody
deficiency [35].
| Drug |
Administration |
Composition |
Swiss market authorisation (regarding secondary
immunodeficiency disease) |
| Privigen® (CSL Behring) |
i.v. |
Human plasma protein, ≥98% IgG; IgG1 69%, IgG2
26%, IgG3 3%, IgG4 2%; Max. 25 µg/ml IgA; anti-A 1:8, anti-B 1:4 |
Indications: severe or recurrent infections,
ineffective antimicrobial treatments and either a proven specific antibody failure
or IgG <4 g/l. Contraindications: IgA-deficiency
with anti-IgA-antibodies; hypogammaglobulinaemia and recurrent bacterial
infections in patients with chronic lymphocytic leukaemia (CLL) in whom
prophylactic treatment with antibiotics has failed or is contraindicated; hypogammaglobulinaemia
and recurrent bacterial infections in patients with multiple myeloma; hypogammaglobulinaemia
in patients before and after allogeneic haematopoietic stem cell transplantation
(HSCT) |
| Octagam® (Octapharma) |
i.v. |
Human plasma protein, ≥95% IgG; IgG1 60%, IgG2
32%, IgG3 7%, IgG4 1%; max. 0.4 µg/ml IgA |
| Intratect® (Biotest) |
i.v. |
Human plasma protein, ≥96% IgG; IgG1 57%, IgG2
37%, IgG3 3%, IgG4 3%; max. 1.8 mg/ml IgA |
| Klovig® (Takeda) |
i.v. |
Human plasma protein, ≥98% IgG; IgG1 ≥56.9%, IgG2
≥26.6%, IgG3 ≥3.4%, IgG4 ≥1.7%; max. ≤140 µg/ml IgA |
| Hizentra® (CSL Behring) |
s.c. |
Human plasma protein, ≥98% IgG; IgG1 69%, IgG2
26%, IgG3 3%, IgG4 2%; max. 50 µg/ml IgA. No information on isoagglutinins |
Indications: severe or recurrent infections,
ineffective antimicrobial treatments and etiher a proven specific antibody failure
or IgG <4 g/l. |
| Cuvitru® (Takeda) |
s.c. |
Human plasma protein, ≥98% IgG; IgG1 ≥56.9%, IgG2
≥26.6%, IgG3 ≥3.4%, IgG4 ≥1.7%; max. 280 µg/ml IgA. No information on
isoagglutinins |
Hypogammaglobulinaemia and recurrent bacterial
infections in patients with chronic lymphocytic leukaemia (CLL) in whom
prophylactic treatment with antibiotics has failed or is contraindicated; hypogammaglobulinaemia
and recurrent bacterial infections in patients with multiple myeloma; hypogammaglobulinaemia
in patients before and after allogeneic haematopoietic stem cell transplantation and
SID |
| HyQvia® (Takeda) |
s.c. |
Human plasma protein, ≥98% IgG; recombinant human
hyaluronidase (rHuPH20), IgG1 ≥56.9%, IgG2 ≥26.6%, IgG3 ≥3.4%, IgG4 ≥1.7% |
Indication: primary immunodeficiency diseases and SID |
Methodological approach
The interdisciplinary group of authors consists
of clinicians from the German- and French-speaking parts of Switzerland. These oncologists,
haematologists and immunologists met to discuss the practical implications of the
extended indication for IVIgs in secondary antibody deficiency and to contribute
to the creation of this paper. The roundtable took place in Zurich on 25 August
2020, with optional virtual attendance via Skype, and was chaired by the first author
(JSG). Participation was based on an invitation to
physicians from the mentioned disciplines with experience in IVIg and SCIg therapy;
we make no claims as to completeness.
The subsequent manuscript, created with the
help of a medical writer (J. Keim, Iaculis GmbH) involved two feedback rounds with
the authors, as well as substantial feedback and critical discussion from two initially
non-participating experts (NK), who contributed to the discussion of the second
manuscript from the infectious disease expert position and ATP, a haematologist,
who contributed the haemolysis paragraph and more detailed product information resulting
in a consensus for the next version. The literature search and further writing
from then on was the sole responsibility of the authors, led by JC and FS. The final
manuscript reflects, to our mind, a representative consensus opinion of the respective
authors on the current practice of IVIg and SCIg use in Switzerland. It does not fulfil
the requirements of formal guidelines but provides structured recommendations
from the authors, based on their clinical experience, on the use of IVIgs to treat
secondary antibody deficiencies.
Identification of patients who can potentially benefit
from IVIgs
To identify
patients who can potentially benefit from IVIgs and to optimise the use of IVIgs
in clinical practice, several parameters, including the occurrence of infections,
serum IgG levels and response to vaccination, can be assessed. The authors suggest
a treatment algorithm that can aid clinical decision-making, which is summarised
in figure 1 and described in more detail below.
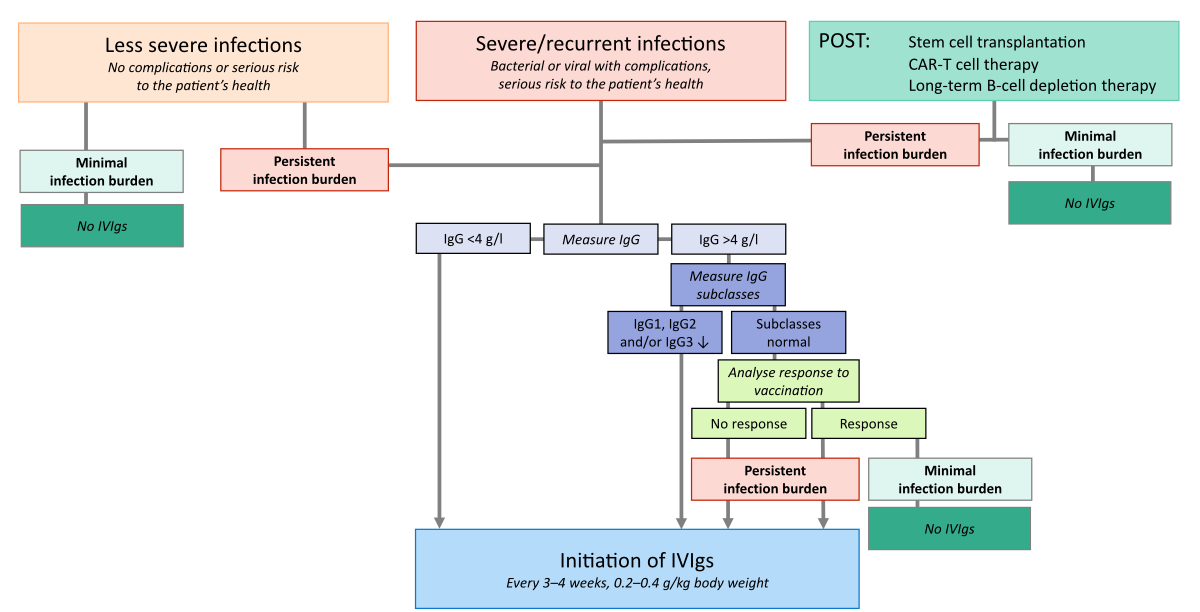
Figure 1
Selecting patients suitable for IVIgs.
A treatment algorithm that can aid clinical decision-making. Severe infections were
those leading to complications or posing a serious risk to the patient’s “health”,
e.g.
infections requiring an acute i.v. intervention, immediate or prolonged hospitalisation,
or emergency intensive care [12]. Recommendations are based on the authors’ clinical
experience.
Infections
The authors agreed that the most obvious and
important reason why patients with secondary antibody deficiencies would potentially
benefit from IVIgs is the presence of recurrent and, most importantly, severe infections
with serious complications. According to a recently published European expert consensus
paper on the topic, recurrent infections can be defined as at least three infections
in a 12-month period despite appropriate anti-infective treatment. Furthermore,
an infection can be regarded as severe if it requires an acute i.v. intervention,
immediate or prolonged hospitalisation, or emergency intensive care [12]. In the recommendations
presented
herein, we decided to use the term severe infections
to refer to infections leading to complications or posing a serious risk to the patient’s
health. In addition to assessing the occurrence of recurrent severe infections,
the authors recommend evaluating the overall clinical status of the patient and
the respective psychological stress when deciding which patients would be good candidates
for IVIgs. Secondary antibody deficiency-related infections can be of bacterial
or viral origin and mainly affect the upper and lower respiratory tract. In patients
with secondary antibody deficiencies, IVIgs are commonly prescribed after two or
more infections or after the first severe infection.
IgG levels
Most importantly, if a patient does not have
an infection, IVIgs are not indicated, irrespective of serum IgG levels. In the
case of recurrent infections, however, serum IgG levels can be an additional marker
indicating whether a patient could benefit from IVIgs. Even though IVIg label specifications
define IgG levels below 4 g/l as the threshold for the use of IVIgs, there are patients
with Ig levels below this threshold who do not show infections and therefore do
not need IVIgs; conversely, there are patients with IgG levels above 4 g/l who have
serious infections. In patients with extremely low IgG levels (<2 g/l), especially
in combination with extremely low IgA levels, initiation of IVIg substitution should
be considered on an individual basis to prevent a first severe and potentially life-threatening
infection. This is in line with the American Society for Transplantation and
Cellular Therapy (ASTCT) consensus recommendations for CAR-T treatment [13].
In patients with normal IgG levels and recurrent
severe infections, determining the levels of the individual IgG subclasses IgG1,
IgG2 and IgG3 can be revealing. IgG subclass deficiency is typically diagnosed when
one or more IgG subclass levels are two standard deviations below the age-adjusted
range in patients with normal total IgG levels [14, 15]. IgG1 and IgG3 production
are induced
by exposure to soluble and membrane protein antigens. IgG2 plays an important role
in the response to bacterial capsular polysaccharide antigens. The clinical significance
of subnormal IgG4 subclass, if any, is unclear. Patients with IgG subclass deficiency
with infections might benefit from IVIgs [16].
Preventive measures
Substitution of Igs in patients with Ig deficiencies
could potentially be prevented or its frequency could be reduced by sufficient preventive
measures. Not least for resource-saving purposes, sufficient vaccination should
be offered to all patients at risk. By analogy with the evidence of patients with
multiple myeloma, who have a minimum NCCN
(National Comprehensive Cancer Network) level of 2b, physicians caring for patients
with Ig deficiencies should promote all indicated immunisations, particularly those
for seasonal influenza viruses. Passive immunisation should be considered in patients
with Ig deficiencies after exposure with hepatitis A, varicella or measles and active
immunisation with varicella zoster vaccine where indicated, regardless of vaccination
status (NCCN level 2b). For best use of vaccinations,
outside the scope of this paper, please refer to Kroger A, et al. (Best
Practice Guidelines for Immunization, https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/intro.pdf,
accessed on 5 May 2024). Alternatively, the recommendations of the Federal
Office of Public Health (FOPH) can serve as a reference in this regard [40].
Prophylactic antibiotic therapy is not recommended
for patients with Ig deficiencies; however, early use of antibiotics in infections
in these vulnerable populations should be considered and ideally discussed with
an expert in infectiology.
Response to vaccination
Bacterial antigens are either proteins or complex
polysaccharides. When evaluating suspected immunodeficiency, responsiveness to vaccines
containing each distinct type of antigen should be assessed separately. Depending
on the immunological defect present, a patient may respond poorly to one or both
types.
The polysaccharide pneumococcal vaccine is usually
used to assess the response to polysaccharide antigens, which requires functional
B-cells only. The response to pneumococcal vaccines can be analysed with two consecutive
standard serology assays. Protein vaccines, such as tetanus, are the most common
vaccines used to evaluate the antibody-mediated response to protein antigens. Responses
to protein antigens require intact B and T cell function. An adequate immune
response is demonstrated if at least a 2-fold rise in IgG antibody titre to pneumococcal
polysaccharide and polypeptide antigen vaccines is measured after four weeks. It
is important to consider that potential co-medication, such as chemotherapy in haematological
conditions, can affect the response to vaccination. However, in the case of continuous
co-medication, the response to vaccination would partially reflect the patient’s
clinical situation.
Pathological conditions and respective treatments that
are typical for patients needing IVIgs
Based on their clinical experience, the authors
have identified patient populations that are more likely to require IVIgs to a certain
extent. Table 2 contains an overview of these treatment groups. Table 1 summarises
the available products for immunoglobulin replacement therapy in Switzerland and their
respective indications.
Table 2Therapies associated with secondary
antibody deficiencies and the resulting potential benefit forIVIgs.
Recommendations by the authors based on clinical evidence. Level of evidence (LoE).
| Treatment |
Mode of action |
Examples |
Potential benefit for IVIgs |
Comments / recommendations by the expert
panel |
| Anti-B-cell monoclonal and bispecific antibodies |
Deplete B-cells or plasma cells* |
Rituximab (a-CD20) |
Often (symptomatic hypogammaglobulinaemia
in patients receiving rituximab that prompts immunoglobulin replacement
therapy is 6.6% LoE 3 [36]; Bispecific antibodies in multiple myeloma hypogammaglobulinaemia
in 34 (87%) patients, immunoglobulin replacement therapy needed in 18 (53%) LoE
3 [37]) |
Depends on the dosage, the treatment duration
and respective concomitant immunosuppression. |
| Belimumab (a-BlyS/BAFF) |
| Blinatumomab (a-CD3/CD19) |
| Glofitamab
(a-CD3/CD20) |
| Daratumomab*
(a-CD38) |
| Teclistamab*
(a-CD3/BCMA) |
| Tyrosine kinase inhibitors |
Inhibit B-cell proliferation and survival |
Imatinib |
Rarely |
|
| Dasatinib |
| Ibrutinib |
| Bosutinib |
| Nilotinib |
| Asciminib |
| BCL-2 inhibitors |
Induce apoptosis of B-cells |
Venetoclax |
Occasionally |
|
| Janus kinase inhibitors |
Modulate cytokine response and proliferation
factors |
Ruxolitinib |
Rarely (increased infectious risk for herpes
zoster 8%, bronchitis 6% and urinary tract infections 6%LoE 3 [22]) |
|
| Purine analogues |
Supress T-cells and B-cells |
Azathioprine |
Rarely |
|
| Fludarabine |
Occasionally |
|
| Cladribine |
| CAR-T |
Suppress B-cells |
Tisagenlecleucel (Kymriah®) |
Kymriah® (Hypogammaglobulinaemia
45% in ALL patients. 15% in DLBCL patients; immunoglobulin replacement
therapy in 19% (DLBCL) [38]) |
|
| Axicabtagen ciloleucel (Yescarta®) |
| Yescarta® (11% hypogammaglobulinaemia
prolonged hypogammaglobulinaemia 6%; immunoglobulin replacement therapy in 16.5%
[39]) |
| Alkylating agents |
B-cell and T-cell death |
Cyclophosphamide |
Rarely |
|
| Chlorambucil |
| Melphalan |
| Anticonvulsants |
Arrested B-cell development |
Carbamazepine |
Rarely |
|
| Valproate |
| Phenytoin |
| Lamotrigine |
| Autologous and allogeneic stem cell transplantation
(SCT) |
Replenishment of blood cells |
|
Occasionally (but routine prophylaxis is not
recommended. LoE 1 [13]) |
More frequent if SCT is due to haematological
malignancies than due to solid tumours. Revaccination after stem cell transplantation. |
| BTKi |
Suppress B-cells |
Ibrutininb |
11.4% serious infections in the 1st
year, 14% of these patients died [21] |
The risk of fungal infections appears to be
increased |
| Acalabrutinib |
|
| Zanubrutinib |
| Pirtobrutinib |
| Other |
|
Clozapine |
Rarely |
Long-term steroid therapy in combination with
other immunosuppressive drugs may be an indication for IVIgs. |
| Steroids |
| Methotrexate |
| Mycophenolate |
| Hydroxychloroquine |
Patients with haematological malignancies
Patients with chronic lymphatic leukaemia (CLL) or multiple myeloma (MM) are at
an increased risk of developing secondary antibody deficiencies and are therefore
more vulnerable to infections [17, 18]. Consequently, IVIgs are frequently indicated
to prevent infections in these malignancies, but their use should be considered
on an individual basis. Over 30 years ago, a study demonstrated that administering
IVIgs to patients with CLL, hypogammaglobulinaemia or a history of infection significantly
lowered bacterial infections over one year compared to those treated with a placebo
[6]. In the more recent German prospective non-interventional SIGNS study, including
307 patients with secondary immunodeficiency disease due to CLL, multiple
myeloma, indolent lymphoma or other malignancies, immunoglobulin treatment was associated
with a decrease in overall infection rates and improved quality of life. Additionally,
IVIgs were reported to have very good tolerability [18]. Besides a reserved use
of immunoglobulin replacement therapy in CLL, the European Society for Medical
Oncology (ESMO) practice guidelines recommend restricting the use of immunosuppressive
agents, e.g. corticosteroids [19].
For patients with multiple myeloma the European
Myeloma Network (EMN) has defined a small subset with an immunoglobulin
replacement therapy indication. This subset encompasses: patients with a high tumour
burden, sepsis with organ dysfunction (neutropenia or renal failure) [20].
Both guidelines (ESMO and EMN) limit immunoglobulin
replacement therapy to patients with serum IgG concentrations below 400 mg/dL and
who have severe and recurrent infections by encapsulated bacteria (or other pathogens
reasonably thought to be due to hypogammaglobulinaemia), despite appropriate antimicrobial
prophylaxis and immunisation (NCCN level 2A) [19, 20].
According to the clinical experience of the
authors, patients with Hodgkin’s lymphoma hardly ever need IVIgs, whereas they are
frequently administered to patients with Burkitt’s lymphoma.
Generally, the type of treatment rather than
the pathological condition itself is considered to affect the severity of immunodeficiencies.
Based on clinical experience, patients who undergo long-term B-cell depletion therapies
often require IVIgs. Some patients with follicular lymphoma who receive rituximab
as a long-term maintenance treatment may be at risk of developing secondary
antibody deficiencies. Additionally, the use of cladribine and fludarabine, or a
combination of fludarabine and rituximab, can lead to significant and lasting secondary
antibody deficiencies.
There is also some, probably non-representative,
clinical experience with patients treated with the tyrosine kinase inhibitor (TKI)
ibrutinib. Even though IgG levels are often low during ibrutinib treatment, most
patients do not develop infections and therefore do not require IVIgs. This could
imply that ibrutinib and other tyrosine kinase inhibitors might be less immunosuppressive
than classic chemo-immunotherapies. However, serious infections have been reported
for ibrutinib [21] and should be considered when treating and monitoring patients
taking Bruton's tyrosine kinase inhibitor (BTKi).
For patients on the BCL2 inhibitor venetoclax,
the clinical experience is limited but may be like that of ibrutinib. It is worth
noting that ruxolitinib, a JAK1/2 inhibitor, displays significant immunosuppressive
capabilities and carries a heightened risk of infections [22]. In summary, patients
under
chronic tyrosine kinase inhibitor treatment should be carefully monitored for infections
and have their Ig levels measured at reasonable intervals.
Based on clinical experience, patients who undergo
CAR-T cell therapy often have low levels of lymphocytes and IgG, yet they do not
frequently experience infections. However, long-term data are so far limited, as
CAR-T cell therapy has only recently become available in clinical practice. It should
therefore be carefully considered in these patients whether the administration of
IVIgs is necessary, and management should be focused on prophylactic treatment,
e.g. with cotrimoxazole, acyclovir or valacyclovir. Furthermore, patients with haematological
malignancies may require IVIgs following autologous or allogeneic stem cell transplantation
[23].
Patients with solid tumours
Patients with solid tumours are generally considered
to need IVIgs less frequently, as they are usually prone to infection only during
chemotherapy and not after the completion of their treatment. Even patients with
testicular cancer who undergo high-dose chemotherapy along with autologous stem
cell transplantation generally have a good recovery rate, and therefore, may not
require IVIgs.
Patients outside of haematology and oncology
Outside of haematology and oncology, there are
conditions in which patients are also prone to develop secondary antibody
deficiencies. The following paragraph intends to give a concise summary of these
conditions, but it does not claim to be comprehensive. In autoimmune disorders,
including vasculitis and collagenosis, the rate of secondary antibody
deficiencies seems to be low in patients who are treated with conventional disease-modifying
drugs such as methotrexate, azathioprine, mycophenolate and hydroxychloroquine.
However, repeated courses of anti-CD20 treatment in combination with glucocorticoid
therapy or other immunosuppression/chemotherapy, older age and pre-existing hypogammaglobulinaemia
are risk factors for developing hypogammaglobulinaemia. In addition, individuals
with autoimmune conditions who take steroids and other immunosuppressive medications
over an extended period are at a higher risk of developing secondary antibody
deficiencies. It is possible that prolonged exposure to low doses may have had an
impact on B-cells and resulted in a deficiency in immunoglobulins. Assessing baseline
serum IgG, IgA and IgM levels and peripheral lymphocyte counts (B-cells) prior to
the initiation of anti-CD20 therapy, during long-term treatment and after treatment
is recommended. It is worth noting that patients who are taking anticonvulsants
may require IVIgs.
Overall, the authors conclude that most patients
who need IVIgs have haematological/oncological or autoimmune diseases and receive
the respective treatments.
Treatment goals and management of IVIgs
To optimise the use of IVIgs, defining treatment
goals as well as adequate therapeutic management concerning the choice of product,
dosage, administration and potential side effects are of great importance. The authors’
recommendations are illustrated in figure 2 and described below.
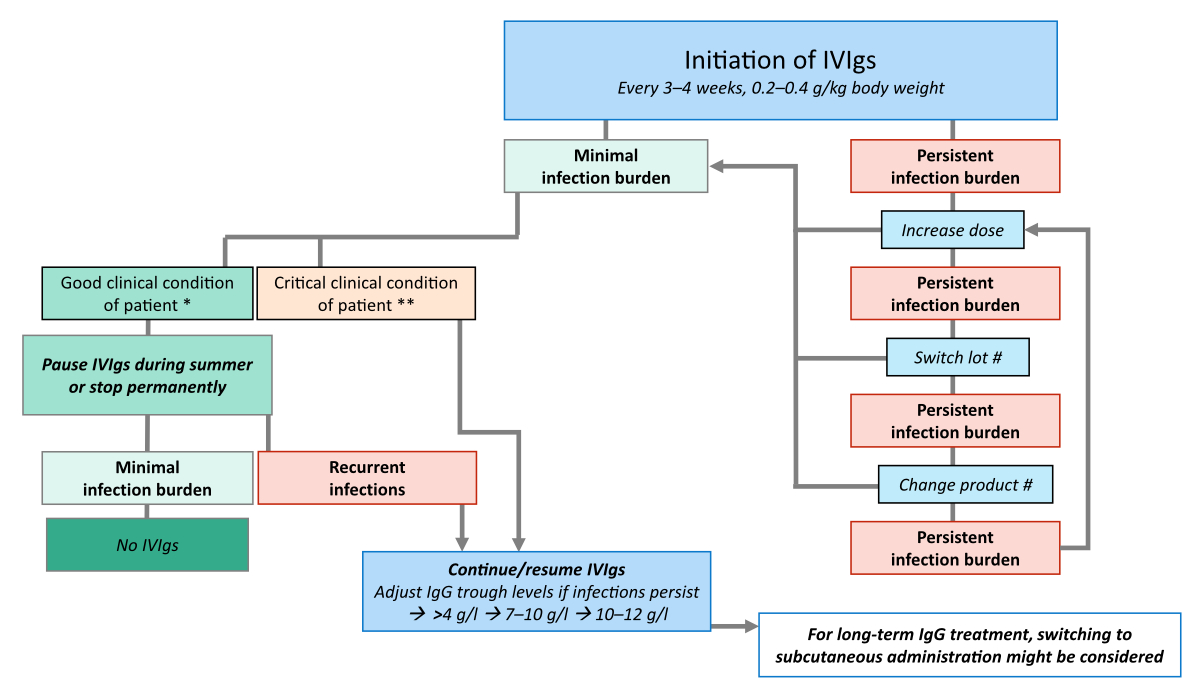
Figure 2
Management of IVIg therapy. Recommendations
are based on the authors’ clinical experience. * e.g. therapy-free and in remission;
** e.g. receiving immunosuppressive therapy and prone to complications; # also in
case of adverse events if lowering the dose/infusion rate or premedication do not
help.
Treatment goals
The primary goal of treating patients with IVIgs
is to prevent infections. Therefore, the dose as well as the administration route
and frequency should be optimised for each patient individually to maintain acceptable
plasma IgG levels and a substantial reduction of infection rate. IgG levels in the
range 7–10 g/l are desirable, lower IgG levels in the range 4–7 g/l or even <4
g/l are acceptable if there are no infections.
Treatment optimisation
If infections still occur with normal IgG levels
(>4 g/l), trough levels of IVIgs can be increased, first to 7–10 g/l and, if
infections persist, to 10–12 g/l. All IVIgs should be considered as individual therapies
and switching from one product to another is an option in situations where inefficacy
or resistance is clinically suspected or likely [24, 25]. Based on their clinical
experience, the authors agree that switching products within the class of IVIgs
and switching to a different batch of the same product can both be considered if
infections continue to occur.
Practical implications of administering IVIgs and managing
adverse reactions
The suggested treatment interval for IVIgs in
secondary antibody deficiency is three to four weeks and the suggested dose is 0.2
to 0.4 g/kg body weight. In general, no premedication is required. Patients who
have adverse reactions to the infusion can be treated with premedication, such as
paracetamol or antihistamines. Steroid treatment is not recommended unless there
is a serious reaction. Furthermore, IVIgs should be administered slowly, and the
infusion should be interrupted if necessary. First administrations can take place
in the ambulatory setting. According to some of the authors, the first treatment
should preferentially be given early in the morning, and the administration rate
should initially be slow. To shorten the duration of the first treatment, administering
a lower starting dose can be considered. If the treatment is well tolerated, dose
and rate can be increased at the next session. However, it must be taken into consideration
that even if the first administration is well tolerated, there is no guarantee that
this will also be the case for subsequent administrations, due to batch-to-batch
variability of IVIgs. In the event of adverse reactions, changing to a different
batch of the same product or changing the product within the IVIg class can also
be considered. If long-term immunoglobulin treatment is needed, switching from intravenous
to subcutaneous administration (subcutaneous Ig can be self-administered by the
patient at home) may be an option, taking the patient’s preferences into consideration.
Clinical situations that require cautious use
of IVIg and potentially repetitive small doses are for example increased blood viscosity,
unstable angina pectoris, renal insufficiency, uncontrolled hypertension and thromboembolic
disorders.
IVIg-associated haemolysis
IVIg administration may result in mild and usually
self-limiting haemolytic reactions. In rare cases, significant haemolysis due to
ABO blood group antibodies (isoagglutinins) contained in the product can occur as
a serious complication of IVIg use and may result in renal and multiorgan failure
and even death.
In clinical trials and observational studies,
the incidence of IVIg-associated haemolysis ranged from 0 to 20% [26].
Factors associated with haemolysis frequency are:
- IVIg doses: higher frequency with
high IVIg doses, i.e. >2 g/kg, as in the context of autoimmune disorders [27],
low incidence
in doses as applied in the context of antibody deficiency (<0.5 g/kg); one trial
reported a frequency of 3.7% [28].
- ABO blood group: higher frequency
for blood groups A and AB, less frequent for blood group B [13, 29].
- IVIg preparation methods: IVIg are
derived from large human plasma pools, evidently comprising donors with very variable
isoagglutinin titres. Most modern Ig manufacturing processes consist of precipitation
and chromatographic steps to separate IgG from albumin and increase IgG purity,
followed by additional purification steps to remove isoagglutinins from the product.
The majority of reported haemolytic events occurred with IVIg products produced
by ethanol‐octanoic acid (OA) fractionation given at a high dose (≥2 g/kg). Only
a few haemolytic events have been reported when products produced by Cohn fractionation,
ethanol‐PEG, immunoaffinity chromatography (IAC) and ethanol‐OA plus IAC were applied
[26, 30, 31].
Patients treated with IVIg should be monitored
for signs of haemolysis and, in case haemolysis occurs, treatment interruption and
product switch should be considered. According to the European Pharmacopoeia, the
anti-A titre in IVIg preparations may at a maximum be 1:64. At the time of writing
this article, anti-A content of the respective products in the vendor’s product
information were for Privigen® 1:8 (1 Oct 2016 – 30 Apr 2019). This was a reduction
from 1:32 (1 Jan 2008 to 31 Dec 2021) by exclusion of donors with high anti-A titres
and a refined isoagglutinin A and B reduction process by immune affinity chromatography
(IAC). Data regarding anti-A/B reduction and titres for Octagam (10%)®, Intratect®
and Kiovig were unavailable [32–35].
Treatment duration, interruption and discontinuation
In the absence of infections, pausing the administration
of IVIgs over the summer months (April–October in the Northern hemisphere) can be
considered. This decision should depend on the individual risk situation of the
patient, which must be carefully and repeatedly assessed. If infections recur, IVIgs
should be reinitiated. In some patients with lymphoma and secondary antibody
deficiencies, B-cells might recover after stopping treatment when the patient is
in long-term remission: in these patients, IVIgs can be stopped.
Discussion and conclusions
IVIgs play a crucial role in assisting patients
with secondary antibody deficiencies by substantially elevating their IgG levels,
reinforcing their immune response, and efficiently averting infections. This is
reflected by the relatively broad indication for the use of IVIgs that has been
implemented by the EMA and endorsed by Swissmedic. The effectiveness of IVIgs in
treating secondary antibody deficiencies varies due to the diverse patient profiles
and the numerous possible underlying causes. The high costs, the limited availability
and an expected increasing worldwide demand for IVIgs derived from the plasma of
healthy donors make optimal use of this symptomatic treatment a necessity. This
necessity became especially evident and important during the global COVID-19 pandemic.
The objective of this paper is to offer practical
guidance to physicians from diverse fields based on the interdisciplinary clinical
experience of the authors with IVIgs.
When making the decision on whether to administer
IVIg treatment to a patient with secondary antibody deficiency, it is crucial to
carefully consider if any severe infections have negatively impacted their overall
health and wellbeing. Patients who are susceptible to infections should be recommended
IVIg treatment. This usually applies to individuals undergoing treatment for haematological/oncological
conditions, occasionally for those with autoimmune disorders and rarely for those
with solid tumours. When making the decision to use IVIgs, it’s important to consider
the levels of both IgG and IgG subclasses. If there are any doubts about whether
there is an indication for IVIgs in a patient, their response to vaccination can
be analysed. When deciding to use IVIgs, it is important to adjust the starting
dose and administration rate based on the individual’s tolerance. If a patient experiences
adverse reactions to an IVIg infusion or if the initially chosen product does not
produce the desired effect, switching to a different IVIg product or batch may be
helpful. For patients with a lower risk of infection, such as those with post-treatment
CLL in long-term remission, stopping IVIgs may be an option, but treatment should
be resumed if recurrent infections occur. The treatment of various secondary
immunodeficiency disease patients is continually improving through clinical experience,
and it is vital to have interdisciplinary discussions to establish the best criteria
for using IVIgs.
Acknowledgments
The authors thank Dr. sc. nat. Jennifer Keim
(Iaculis GmbH) for medical writing support of the first draft.
PD Dr Jeroen S. Goede
Clinic for Medical Oncology and Haematology
Winterthur Cantonal Hospital
CH-8400 Winterthur
Jeroen.Goede[at]ksw.ch
Prof. Dr Frank Stenner
Basel University Hospital
Clinic for Medical Oncology and Haematology
CH-4031 Basel
Frank.Stenner[at]usb.ch
References
1. Na IK, Hensel M, Maschmeyer G, Scheibenbogen C, Wehr C. Daniel Wolff KW. Onkopedia
Leitlinie: Immundefekte sekundär. 2019. Available from: https://www.onkopedia.com/de/onkopedia/guidelines/immundefekte-sekundaer/@@guideline/html/index.html
2. Sánchez-Ramón S, Bermúdez A, González-Granado LI, Rodríguez-Gallego C, Sastre A, Soler-Palacín P;
ID-Signal Onco-Haematology Group. Primary and secondary immunodeficiency diseases
in oncohaematology: warning signs, diagnosis, and management. Front Immunol. 2019 Mar;10(MAR):586.
doi: https://doi.org/10.3389/fimmu.2019.00586
3. Patel SY, Carbone J, Jolles S. The expanding field of secondary antibody deficiency:
Causes, diagnosis, and management. Front Immunol. 2019 Feb;10:33. doi: https://doi.org/10.3389/fimmu.2019.00033
4. Compagno N, Malipiero G, Cinetto F, Agostini C. Immunoglobulin replacement therapy
in secondary hypogammaglobulinemia. Front Immunol. 2014 Dec;5(DEC):626.
5. Vivarelli E, Matucci A, Bormioli S, Parronchi P, Liotta F, Cosmi L, et al. Effectiveness
of low-dose intravenous immunoglobulin therapy in minor primary antibody deficiencies:
A 2-year real-life experience. Clin Exp Immunol. 2021 Sep;205(3):346–53. doi: https://doi.org/10.1111/cei.13629
6. Gale RP, Chapel HM, Bunch C, Rai KR, Foon K, Courter SG, et al.; Cooperative Group
for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia. Intravenous immunoglobulin
for the prevention of infection in chronic lymphocytic leukemia. A randomized, controlled
clinical trial. N Engl J Med. 1988 Oct;319(14):902–7. doi: https://doi.org/10.1056/NEJM198810063191403
7. Lancman G, Parsa K, Kotlarz K, Avery L, Lurie A, Lieberman-Cribbin A, et al. IVIg
Use Associated with Ten-Fold Reduction of Serious Infections in Multiple Myeloma Patients
Treated with Anti-BCMA Bispecific Antibodies. Blood Cancer Discov. 2023 Nov;4(6):440–51.
doi: https://doi.org/10.1158/2643-3230.BCD-23-0049
8. Novaretti MC, Dinardo CL. Immunoglobulin: production, mechanisms of action and formulations.
Rev Bras Hematol Hemoter. 2011;33(5):377–82. doi: https://doi.org/10.5581/1516-8484.20110102
9. European Medicines Agency. Guideline on core SmPC for human normal immunoglobulin
for intravenous administration (IVIg). IVIg) Eur Med Agency Comm Med Prod Hum Use
(CHMP). 2018 Jun;44(June 2018). Available from: http://www.ema.europa.eu/htms/human/qrd/docs/convention.pdf
10. Bundesamt fuer Gesundheit (BAG). Specialty list, state August 2020. 2020 Aug. Available
from: www.spezialitätenliste.ch
11. Drayson MT, Bowcock S, Planche T, Iqbal G, Pratt G, Yong K, et al.; TEAMM Trial Management
Group and Trial Investigators. Levofloxacin prophylaxis in patients with newly diagnosed
myeloma (TEAMM): a multicentre, double-blind, placebo-controlled, randomised, phase
3 trial. Lancet Oncol. 2019 Dec;20(12):1760–72. doi: https://doi.org/10.1016/S1470-2045(19)30506-6
12. Jolles S, Michallet M, Agostini C, Albert MH, Edgar D, Ria R, et al. Treating secondary
antibody deficiency in patients with haematological malignancy: european expert consensus.
Eur J Haematol. 2021 Apr;106(4):439–49. doi: https://doi.org/10.1111/ejh.13580
13. Jain T, Bar M, Kansagra AJ, Chong EA, Hashmi SK, Neelapu SS, et al. Use of Chimeric
Antigen Receptor T Cell Therapy in Clinical Practice for Relapsed/Refractory Aggressive
B Cell Non-Hodgkin Lymphoma: An Expert Panel Opinion from the American Society for
Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019 Dec;25(12):2305–21.
doi: https://doi.org/10.1016/j.bbmt.2019.08.015
14. Abrahamian F, Agrawal S, Gupta S. Immunological and clinical profile of adult patients
with selective immunoglobulin subclass deficiency: response to intravenous immunoglobulin
therapy. Clin Exp Immunol. 2010 Mar;159(3):344–50. doi: https://doi.org/10.1111/j.1365-2249.2009.04062.x
15. Leitao Filho FS, Ra SW, Mattman A, Schellenberg RS, Criner GJ, Woodruff PG, et al.;
Canadian Respiratory Research Network (CRRN). Serum IgG subclass levels and risk of
exacerbations and hospitalizations in patients with COPD. Respir Res. 2018 Feb;19(1):30.
doi: https://doi.org/10.1186/s12931-018-0733-z
16. Umetsu DT, Ambrosino DM, Quinti I, Siber GR, Geha RS. Recurrent sinopulmonary infection
and impaired antibody response to bacterial capsular polysaccharide antigen in children
with selective IgG-subclass deficiency. N Engl J Med. 1985 Nov;313(20):1247–51. doi: https://doi.org/10.1056/NEJM198511143132002
17. Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin
prophylaxis in chronic lymphocytic leukemia and multiple myeloma: systematic review
and meta-analysis. Leuk Lymphoma. 2009 May;50(5):764–72. doi: https://doi.org/10.1080/10428190902856824
18. Reiser M, Borte M, Huscher D, Baumann U, Pittrow D, Sommer C, et al. Management of
patients with malignancies and secondary immunodeficiencies treated with immunoglobulins
in clinical practice: long-term data of the SIGNS study. Eur J Haematol. 2017 Aug;99(2):169–77.
doi: https://doi.org/10.1111/ejh.12900
19. Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al.; ESMO Guidelines
Committee. Electronic address: clinicalguidelines@esmo.org. Chronic lymphocytic leukaemia:
ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
2021 Jan;32(1):23–33. doi: https://doi.org/10.1016/j.annonc.2020.09.019
20. Raje NS, Anaissie E, Kumar SK, Lonial S, Martin T, Gertz MA, et al. Consensus guidelines
and recommendations for infection prevention in multiple myeloma: a report from the
International Myeloma Working Group. Lancet Haematol. 2022 Feb;9(2):e143–61. doi: https://doi.org/10.1016/S2352-3026(21)00283-0
21. Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, et al. Serious Infections
in Patients Receiving Ibrutinib for Treatment of Lymphoid Cancer. Clin Infect Dis.
2018 Aug;67(5):687–92. doi: https://doi.org/10.1093/cid/ciy175
22. Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections:
A systematic review and meta-analysis. Am J Hematol. 2018 Mar;93(3):339–47. doi: https://doi.org/10.1002/ajh.24976
23. Serra Font S, López-Granados L, Sisinni L, Serna Berna JV, Martínez Martínez L, Fernández
de Gamarra-Martínez E, et al. Chronic hypogammaglobulinemia after allogeneic stem
cell transplantation and their treatment with subcutaneous immunoglobulin in pediatric
patients. An Pediatr (Engl Ed). 2022 Aug;97(2):103–11. doi: https://doi.org/10.1016/j.anpede.2021.08.010
24. Gelfand EW. Differences between IGIV products: impact on clinical outcome. Int Immunopharmacol.
2006 Apr;6(4):592–9. doi: https://doi.org/10.1016/j.intimp.2005.11.003
25. Gelfand EW. Critical decisions in selecting an intravenous immunoglobulin product.
J Infus Nurs. 2005;28(6):366–74. doi: https://doi.org/10.1097/00129804-200511000-00003
26. Cuesta H, El Menyawi I, Hubsch A, Hoefferer L, Mielke O, Gabriel S, et al. Incidence
and risk factors for intravenous immunoglobulin-related hemolysis: A systematic review
of clinical trial and real-world populations. Transfusion. 2022 Sep;62(9):1894–907.
doi: https://doi.org/10.1111/trf.17028
27. Nolan BE, Wang Y, Pary PP, Luban NL, Wong EC, Ronis T. High-dose intravenous immunoglobulin
is strongly associated with hemolytic anemia in patients with Kawasaki disease. Transfusion.
2018 Nov;58(11):2564–71. doi: https://doi.org/10.1111/trf.14879
28. Quinti I, Pulvirenti F, Milito C, Granata G, Giovannetti G, La Marra F, et al. Hemolysis
in patients with antibody deficiencies on immunoglobulin replacement treatment. Transfusion.
2015 May;55(5):1067–74. doi: https://doi.org/10.1111/trf.12939
29. Pendergrast J, Armali C, Callum J, Cserti-Gazdewich C, Jiwajee A, Lieberman L, et
al.; QUEST Research Program. A prospective observational study of the incidence, natural
history, and risk factors for intravenous immunoglobulin-mediated hemolysis. Transfusion.
2021 Apr;61(4):1053–63. doi: https://doi.org/10.1111/trf.16232
30. Bellac CL, Hottiger T, Jutzi MP, Bögli-Stuber K, Sänger M, Hanschmann KM, et al. The
role of isoagglutinins in intravenous immunoglobulin-related hemolysis. Transfusion.
2015 Jul;55(S2 Suppl 2):S13–22. doi: https://doi.org/10.1111/trf.13113
31. Wallenhorst C, Patel A, Shebl A, Hubsch A, Simon TL, Martinez C. Anti-A/B isoagglutinin
reduction in an intravenous immunoglobulin product and risk of hemolytic anemia: a
hospital-based cohort study. Transfusion. 2020 Jul;60(7):1381–90. doi: https://doi.org/10.1111/trf.15859
32. Product information. Cuvitru®. 2021 Jun.
33. Product information. Hizentra®. 2022 Oct.
34. Product information. Cutaquiq®. 2022 Mar.
35. Product information. Hyqvia®. 2018 Dec.
36. Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients
receiving rituximab and the use of intravenous immunoglobulin for recurrent infections.
Clin Lymphoma Myeloma Leuk. 2013 Apr;13(2):106–11. doi: https://doi.org/10.1016/j.clml.2012.11.011
37. Sim BZ, Longhitano A, Er J, Harrison SJ, Slavin MA, Teh BW. Infectious complications
of bispecific antibody therapy in patients with multiple myeloma. Blood Cancer J.
2023 Mar;13(1):34. doi: https://doi.org/10.1038/s41408-023-00808-8
38. Ali S, Kjeken R, Niederlaender C, Markey G, Saunders TS, Opsata M, et al. The European
Medicines Agency Review of Kymriah (Tisagenlecleucel) for the Treatment of Acute Lymphoblastic
Leukemia and Diffuse Large B-Cell Lymphoma. Oncologist. 2020 Feb;25(2):e321–7. doi: https://doi.org/10.1634/theoncologist.2019-0233
39. Westin JR, Oluwole OO, Kersten MJ, Miklos DB, Perales MA, Ghobadi A, et al.; ZUMA-7
Investigators; Kite Members. Survival with Axicabtagene Ciloleucel in Large B-Cell
Lymphoma. N Engl J Med. 2023 Jul;389(2):148–57. doi: https://doi.org/10.1056/NEJMoa2301665
40. https://www.bag.admin.ch/dam/bag/de/dokumente/mt/i-und-b/richtlinien-empfehlungen/empfehlungen-risikogruppen-risikosituationen/empfehlungen-onkologie.pdf.download.pdf/BU_20_22_Impfempfehlung_Onkologie_DE.pdf

