a Divisions of Gastroenterology and Hepatology and of Clinical Pathology, University Hospital, Genève, Switzerland
b Swiss HPB (Hepato-Pancreato-Biliary) Center and Department of Gastroenterology and Hepatology, University Hospital Zürich, Switzerland
c Division of Gastroenterology & Hepatology, Cantonal Hospital St. Gallen, Switzerland
d CDA Foundation, Lafayette, Colorado, USA
e Arud Centre for Addiction Medicine, Zurich, Switzerland
f Gastroenterology and Hepatology, Ente Ospedaliero Cantonale, Lugano, Switzerland
g Faculty of Biomedical Sciences, Università della Svizzera Italiana, Lugano, Switzerland
h Private Practice, Pully, Switzerland
i Division of Gastroenterology and Hepatology, Lausanne University Hospital (CHUV) and University of Lausanne, Lausanne, Switzerland
Summary
BACKGROUND AND AIMS: Chronic hepatitis B infection (defined as sustained detection of hepatitis B virus [HBV] surface antigen [HBsAg] protein in serum) is a leading cause of cirrhosis, hepatocellular carcinoma and liver-related deaths. A situation analysis carried out by the Swiss Federal Office of Public Health estimated the HBsAg prevalence in Switzerland to be 0.53% (95% CI: 0.32–0.89%) in 2015 (~44,000 cases). A lower prevalence of chronic HBV in the younger generation and the adoption of universal coverage in the first year of life are expected to decrease the burden of HBV; however, a number of people in key populations (including migrants) remain undiagnosed and untreated, and infected individuals remain at risk of progressing to cirrhosis, hepatocellular carcinoma and death. Our primary objective was to examine the current and estimate the future disease burden of HBV in Switzerland and the impact of migration. The secondary objective was to estimate the impact of changing future treatment numbers.
METHODS: A modelling study was performed using an existing, validated model (PRoGReSs Model) applied to the Swiss context. Model inputs were selected through a literature search and expert consensus. Population data from the Federal Statistical Office were used alongside prevalence data from the Polaris Observatory to estimate the number of HBV infections among people born abroad. The PRoGReSs Model was populated with and calibrated to the available data and what-if scenarios were developed to explore the impact of intervention on the future burden of disease. A Monte Carlo simulation was used to estimate 95% uncertainty intervals (95% UIs).
RESULTS: In 2020, there were an estimated 50,100 (95% UI: 47,500–55,000) HBsAg+ cases among people born abroad. Among people born in Switzerland, there were approximately 62,700 (UI: 58,900–68,400) total HBV infections (0.72% [UI: 0.68–0.79%] prevalence). Prevalence among infants and children under the age of 5 were both <0.1%. By 2030, prevalence of HBV is expected to decrease, although morbidity and mortality will increase. Increasing diagnosis (90%) and treatment (80% of those eligible) to meet the global health sector strategy on viral hepatitis programme targets could prevent 120 cases of hepatocellular carcinoma and 120 liver-related deaths.
CONCLUSIONS: Thanks to the historical vaccination programmes and the continued rollout of universal 3-dose coverage in the first year of life, Switzerland is expected to exceed the global health sector strategy targets for the reduction of incidence. While overall prevalence is decreasing, the current diagnosis and treatment levels remain below global health sector strategy targets.
Introduction
Globally, the hepatitis B virus (HBV) chronically infects approximately 292 million individuals and is a leading cause of cirrhosis, hepatocellular carcinoma and liver-related deaths [1]. Chronic infection is defined as the persistence of hepatitis B surface antigen (HBsAg) in serum for at least 6 months after the acute infection. A situation analysis carried out by the Swiss Federal Office of Public Health estimated the HBsAg prevalence in Switzerland to be 0.53% (95% CI 0.32–0.89%) in 2015 (~44,000 cases) [2, 3]. Although reported cases of acute HBV have declined in the country, more than 80% of reported chronic cases in 2015 were of foreign origin, which suggests that migrants are a key population for efforts to eliminate HBV [4]. The first Swiss Hepatitis Strategy (SHS) was developed in 2014 as a living document process paper, with the goal of eliminating viral hepatitis by 2030 [5]. The targets outlined in the SHS were chosen to eliminate the burden of HBV, including an 80% reduction in chronic infections by 2030 [5]. Similarly, in 2016, the World Health Assembly approved the first global health sector strategy on viral hepatitis, including targets to reduce the burden of HBV by 2030 (95% reduction in incidence; 65% reduction in mortality) through increased diagnosis (90% diagnosed) and increased treatment (80% of eligible patients treated) [6]. This strategy was expanded in 2022 to include absolute targets for the annual number of new HBV infections (2 per 100,000) and deaths from HBV (4 per 100,000) by 2030 [7].
Switzerland has been providing routine vaccination to adolescents since 1998 and in 2019 officially recommended that infants receive the hexavalent vaccine in the first year of life [2, 8]. Studies have shown a lower prevalence of chronic HBV in the younger generation and the adoption of universal coverage in the first year of life will certainly continue to decrease the burden in this group [2, 3]. However, a number of people in key populations (including migrants) remain undiagnosed and untreated, and infected individuals remain at risk of progressing to cirrhosis, hepatocellular carcinoma and death [4]. Current therapies are not curative and, while they have been shown to slow the progression of fibrosis, some treated individuals will still develop hepatocellular carcinoma [9–12]. The primary objective of this study was to examine the current and estimate the future disease burden of HBV in Switzerland and to examine the impact of migration. The secondary objective was to estimate the impacts of changing future treatment numbers.
Materials and methods
A modelling study was performed using an existing, validated model (PRoGReSs Model [1]) applied to the Swiss context. The model is described briefly below and in more detail in Appendix Section 1. Model inputs were selected through a literature search and expert consensus as described briefly below and in more detail in Appendix Section 2. First, a non-systematic literature review was conducted using PubMed and government databases to gather publicly available epidemiological data from published and grey literature. Then two virtual meetings were held with a panel of local clinical and research experts to build consensus on the most accurate input data for the PRoGReSs Model. These experts also provided additional studies and data, which are described in more detail throughout the methodology. The present study analysed previously collected published or unpublished aggregate data so ethical approval was not required. No identifiable information was accessed over the course of the study. As this analysis was conducted on previously collected data, a study protocol was not prepared.
Structure of the HBV transmission and disease burden model (PRoGReSs Model)
Historic Swiss-specific background population, mortality and epidemiological HBV data was used to populate a fully dynamic transmission and Markov disease burden model. This model tracked the distribution of HBsAg across sex, age (1-year age cohorts), year (from 1950‒2050), disease stage (acute, chronic, cirrhosis, decompensated cirrhosis, hepatocellular carcinoma and death) and viral load (categorical). The PRoGReSs model has been previously described in detail [1]. Age- and sex-specific progression rates, including for spontaneous clearance and acute liver failure, were used to advance individuals through the disease stages over time. Horizontal and vertical transmission of disease were estimated considering historical and current prophylactic measures including adolescent immunisation efforts. Swiss-specific historical data inputs (described in the next section) included HBsAg prevalence by age, portion of the infected population with a viral load greater or equal to 20,000 IU/ml, prophylaxis coverage rates by year, annual number of liver transplants, treatment and diagnosis (table 1). After the last year of available data, the number of liver transplants, annual newly diagnosed and prophylaxis coverage were assumed to remain constant. The total number of patients on treatment was assumed to change at the same rate as past treatment. The number of total diagnosed patients was calculated in the model annually considering newly diagnosed and mortality. Prevalence was calculated annually in the model considering incidence and mortality. To estimate the current burden of HBV in Switzerland, the PRoGReSs Model accounted for the impact of treatment and prevention on the transmission and disease burden over time. The PRoGReSs Model was developed in Microsoft Excel (Microsoft Corporation, Redmond, WA, United States) and thus there are no code, software libraries, frameworks or packages used in this publication.
Input parameters
Swiss-specific background data from the United Nation’s Department of Economic and Social Affairs, Population Division was used for population and mortality by sex and 1-year age cohort for every year from 1900–2030 [13]. HBsAg prevalence and prevalence by age and sex were calculated considering country of birth using population data from the Swiss Federal Statistical Office and HBsAg prevalence data from the Polaris Observatory (based on previously published work) [1, 14]. This methodology is described in detail in the next section.
Since 1998, individuals aged 11–15 years have been routinely vaccinated for HBV, with 71% coverage achieved in 2014–2016 by recent estimates [15]. Data regarding the HBV vaccination coverage of 2-year-olds has been available since 2005 (55% of 2-year-olds received at least two doses of vaccine as of the 2014–16 survey); and, in 2019, it was recommended that all children receive hexavalent vaccination in the first year of life [15, 8]. Studies have consistently shown high rates of screening for HBV among pregnant women, with concomitant high levels of administration of a timely birth dose and hepatitis B immunoglobulin to infants born to HBsAg+ mothers [16, 17]. Based on expert feedback, it was estimated that these levels of coverage remained high and that by 2016, 95% of infants born to HBsAg+ mothers had received full vaccination coverage.
The annual number of chronically infected individuals diagnosed was available for 1988–2019. On the basis of historical data, ageing of diagnosed individuals and mortality among them, it was estimated that 31,300 individuals were diagnosed with HBV in 2015 and were still alive (table 1) [18]. After 2015, the annual number of individuals newly diagnosed with HBV in Switzerland was obtained from the Federal Office of Public Health, and included in the model [19]. In 2019, there were 1100 individuals newly diagnosed with HBV in Switzerland (table 1) [19].
Table 1Model inputs.
| Category | Item | Year | Value | Source |
| Disease Burden Model Parameters | HBsAg+ prevalence rate | 2020 | 0.72% (0.32–0.89%) | [14, 1–3] |
| HBsAg+ prevalence – age and sex distribution | 2020 | – | [14, 1, 18] | |
| Viral load ≥20,000 IU/ml | 2017 | 19% | Expert Input | |
| Total diagnosed | 2015 | 31,300 | [18], Expert input | |
| Newly diagnosed | 2019 | 1097 | [19] | |
| Total treated | 2019 | 3050 | [2], Expert input | |
| Historical treatment | 2013–2018 | – | [2], Expert input | |
| Annual liver transplants | 2019 | 168 | [21] | |
| Liver transplants due to HBV | 2015 | 10% | [2] | |
| Timely birth dose coverage (All births) | 2019 | 0% | [25, 8, 15] | |
| HepB 3-dose coverage (All births) | 2019 | 69% | [25, 8, 15] | |
| Timely birth dose coverage (Infants born to HBsAg+ mothers) | 2019 | 99% | [17], Expert Input | |
| HepB 3-dose coverage (Infants born to HBsAg+ mothers) | 2019 | 95% | [17], Expert Input | |
| HBIG coverage (Infants born to HBsAg+ mothers) | 2019 | 100% | [17], Expert Input |
HBsAg+: hepatitis B surface antigen-positive; IU/ml: international units per millilitre; HBV: hepatitis B virus; HepB 3: hepatitis B 3-dose vaccination; HBIG: hepatitis B immune globulin.
Switzerland generally follows the European Association for the Study of Liver (EASL) Clinical Practice Guidelines [20]. These specify that all individuals with a viral load >2000 IU/ml, an alanine transaminase (ALT) level >Upper Limit of Normal (ULN, 40 U/L) and/or at least moderate necroinflammation or fibrosis on histology should be treated [20]. Furthermore, all patients with detectable HBV DNA and cirrhosis should be treated, irrespective of ALT levels. Additionally, all individuals with a viral load >20,000 IU/ml and an ALT ≥2 × ULN should also be treated regardless of the fibrosis stage as may be individuals with an HBsAg+ HBV infection aged over 30 with a viral load ≥2 million IU/ml [20]. Under these criteria, an estimated 21% of the HBV-infected population (n = 13,400 in 2019) would be eligible for treatment, which is in line with expert consensus from the country. From 2013 to 2015, the annual number of individuals on treatment increased from 2000 to 2200 [2]. By 2019, it was estimated that there were 3050 individuals on treatment (23% of an estimated 13,300 eligible [diagnosed or undiagnosed] patients) (table 1); treatment was assumed to have increased linearly between 2015 and 2019.
Annual liver transplantation data was available for 2003–2019 from SwissTransplant (table 1) [21]. Based on the liver transplant waiting list data, it was assumed that 10% of all transplants were due to HBV (table 1) [2].
Estimating HBsAg prevalence by country of birth
Swiss Federal Statistical Office population counts were retrieved by age, sex and country of birth (199 countries) for the resident population (permanent residents and nonpermanent residents) in the year 2020 [14]. Next, modelled HBsAg prevalence (%) in 2020 was retrieved by age, sex and country (166 countries) from the Polaris Observatory based on previously published work [1]. For countries in which models were not available, population data was proportionally allocated to countries with models within the Global Burden of Disease region. To estimate prevalence among foreign-born people, prevalence (%) was multiplied by population (#), by age, sex and country of birth. Finally, HBV infections among foreign-born people were summed across countries by age and sex. The number of infections in the Swiss population was estimated based on Federal Office of Public Health notification data from 2015 which indicate that ~80% of infections were among people born abroad [4].
Scenario development and assessment
After populating and calibrating the PRoGReSs model with the Swiss-specific input data mentioned above, scenarios were developed to examine the future impact of different treatment rates, compared to the current treatment paradigm. Changes to scenarios were modelled to begin in 2022, with the impact measured annually up to 2030. Scenario inputs are described briefly below with annual inputs shown in table 2.
Table 2Annual number diagnosed and total number of patients on treatment, by scenario, 2021–2028.
| Year of input | |||||||
| Scenario input | Scenario | 2021 | 2022 | 2023 | 2024–2025 | 2026–2027 | ≥2028 |
| Annual newly diagnosed | Base | 1100 | 1100 | 1100 | 1100 | 1100 | 1100 |
| Stop Tx | 1100 | – | – | – | – | – | |
| Tx all – Curr Guide | 1100 | 1100 | 1100 | 1500 | 2500 | 2500 | |
| Total on treatment | Base | 3500 | 3700 | 4000 | 4300 | 4900 | 5600 |
| Stop Tx | 3500 | – | – | – | – | – | |
| Tx all – Curr Guide | 3500 | 4000 | 5000 | 6000 | 7000 | 9900 | |
Stop Tx: Stop treatment; Tx all – Curr Guide: treat all patients as per current guidelines.
The base case: The first scenario was designed to measure the future impact on disease burden of maintaining current levels of screening and treatment initiation. In the absence of better information, the estimated annual number of newly diagnosed patients (n = 1100) in 2019 was assumed to remain constant in the future. Historically the total number of patients on treatment has increased by 7% annually; this was assumed to remain constant in the future thus increasing the total treated by 7% per year.
Stop Tx: This scenario examined the impact of stopping all treatment (including patients previously initiated on treatment) starting in 2022.
Tx All – Curr Guide: This scenario examined the impact of maintaining the current guidelines but scaling up diagnosis to identify 90% of all HBV infections, and scaling up treatment to reach 80% of diagnosed and eligible. Diagnosis and treatment increase stepwise to a maximum of 2500 and 9900, respectively, by 2028.
In all scenarios it was assumed that treatment eligibility was between the ages of 15 and 85 and that the efficacy of treatment in achieving sustained viral suppression was 90%. Given the already high vaccination coverage, the current scenarios did not examine the impact of expanding the prevention efforts, assuming a continuation of the 2019 rates into the future.
Uncertainty analysis
The uncertainty interval (UI) around prevalence in 2020 was calculated via a sensitivity analysis using Crystal Ball release 11.1.2.3.500. Ranges around prevalence were defined for each country of origin using β-PERT distributions [22]. A Monte Carlo simulation estimated 95% UIs, with 1000 simulations. The UIs for other outcomes were calculated using low and high range inputs for prevalence, transmission rates, transition rates and mortality rates [1, 23].
Results
HBsAg prevalence by country of birth
In 2020, there were an estimated 50,100 (95% UI: 47,500–55,000) HBsAg+ cases among people born abroad (prevalence of 1.94% [UI: 1.84–2.13%] using a population of 2.6 million people born abroad) or approximately 62,700 (UI: 59,400–68,700) total cases (80% foreign-born, 20% Swiss-born) (prevalence of 0.72% [UI: 0.68–0.79%] using the Swiss population of 8.7 million people). More than 50% of cases born abroad were from Western Europe, Central Europe or North Africa/Middle East. The distribution of cases by country of birth is shown in figure 1, with Turkey, Portugal, Eritrea and the Philippines accounting for more than 2000 cases each (collectively these four countries accounted for approximately 20% of cases born abroad).
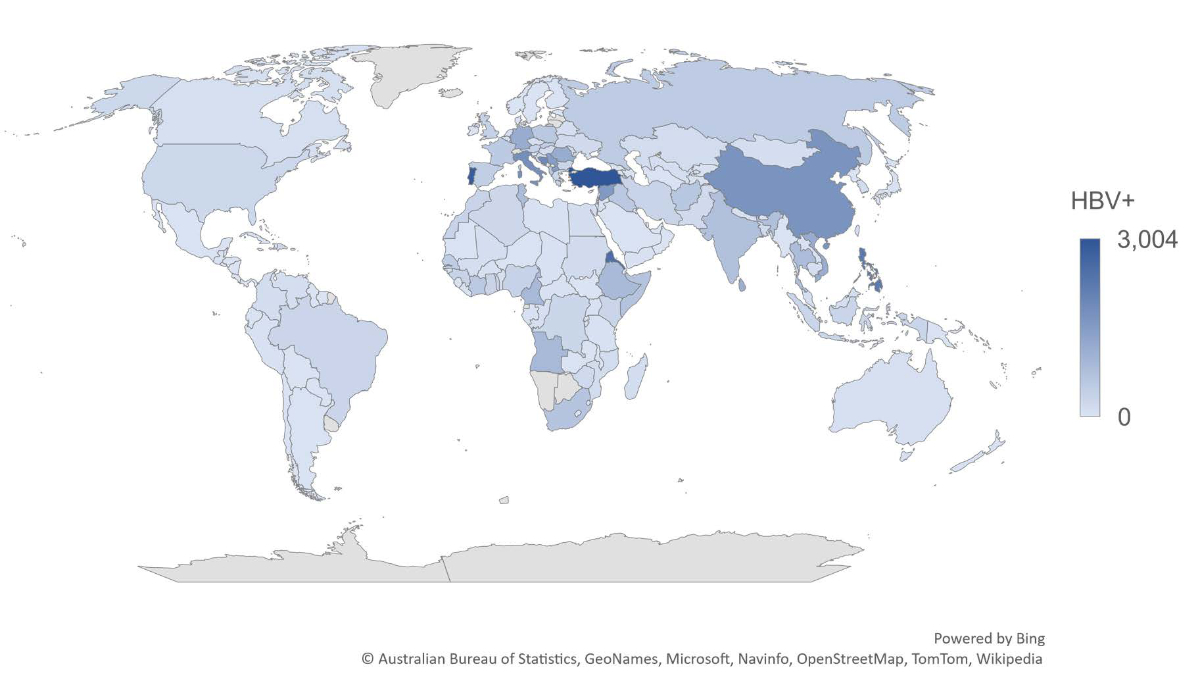
Figure 1Map of foreign-born HBV cases by country of birth (excl. Switzerland) + Swiss Residents, 2020.
The disease burden
The calculated Swiss prevalence (0.72% [0.68–0.79%]) was higher than, but within the range of, previous estimates from the Swiss Federal Office of Public Health situation analysis (0.53% [95% CI: 0.32–0.89%]) [3]. In 2020, this corresponded to 62,700 (UI: 58,900–68,400) HBsAg+ cases (figure 2). Due to the impact of mortality and the effects of prophylactic measures, this figure is expected to decrease to 56,900 (UI: 53,000–62,900) or 0.62% (0.58–0.68%) by 2030 (figure 3). Among the total infected population, we estimated that 61% of cases were between the ages of 35 and 59 (figure 4). In 2020, there were an estimated 48 (UI: 42–58) incident cases of decompensated cirrhosis, 173 (UI: 162–211) incident cases of hepatocellular carcinoma and 198 (UI: 178–241) liver-related deaths. HBsAg+ prevalence was estimated to be <0.1% among both infants and 5-year-olds in 2020. Applying the EASL treatment guidelines to the Swiss infected population, the model estimates that 21.0% (n = 13,400) of all infected individuals would be eligible for treatment.
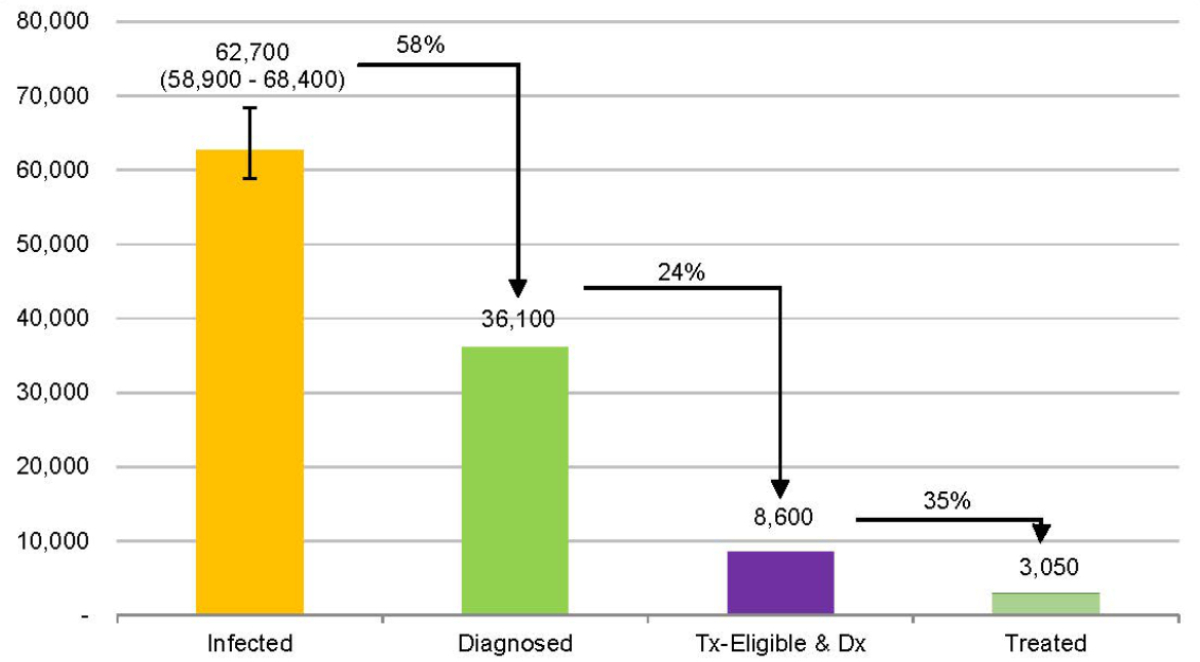
Figure 2HBV Cascade of Care in Switzerland, 2020.
Tx: treatment; Dx: diagnosis.
The base case: If the current treatment and diagnostic levels were continued in the future, the incidence of decompensated cirrhosis would decrease by 8% (from 48 [42–58] in 2020 to 43 [37–54] in 2030), while hepatocellular carcinoma and liver-related deaths would increase, respectively, by 21% (from 173 [162–211] in 2020 to 209 [187–289] in 2030) and 22% (from 198 [178–241] in 2020 to 238 [216–311] in 2030) as the infected population advances in disease state and age between 2020 and 2030.
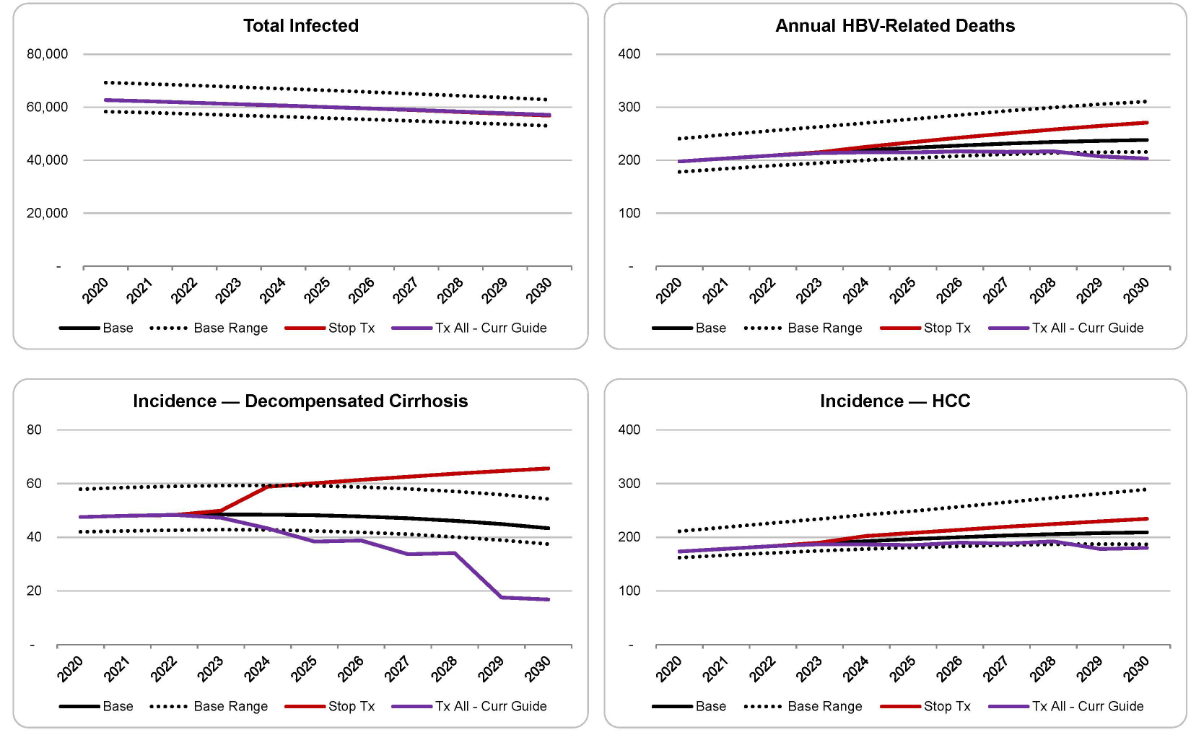
Figure 3HBV morbidity and mortality in Switzerland, 2020–2030.
HCC: hepatocellular carcinoma; Stop Tx: “Stop treatment” scenario; Tx all – Curr Guide: “Treat all patients as per current guidelines” scenario.
Stop Tx: If all treatments were stopped in 2022, there would be an estimated 38% increase in the incidence of decompensated cirrhosis (from 48 [42–58] in 2020 to 66 [58–78] in 2030), a 35% increase in the incidence of hepatocellular carcinoma (from 173 [162–211] in 2020 to 234 [216–266] in 2030) and a 37% increase in liver-related deaths (from 198 [178–241] in 2020 to 271 [242–314] in 2030) by 2030. Compared to the Base Case, this scenario would result in an additional 110 cases of decompensated cirrhosis, 120 cases of hepatocellular carcinoma and an additional 140 lives lost.
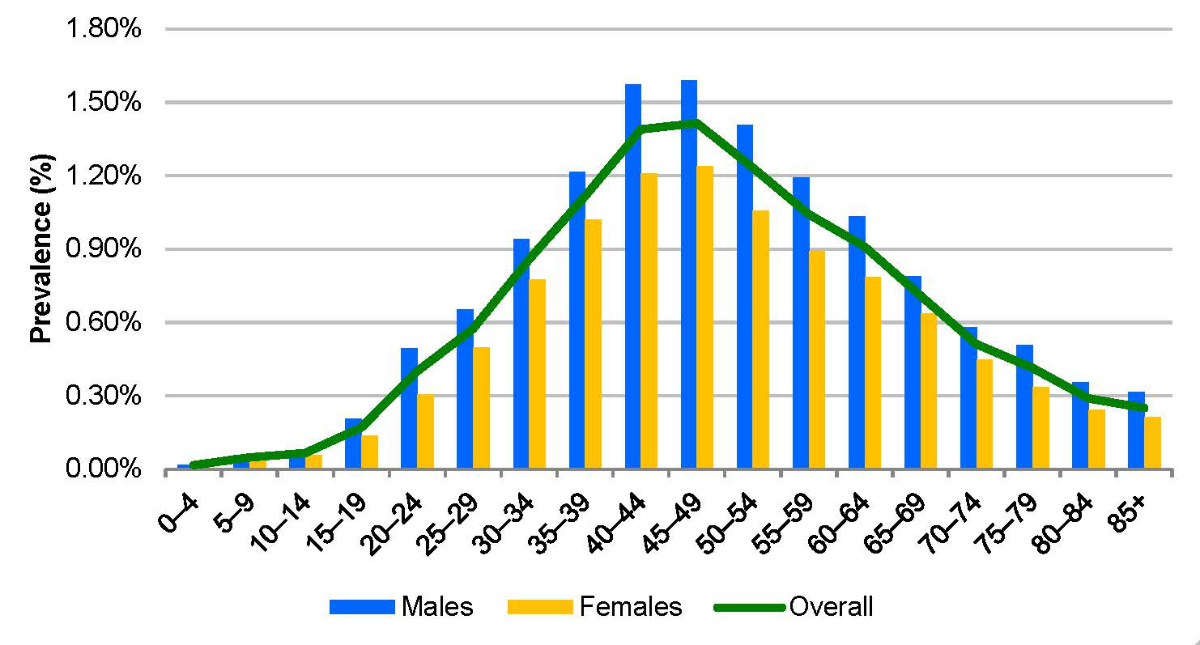
Figure 4HBV prevalence by sex and age group in Switzerland, 2020.
Tx All – Curr Guide: By increasing diagnosis and linkage to care so that 80% of individuals eligible for treatment receive treatment, there would be an estimated 65% reduction in the incidence of decompensated cirrhosis (from 48 [42–58] in 2020 to 17 [11–26] in 2030), a 4% increase in the incidence of hepatocellular carcinoma (from 173 [162–211] in 2020 to 180 [131–353] in 2030) and a 3% increase in liver-related deaths (from 198 [178–241] in 2020 to 203 [163–349] in 2030] by 2030. Compared to the Base Case, this scenario would prevent 100 cases of decompensated cirrhosis, 120 cases of hepatocellular carcinoma and save 120 lives.
Uncertainty analysis
Given that the 95% CI in the Swiss situation analysis (by the Federal Office of Public Health) provided a wider range than was identified in our immigration analysis, a second scenario was developed where we populated the PRoGReSs model with a base prevalence of 0.72% and a range of 0.32% to 0.89%. The outcomes from this scenario are included in Appendix Section 3.
Discussion
Our analysis used a bottom-up approach to estimate the prevalence of HBV in Switzerland, combining census data by age and country of birth with country-level HBV prevalence estimates by age. This resulted in a baseline prevalence estimate that was higher than the Swiss Federal Office of Public Health situation analysis (0.72% vs 0.53%), but within the range of the situation analysis confidence interval (0.32% to 0.89%). The reason for the baseline discrepancy is unknown, but it could indicate that our calculations overestimated the true prevalence or that the situation analysis underestimated the true prevalence. Alternatively, both estimates could be correct for their respective years, although this would suggest that the number of HBV infections in Switzerland has grown by 18,000 people in the past five years. While this is possible, it is unlikely given that global vaccination campaigns have contributed to a declining incidence of HBV.
Thanks to historical vaccination programmes in Switzerland (including adolescent catch-up and infant vaccination campaigns) as well as the continued rollout of universal 3-dose coverage in the first year of life, the incidence of HBV in the country has been declining and an increasing proportion of the population is no longer susceptible to infection. Unfortunately, there is a sizeable population that was infected prior to these campaigns, which remains at risk of HBV-related morbidity and mortality. As a result, the number of incident cases of decompensated cirrhosis, hepatocellular carcinoma and liver-related deaths are expected to increase up to 2030 if no changes are made to the current levels of treatment. The worst-case scenario, which stopped all treatment in 2022, projected a further increase in the cumulative numbers of these indicators. Meanwhile, a scenario to increase diagnosis rates and link all eligible patients to treatment projected that 100 cases of decompensated cirrhosis, 120 cases of hepatocellular carcinoma and 120 liver-related deaths could be prevented.
We nevertheless acknowledge some limitations of the current study. This study took a population-based approach at the national level without explicitly examining subpopulations that may have higher or lower burdens of hepatitis B. A national approach may also overlook heterogeneity in access to prophylaxis or treatment occurring at the canton level. While the model considers the impact of past migration into Switzerland, the future impact of immigration on the future burden of HBV has not been estimated. As it is known that immigrants are a major source of “new” cases in Switzerland, this omission could lead to a more optimistic forecast. The model does not currently consider the impact of hepatitis delta virus (HDV) coinfection. HDV causes faster fibrosis progression and markedly increases hepatocellular carcinoma risk [24]. It requires different treatment than the nucleotide analogues that are used for HBV mono-infected patients. Based on unpublished data, it is estimated that approximately 7.2% of HBsAg-positive Swiss patients are also anti-HDV-positive with 70% of those being HDV RNA-positive. Thus, the base model does not consider the additional morbidity and mortality due to HDV, nor the additional treatment requirements. Although studies have provided evidence that long-term treatment of the currently available therapies can result in the regression of fibrosis, the model assumes that disease progression stops when an individual responds successfully to treatment [10]. This assumption has exceptions within the model to as the transition probabilities of individuals progressing to hepatocellular carcinoma which are reduced but not eliminated by treatment [9–12]. This could result in underestimating the impact of treatment. The current model and scenarios do not consider any possible advances in treatment which may or may not be available by 2030. Uncertainty in input parameters may also affect model outcomes. Wherever possible, the ranges around input parameters have been used to develop the 95% uncertainty intervals; however, there may be other unforeseen sources of uncertainty that have not been captured here.
Based on the current assumptions and outputs of this model, Switzerland is expected to exceed the global health sector strategy targets for the reduction of incidence. Unfortunately, Switzerland is not on track to achieve the other targets, including diagnosing 90% of the population and treating 80% of the eligible population. Greatly increasing the linkage to care under the current guidelines is projected to have large impacts on the future HBV-related morbidity and mortality.
Notes
Funding for this project was provided by Gilead Sciences.
The funders had no role in the study design, data collection, analysis, interpretation of data, or preparation of the manuscript. All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. SB, DRS: Employee – CDA Foundation; Research (Grants to CDA Foundation) – Gilead, AbbVie, Assembly Biosciences, Intercept, Pfizer. HR: Employee – CDA Foundation, Advisory Board – Gilead, AbbVie, VBI Vaccine, Merck, Jansson, Roche; Research (Grants to CDA Foundation) – Gilead, AbbVie, Assembly Biosciences, Intercept, Pfizer. PB: Research, project and travel (Grants to Arud) – Abbvie and Gilead.
References
1. Razavi-Shearer D, Gamkrelidze I, Nguyen MH, Chen DS, Van Damme P, Abbas Z, et al.; Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018 Jun;3(6):383–403.
2. Swiss Federal Office of Public Health. Zahnd C, Brezzi M, Bertisch B, Giudici F, Keizer O [Situation Analysis of Hepatitis B and C in Switzerland]Bern: Federal Office of Public Health; 2017.
3. Brezzi MB, B.; Keiser, O. [Erratum to: Situation Analysis of Hepatitis B and C in Switzerland 23 March 2017]. Geneva2017 19 June 2017.
4. Richard JL, Schaetti C, Basler S, Masserey Spicher V. Reduction of acute hepatitis B through vaccina-tion of adolescents with no decrease in chronic hepatitis B due to immigration in a low ende-micity country. Swiss Med Wkly. 2017 Feb;147(708):w14409.
5. Swiss Hepatitis Strategy 2014-2030. Time to Act Now! Process Paper - A Living Document. http://www.hepatitis-schweiz.ch/files/Dokumente/Process_Paper_2nd_version_final.pdf. 2015.
6. World Health Organization. Global health sector strategy on viral hepatitis 2016–2021, Towards ending viral hepatitis. Geneva, Switzerland: World Health Organization2016 June 2016.
7. World Health Organization. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030. Geneva, Switzerland: World Health Organization; 2022.
8. Federal Office of Public Health and Federal Commission for Vaccination Issues. Swiss Vaccination Plan 20202020.
9. Nguyen MH, Yang HI, Le A, Henry L, Nguyen N, Lee MH, et al. Reduced Incidence of Hepatocellular Carcinoma in Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis B Treated With Tenofovir-A Propensity Score-Matched Study. J Infect Dis. 2019 Jan;219(1):10–8.
10. Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013 Feb;381(9865):468–75.
11. Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, van Boemmel F, et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017 Nov;66(5):1444–53.
12. Buti M, Tsai N, Petersen J, Flisiak R, Gurel S, Krastev Z, et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015 May;60(5):1457–64.
13. World Population Prospects. 2019, Online Edition [database on the Internet]. United Nations, Department of Economic and Social Affairs, Population Division. 2019. Available from: https://population.un.org/wpp/. Accessed: 2019-09-20
14. Swiss Federal Statistical Office. Permanent and non permanent resident population by Year, Canton, Citizenship (selection), Population type, Sex, Age class and Country of birth. Population and Household Statistics. 06/01. 2021 ed. Neuchâtel, Switzerland: Demography and Migration Section; 2021.
15. Bundesamt für Gesundheit (BAG). Vaccination of 2, 8 and 16 year old children in Switzerland, 2014–2016. BAG-Bulletin. 2018;24(11):13–8.
16. Beckers K, Schaad UB, Heininger U. Compliance with antenatal screening for hepatitis B surface antigen carrier status in pregnant women and consecutive procedures in exposed newborns. Eur J Pediatr. 2004 Nov;163(11):654–7.
17. Heininger U, Vaudaux B, Nidecker M, Pfister RE, Posfay-Barbe KM, Bachofner M, et al. Evaluation of the compliance with recommended procedures in newborns exposed to HBsAg-positive mothers: a multicenter collaborative study. Pediatr Infect Dis J. 2010 Mar;29(3):248–50.
18. Federal Office of Public Health. Number of hepatitis B cases reported in Switzerland between 1988 and 2016 by age (mandatory notification of laboratory confirmed cases): FOPH/ID/EPI/RIC2017.
19. Federal Office of Public Health. Swiss mandatory notifications for hepatitis B per canton, FOPH/CD/EPI/RIC2020.
20. Lampertico P, Agarwal K, Berg T, Buti M, Janssen HL, Papatheodoridis G, et al.; European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017 Aug;67(2):370–98.
21. Swiss National Foundation for Organ Donation and Transplantation. Annual Report 2019. 2019. https://www.swisstransplant.org/fileadmin/user_upload/Swisstransplant/Jahresbericht/2019/Swisstransplant-Jahresbericht_2019.pdf
22. Malcolm DG, Roseboom JH, Clark CE, Fazar W. Application of a Technique for Research and Development Program Evaluation. Oper Res. 1959;7(5):646–69. 10.1287/opre.7.5.646
23. Li X, Mukandavire C, Cucunubá ZM, Echeverria Londono S, Abbas K, Clapham HE, et al.; Vaccine Impact Modelling Consortium. Estimating the health impact of vaccination against ten pathogens in 98 low-income and middle-income countries from 2000 to 2030: a modelling study. Lancet. 2021 Jan;397(10272):398–408.
24. Kamal H, Fornes R, Simin J, Stål P, Duberg AS, Brusselaers N, et al. Risk of hepatocellular carcinoma in hepatitis B and D virus co-infected patients: A systematic review and meta-analysis of longitudinal studies. J Viral Hepat. 2021 Oct;28(10):1431–42.
25. WHO/UNICEF. Reported official target population, number of doses administered and official coverage., http://www.who.int/immunization/monitoring_surveillance/data/en/. 2020. http://www.who.int/immunization/monitoring_surveillance/data/en/. Accessed 01 Nov 2020 2020.
Appendix: supplementary data
The appendix is available for download as a separate file at https://doi.org/10.57187/smw.2023.40086.