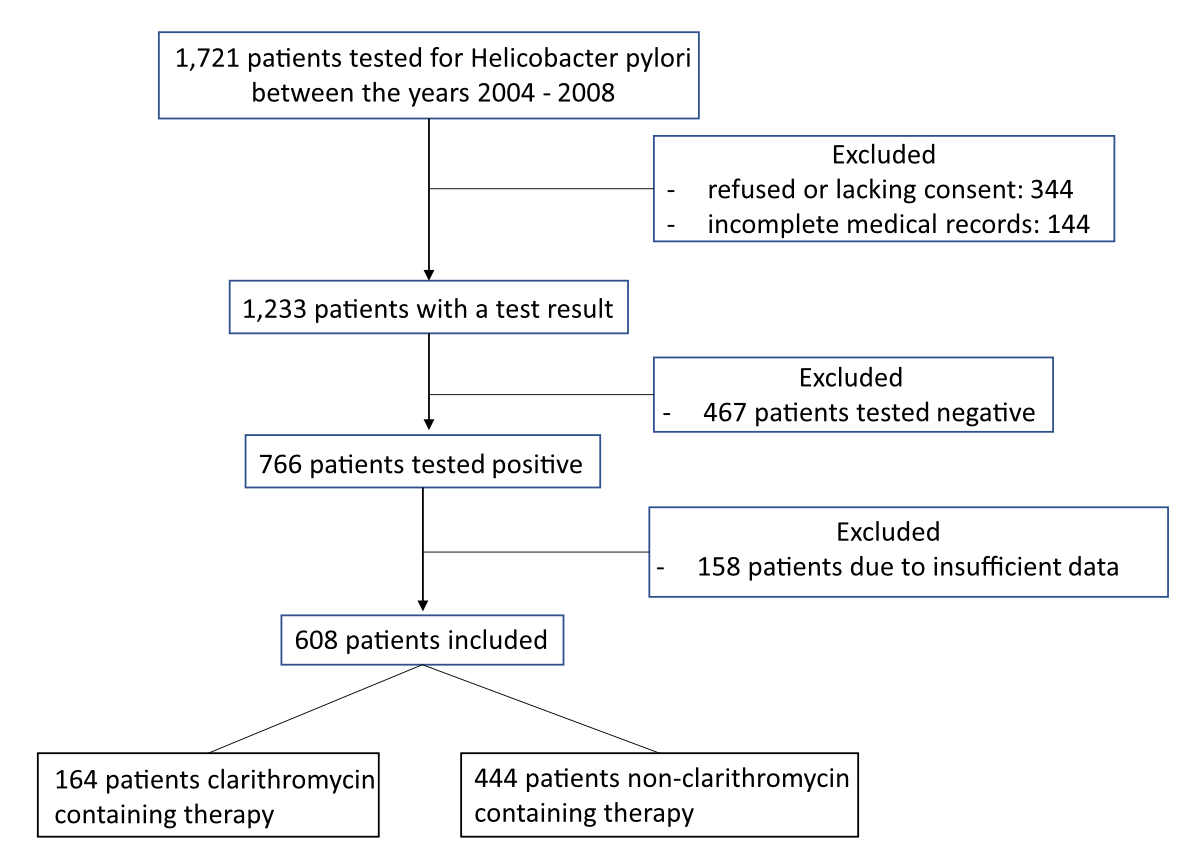
Figure 1 Overview of the total number of patients who were analysed and how many were included or excluded.
DOI: https://doi.org/10.57187/smw.2023.40024
Helicobacter pylori is a Gram-negative bacterium that infects the epithelial lining of the stomach and induces chronic inflammation of the underlying mucosa. It is found in most patients with active chronic gastritis, duodenal ulcer or gastric ulcer [1]. It is a human pathogen, usually contracted at a very young age by – mostly intrafamilial [2] – human to human transmission [3] and via contaminated water supplies [4]. H. pylori is inclined to persist indefinitely, unless treated [5]. Its prevalence varies enormously with socioeconomic status and tends to increase in older age groups, probably owing to cluster effects of children living in poorer conditions in previous decades [5]. It is estimated that in 2015 4.4 billion people globally were infected with H. pylori [6]. Published worldwide prevalence rates of individual countries show that Switzerland had the lowest at merely 18.9% and Nigeria the highest at 87.7% [6]. However, since most people infected with H. pylori are often asymptomatic, screening for H. pylori is not routinely undertaken and the prevalence data reported by various countries have to be interpreted with caution [5].
Circumstances in which to screen patients for H. pylori and the choice of eradication regimen due to antibiotic resistance have become matters of debate [3]. Different eradication regimens have been used over the years: For a long time, the primary first-line eradication therapy was a combination of clarithromycin and a proton pump inhibitor (PPI) with either amoxicillin (French standard triple therapy) or metronidazole (Italian standard triple therapy) for a period of 7 to 10 days [7]. Due to increasing clarithromycin-resistance [7–9], this approach has been shown to be becoming more and more inefficient. According to the Maastricht 2017 consensus report [3], in areas of high clarithromycin resistance (>15%) and low metronidazole-resistance, PPI-amoxicillin-metronidazole triple therapy is recommended. In areas of low dual clarithromycin and metronidazole resistance (<15%), a four-drug regimen such as bismuth quadruple therapy (PPI, bismuth, tetracycline, nitroimidazole/metronidazole) or non-bismuth containing quadruple therapies are recommended. Finally, in areas of high dual clarithromycin and metronidazole resistance (>15%), bismuth-containing quadruple therapies are recommended [3]. Recently reported clarithromycin resistance rates in various European countries were above 20% and metronidazole resistance rates were up to 35% in 2013 [8]. For Switzerland, reliable data about risk factors associated with clarithromycin resistance, prevalence of clarithromycin resistant H. pylori strains and outcome data of H. pylori eradication regimens are scarce.
The goal of our study was to investigate the H. pylori eradication success of clarithromycin- and non-clarithromycin-containing treatments in order to further examine and potentially corroborate changes in antibiotic resistance rates, as well as to investigate how various patient characteristics (e.g., obesity, demographics) impact the performance of different therapeutic schemes. Moreover, we examined which regimens were selected for second-/third-line therapies in recent years.
This retrospective cohort study was approved by the Zurich Ethics Committee (BASEC-Nr. 2018-00665) and was carried out in accordance with principles in the current version of the Declaration of Helsinki, the Essentials of Good Epidemiological Practice issued by Public Health Schweiz (EGEP), the Swiss Law and Swiss regulatory authority’s requirements.
Data of 1721 patients from the department of Gastroenterology and Hepatology were analysed by extensive review of their medical records. A total of 608 patients aged ≥18 years who were treated within our department were included in the final analysis (for details see figure 1). All these patients fulfilled the following criteria: a urea breath test (UBT) between 2004 and 2018 that was positive for H. pylori, and at least one subsequent eradication therapy with eradication confirmed (between 2005 and 2018, at least 4–8 weeks after therapy completion). As can be seen in figure 1, 158 patients were excluded because of insufficient data, meaning they either did not receive an eradication therapy to our knowledge or there were not enough data about the therapy they received, or there was no confirmation of eradication. The urea breath test was preferred for checking eradication due to its high diagnostic sensitivity (96%) and specificity (93%) [10], as well as its convenience. If the first-line treatment was not successful, most patients were treated with a second-line, and some subsequently with a third-line regimen.

Figure 1 Overview of the total number of patients who were analysed and how many were included or excluded.
We collected from patients’ medical files information about age, gender, origin (place of birth), job description, cardiovascular risk factors, chronic medical conditions, previous antibiotic use, gastrointestinal symptoms, biopsy and endoscopy findings from the gastrointestinal tract, and all data related to H. pylori and its eradication. However, incomplete information in any of these areas was not a reason for exclusion, as long as they met all criteria mentioned above.
The main endpoint was the success rate of the different H. pylori eradication regimens, both clarithromycin- and non-clarithromycin-containing. Successful H. pylori eradication was defined as a negative UBT at least 2 weeks after PPI discontinuation and at least 4 weeks after antibiotic discontinuation, in accordance with the Maastricht 2017 consensus report [3] and British Society of Gastroenterology (BSG) guidelines. Secondary endpoints were the influence of various factors including therapy duration, demographics, and potential risk factors such as age, gender, and body mass index (BMI) [11, 12]. Therapy duration was either 7 to 10, or 14 days. For demographics, we assessed the patients’ place of birth/origin and classified it into two categories: Europe versus Middle East and other countries (South America, Africa, Asia, East [Russia, Ukraine, Armenia, Azerbaijan]). In addition, we categorised patients according to their job description as “academic” (e.g., lawyer, medical doctor, professor, architect, banker, fund-manager, consultant, IT specialist, student, engineer, teacher, business/asset manager, fiduciary, etc.) or “non-academic” (e.g., cleaner, secretary, construction worker, painter, farmer, electrician, salesperson, etc.). All secondary endpoints were analysed only in relation to their first-line therapy regimen.
We assorted the various therapy regimens into seven main categories:
Additionally, we categorised them into clarithromycin-containing [1, 2, 6] vs non-clarithromycin-containing [3–5, 7] therapies. We examined which therapies were administered in a second eradication attempt in relation to the various first-line therapies and whether certain therapies were more successful than others.
Groups were compared using contingency tables and chi-square tests for categorical variables. For continuous variables with non-normal distribution, Wilcoxon rank sum tests were used. A p-value <0.05 was regarded as statistically significant. Additionally, a logistic regression analysis was performed. To minimise risk of bias, overfitting and optimism no data-driven selection of variables was applied. On the basis of clinical relevance and availability in the dataset, we added prespecified predictors to the model. Predictors included “therapy regimen” (“medication”, “duration”), “age”, “gender”, “bmi”, “origin” and “profession”. All analyses were performed with R software, version 3.5.2 (2018-12-2).
Research where humans or their data is involved require an official government permit in Switzerland. We strictly obeyed all requirements; data analysis and manuscript preparation were planned in advance and in accordance with the Swiss Ethic Commission in Zurich. This study was reviewed and approved by the official Zurich Ethics Committee, Switzerland (BASEC-Nr. 2018-00665). In accordance with the Zurich Ethics Committee anonymised data from patients between 2005 and 2015 were included – even if the patients did not sign a written consent form – unless they explicitly declined the use of their data for research purposes. All patients included since 2016 have given their written informed consent.
Data of 608patients (57% female, median age 45 years; details in table 1) were analysed.
Table 1Baseline clinical characteristics.
| Overall (n = 608) | With clarithromycin | Without clarithromycin | |
| Age (years), median (range) | 45 (18–90) | 45 (18–84) | 44 (18–90) |
| Gender female, n (%) | 346 (57%) | 94 (57%) | 252 (57%) |
| BMI (kg/m2), median (range) | 29 (16–77) | 28 (17–77) | 29 (16–73) |
| Origin, Europe1, n (%) | 348 (65%) | 101 (67%) | 247 (64%) |
| Academic2, n (%) | 91 (16%) | 33 (21%) | 58 (14%) |
| Therapy duration [7–10, 14 days], n | [491, 113] | [105, 56] | [386, 57] |
BMI: body mass index
1 Origin / place of birth was classified into the following categories: Europe vs middle east and other countries (South America, Africa, Asia, East [Russia, Ukraine, Armenia, Azerbaijan])
2 Academic includes, for example, lawyer, medical doctor, professor, architect, banker.
Successful H. pylori eradication was confirmed by UBT in 94.2%, by biopsy in 5.3% and by stool antigen test in 0.5%.
The most common first-line therapy was PPI-amoxicillin-metronidazole in 432 (71%) of all cases during the analysed period between 2004 and 2018, followed by PPI-amoxicillin-clarithromycin in 126 (21%) and PPI-metronidazole-clarithromycin in 36 (6%). The distribution of different first-line therapies for each year is shown in figure 2.
There were 164 (27%) clarithromycin-containing and 444 (73%) non-clarithromycin-containing first-line therapies.
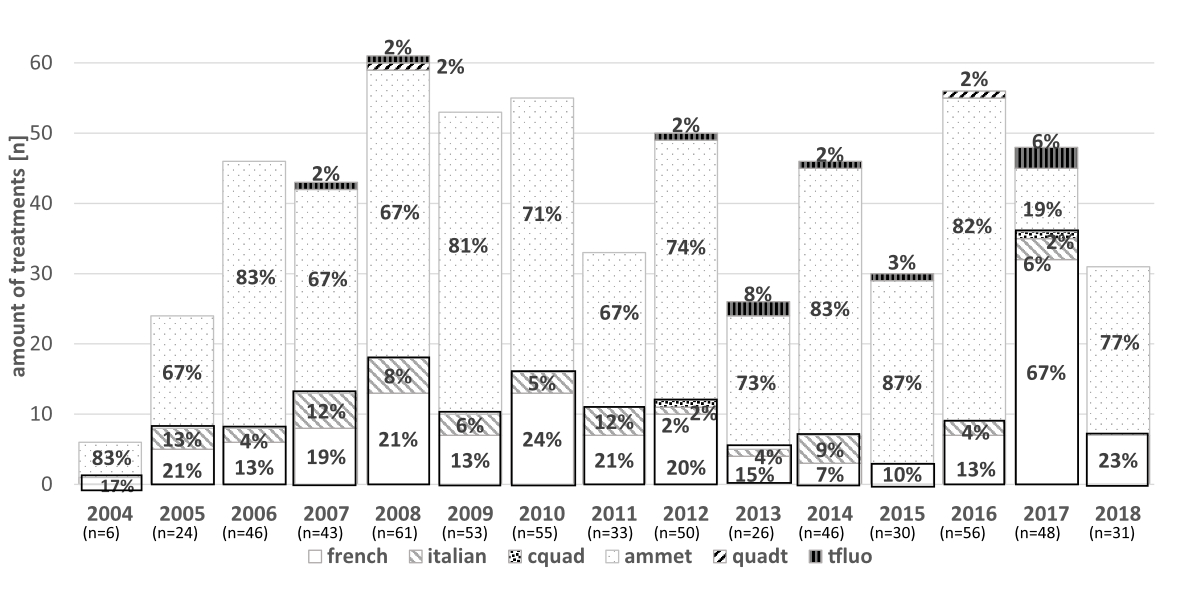
Figure 2 Different first-line Helicobacter pylori eradication regimens in over the years.
french: PPI-amoxicillin-clarithromycin*; italian: PPI-metronidazole-clarithromycin*; cquad: PPI-clarithromycin-amoxicillin-metronidazole*; ammet: PPI-amoxicillin-metronidazole**; quadt: PPI-tetracycline-metronidazole-bismuth**; tfluo: PPI-amoxicillin-levofloxacin/moxifloxacin** (* containing clarithromycin / ** not containing clarithromycin).
The rate of overall H. pylori eradication success was 71%. There was no difference in H. pylori eradication success between clarithromycin- and non-clarithromycin-containing therapies (p = 0.764; table 2).
Table 2Univariate analyses of clinical factors related to Helicobacter pylori eradication success (smaller OR meaning less H. pylori after eradication therapy i.e., better eradication success).
| Univariate analyses | OR | 95% CI | p-value | ||
| 2.5% | 97.5% | ||||
| Clarithromycin vs non-clarithromycin | 0.96 | 0.64 | 1.42 | 0.839 | |
| Age (per 1 year) | 0.99 | 0.98 | 1.00 | 0.338 | |
| Gender (female vs male) | 0.87 | 0.61 | 1.24 | 0.440 | |
| BMI (per kg/m2) | 0.98 | 0.96 | 1.00 | 0.060 | |
| Year of eradication (per 3 years) | 2004–2006 | 0.43 | 0.26 | 0.70 | <0.001 |
| 2007–2009 | 0.66 | 0.36 | 1.23 | 0.189 | |
| 2010–2012 | 1.04 | 0.57 | 1.93 | 0.892 | |
| 2013–2015 | 1.42 | 0.76 | 2.71 | 0.270 | |
| 2016–2018 | 0.90 | 0.49 | 1.69 | 0.745 | |
| Origin (Europe vs others)1 | 0.56 | 0.39 | 0.82 | 0.003 | |
| Academic vs non-academic2 | 0.55 | 0.31 | 0.93 | 0.033 | |
| Duration (14 days vs 7–10 days) | 0.89 | 0.56 | 1.401 | 0.628 | |
BMI: body mass index; CI: confidence interval; OR: odds ratio
1 Origin / place of birth was classified into the following categories: Europe vs middle east and other countries (South America, Africa, Asia, East [Russia, Ukraine, Armenia, Azerbaijan])
2 Academic includes, for example, lawyer, medical doctor, professor, architect, banker.
For details of eradication success over the years and according to clarithromycin vs non-clarithromycin see figure 3.

Figure 3 Success rate after different eradication regimens for Helicobacter pylori.
(A) Overall success rate over the years after first eradication therapy.
(B) Eradication success after first-line therapies (2004–2018) categorised into clarithromycin-containing (french, italian, cquad) and non-clarithromycin-containing (ammet, quadt, tfluo).
Hp: Helicobacter pylori; french: PPI-amoxicillin-clarithromycin*; italian:PPI-metronidazole-clarithromycin*; cquad: PPI-clarithromycin-amoxicillin-metronidazole*; ammet: PPI-amoxicillin-metronidazole**; quadt: PPI-tetracycline-metronidazole-bismuth**; tfluo: PPI-amoxicillin-levofloxacin/moxifloxacin** (* containing clarithromycin / ** not containing clarithromycin)
There was no difference in eradication success between 14 and 7–10 days of therapy in first-line treatments (p = 0.430). We found that the eradication success was higher in patients of European origin than Middle Eastern and other countries (odds ratio [OR] 0.48, 95% confidence interval [CI] 0.30–0.78). No difference could be found according to gender or BMI. For details see tables 2 and 3.
Table 3Multivariate analyses of clinical factors related to Helicobacter pylori eradication success (smaller OR meaning less H. pylori after eradication therapy, i.e., better eradication success).
| Multivariate analyses | OR | 95% CI | p-value | |
| 2.5% | 97.5% | |||
| Clarithromycin vs non-clarithromycin | 0.92 | 4 | 1.56 | 0.764 |
| Age (per 1 year) | 0.99 | 0.98 | 1.01 | 0.628 |
| Gender (female vs male) | 1.01 | 0.63 | 1.62 | 0.973 |
| BMI (per kg/m2) | 0.98 | 0.96 | 1.01 | 0.133 |
| Origin (Europe vs others)1 | 0.48 | 0.30 | 0.78 | 0.003 |
| Academic vs non-academic2 | 0.56 | 0.26 | 1.11 | 0.110 |
| Therapy duration (14 days vs 7–10 days) | 0.77 | 0.40 | 1.44 | 0.430 |
BMI: body mass index; CI: confidence interval; OR: odds ratio
1 Origin / place of birth was classified into the following categories: Europe vs middle east and other countries (South America, Africa, Asia, East [Russia, Ukraine, Armenia, Azerbaijan])
2 Academic includes, for example, lawyer, medical doctor, professor, architect, banker.
Second line therapies were used in 130 patients. The most common was PPI-amoxicillin-metronidazole in 43 (33%) of all cases, followed by PPI-amoxicillin-levofloxacin/moxifloxacin in 41 (32%), PPI-amoxicillin-clarithromycin in 31 (24%), PPI-metronidazole-clarithromycin in 10 (8%), PPI-tetracycline-metronidazole-bismuth in 3 (2%) and PPI-clarithromycin-amoxicillin-metronidazole in 2 (2%) cases. The distribution of different second-line therapies for each year can be seen in figure 4.
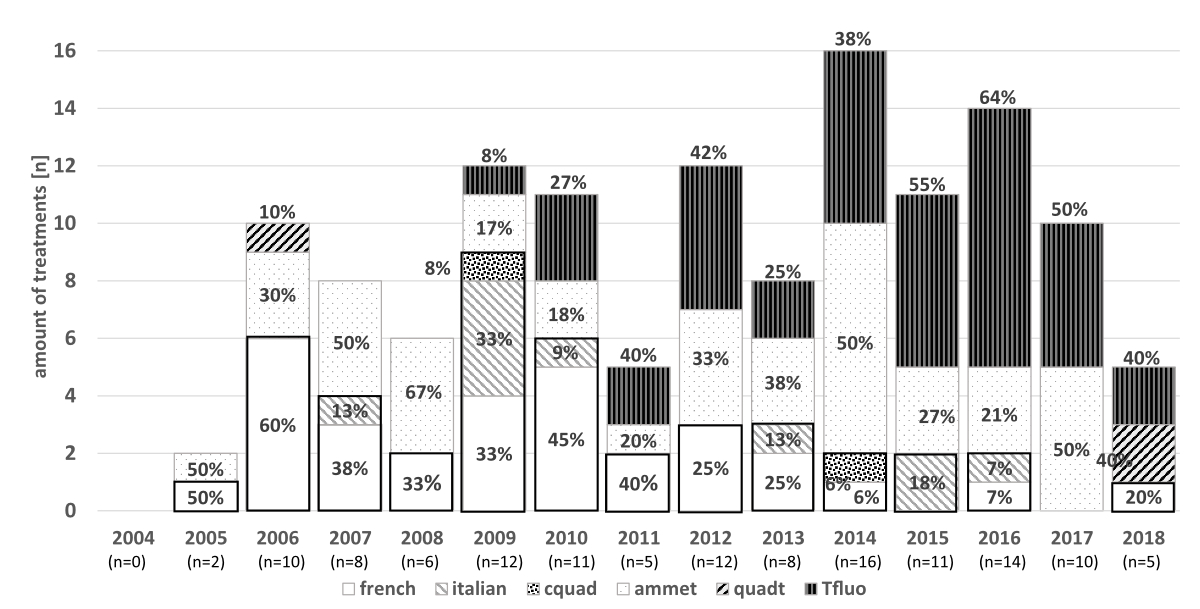
Figure 4 Different second-line Helicobacter pylori eradication therapy regimens over the years.
french: PPI-amoxicillin-clarithromycin*; italian:PPI-metronidazole-clarithromycin*; cquad:PPI-clarithromycin-amoxicillin-metronidazole*; ammet:PPI-amoxicillin-metronidazole**; quadt:PPI-tetracycline-metronidazole-bismuth**; tfluo: PPI-amoxicillin-levofloxacin/moxifloxacin**(* containing clarithromycin / ** not containing clarithromycin)
There were 43 (33%) clarithromycin-containing and 87 (67%) non-clarithromycin-containing second line therapies. The overall rate of H. pylori eradication success was 63%. There was no difference in H. pylori eradication success between clarithromycin-containing vs non-clarithromycin-containing therapies (p = 0.764; fig. 5A.).
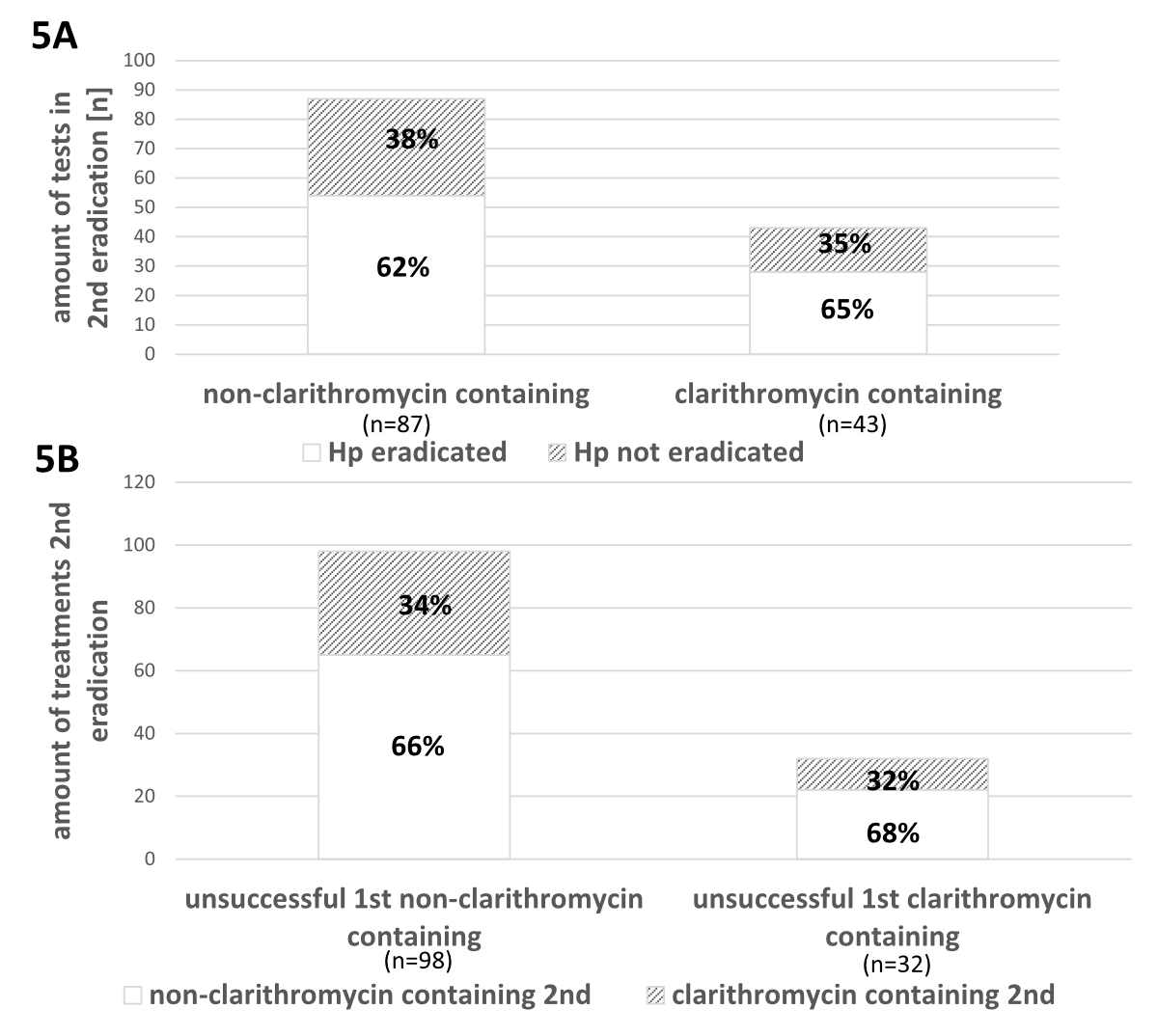
Figure 5 Second-line Helicobacter pylori eradication therapies and their success (between the years 2004 and 2018). 5A: Eradication success after second-line therapies containing clarithromycin- vs non-clarithromycin-containing regimens. 5B: Second-line eradication therapies after different failed first-line regimens.
Hp: Helicobacter pylori
In figure 5B the distribution of second-line therapies containing clarithromycin or not after failed first-line therapy regimens with or without clarithromycin is shown. Their success is shown in the supplementary figure in the appendix.
There was no difference between different durations of second-line therapies.
Overall, third-line therapies (n = 28) were 29% PPI-amoxicillin-clarithromycin (n = 8, eradication success 50%), 18% PPI-amoxicillin-metronidazole (n = 5, eradication success 60%), 43% PPI-amoxicillin-levofloxacin/moxifloxacin (n = 12, eradication success 50%), 4% PPI-metronidazole-clarithromycin (n = 1, eradication success 100%), and 7% PPI-tetracycline-metronidazole-bismuth (n = 2, eradication success 50%). There were 9 (32%) clarithromycin-containing therapies and 19 (68%) non-clarithromycin containing regimens. In the third eradication attempt, the mean eradication rate was 52%.
The relative use of pantoprazole in relation to other PPIs increased from 0% in 2004 up to 61% in 2018. The relative use of esomeprazole decreased from 83% in 2004 to 39% in 2018. Omeprazole was used in 4% overall.
In this retrospective cohort study including 608 patients from a tertiary referral centre we assessed the success rates of different H. pylori eradication regimens, in particular clarithromycin- vs non-clarithromycin-containing first-line therapy. The overall H. pylori eradication success rate was 71%. In contrast to the reported increases in clarithromycin resistance, there was no difference between clarithromycin- and non-clarithromycin-containing therapies. Overall, the average eradication rate was 71% with clarithromycin-based therapies and 71% in non-clarithromycin-based therapies, which is in line with previous studies. In 2007, Chey et al. published H. pylori eradication rates with clarithromycin-based therapy of 70–85% in the US [13]. Similar results were published by Jafri et al. in a meta-analysis of 10 randomised trials in Italy (eradication rate 77%) [14] and by Venerito et al., who showed an eradication rate of 68.9% [15]. In addition, eradication rates of first-line therapy with PPI-amoxicillin-clarithromycin were shown to be 70% and 81% in Spain in 2015 [16, 17].
The eradication rate for PPI-amoxicillin-metronidazole was 70% in our study and in the same range as the clarithromycin-based rate of 71% successful eradication. In contrast to this, Sanchez-Delgado et al. reported an eradication rate between 82% and 88% for PPI-amoxicillin-metronidazole in 2012 in Spain [19]. Nyssen et al. [37] showed a success rate of 80% for triple therapies (most commonly PPI-amoxicillin-clarithromycin), which is based on 30,394 patients from 27 countries during the years of 2013–2018. Possible explanations might be that Nyssen et al. examined more closely how compliant patients were, whereas this information are missing from our analysed data yet represent the real-world scenario. Additionally, our data came from one large tertiary referral hospital with in general more complex patients, whereas Nyssen et al. included a wide variety of centre types (large hospitals vs small outpatient clinics).
Since most people infected with H. pylori are asymptomatic, screening is not routine [5]. However, whenever H. pylori is present, eradication is suggested [3, 20], usually with two or three antibiotics and one PPI either concomitantly or sequentially for 3–14 days [21]. When deciding which first-line therapy to choose, regional antibiotic resistance rates – especially for clarithromycin – must be considered. In Europe, clarithromycin resistance rates have been increasing in the past decade and have been described as 15% in Sweden, 30% in Italy, with an even higher rate of 40% in Turkey [3]. Although outdated, in a study from 2005 in Baden (Switzerland), primary clarithromycin resistance was at 9% [9]. In places with low (<15%) clarithromycin resistance rates (and no history of previous macrolide treatment) H. pylori eradication therapy with PPI-clarithromycin-amoxicillin for 7–14 days is still recommended [3, 5]. We did not find any data pointing to clarithromycin resistance rates lower than 15% in developed nations, such as Europe or the U.S [22]. In the case of penicillin allergy amoxicillin is usually replaced by metronidazole [5]. Whenever high (>15%) clarithromycin resistance rates are suspected three therapeutic options are commonly used:
Although it was widely assumed, that clarithromycin resistance rates were pretty high (>15%), PPI-amoxicillin-clarithromycin was nevertheless prescribed in 21% and, surprisingly, no statistically difference in non-clarithromycin containing therapies was observed. Furthermore, in 2017 the therapy regimen recommendation was altered at the University Hospital of Zurich because of a policy shift to “standard treatment according to guidelines”, rather than directly suggesting PPI-amoxicillin-metronidazole. Interestingly, this led to use of first-line treatment with PPI-amoxicillin-clarithromycin in 67% (n = 32) of patients in 2017 (eradication success 63%), while PPI-amoxicillin-metronidazole was only used in 19% (n = 9) of patients in 2017 (eradication success 67%). The policy shift was abolished in 2018 owing to perceived low success rates with clarithromycin-based therapies, not, however, based on a formal review of data. Unfortunately, there are no recent published data about clarithromycin resistance rates at the University Hospital of Zurich nor in Switzerland in general, but from our study, clarithromycin-based standard triple therapy does not seem to be significantly worse than other first-line therapy regimens used in our patient population.
The average success rate of second-line eradication attempts was 61%. In 2015, a considerably lower second-line eradication rate (45%) was observed, although the first-line eradication rate in 2015 was 60% (second lowest year overall). However, we did not find any explanation for this. First-line treatments and the characteristics of patient population, for example, the proportion of bariatric patients, were comparable to other years.
In our study, only 33% of second-line eradication attempts with PPI-amoxicillin-clarithromycin were successful after an unsuccessful attempt with the same substances as first-line therapy. This finding is in line with findings of Marin et al. [24], who demonstrated a success rate of 46% when repeating a clarithromycin-based therapy after an unsuccessful first attempt. One reason could be a primary resistance to clarithromycin, leading to the recommendation to not use clarithromycin as second-line treatment, unless prior resistance testing confirms H. pylori susceptibility to clarithromycin [5]. Other studies have shown that clarithromycin resistance (in addition to resistance to fluroquinolones and rifabutin) cannot be overcome by an increased dose, duration of treatment or frequency of administration [25–27], Further emphasising a potential benefit of changing therapeutic regimens after a first unsuccessful attempt. Alternatively, Min Li et al. [38] stated in a meta-analysis that vonoprazan instead of conventional PPI-based therapies showed significantly superior eradication rates in clarithromycin-resistant strains in both randomised controlled trials (pooled eradication rates 82.0% vs 40.0%; OR, 6.83; 95% CI, 3.63–12.86; p <0.0001) and nonrandomised controlled trials (pooled eradication rates 80.8% vs 41.8%; OR, 4.98; 95% CI, 2.47–10.03; p <0.0001). Suzuki et al. [39] found that vonoprazan-based H. pylori eradication was associated with a long-term impact on gut microbiota, including effects on the richness of bacterial species. According to these studies, the choice not only of antibiotics but also of the acid suppressant may play a role in eradication success. However, vonoprazan is not (yet) part of H. pylori eradication therapies in Switzerland.
Overall, we observed a decreasing eradication success rate from the first attempt (71%) to the second attempt (61%) to the third attempt (47%). According to Wu et al. the most important reasons for eradication failure are resistant bacteria, poor compliance of patients, rapid metabolism of the PPI and a high bacterial load [28].
Furthermore, we found a statistically significant difference in eradication success between different countries of origin. We attributed these observations mainly to compliance, better socioeconomic living conditions (e.g., hygiene, better access to healthcare facilities) and probably genetic variations, which have been discussed in the literature [31–33].
Interestingly, in our study the duration of treatment only trended towards higher eradication rates with longer treatment periods but did not show statistically significant differences. These findings align with findings of Fuccio et al. [35], who did not find statistically more efficient treatment results with longer treatment periods (14>10>7days) in a meta-analysis of 21 randomised trials in 2007. They stated that, when 14 days (78% eradication success) was compared with 7 days (73% eradication success), only a 5% increase can possibly be achieved. These findings are similar to our stated eradication success rates for PPI-amoxicillin-clarithromycin therapy of 73% (14 days) versus 68% (7 days).
With regard to limitations, we would like to point out that, unfortunately, we did not have a roughly similar number of patients in the first, second and third eradication attempt, which is inevitable with real-world data. Furthermore, our sample size is limited and biased towards a tertiary referral centre, although we are testing patients in an ambulatory out-patient setting with open-referral policy.
Certainly, there are downsides and limitations when evaluating real-world data. However, this study reflects what is really happening on the practitioner level. We can conclude from all the data gathered in this study that H. pylori eradication therapy varied substantially between different physicians. This observation aligns very well with those of Graham et al. [36], who showed that antimicrobial therapy nowadays is mostly based on trial-and-error (comparing different therapies) instead of being susceptibility-based (by analysing different antibiotic resistance rates). It also aligns well with Nyssen et al. [37], who concluded that “management of H. pylori infection by European gastroenterologists is heterogeneous, suboptimal and discrepant with current recommendations.”
As mentioned above, Nyssen et al. [37] observed eradication rates of around 80% for triple therapies (in contrast to our 71%) and hence concluded that only quadruple therapies like “14-day non-bismuth quadruple concomitant therapy (PPI, amoxicillin, clarithromycin, metronidazole), 14-day standard triple plus bismuth (PPI, bismuth, amoxicillin, clarithromycin), and 10-day bismuth quadruple therapy (PPI, bismuth, tetracycline, and metronidazole)” achieve eradication rates of over 90%, hence the need to abandon empirical use of triple therapies.
Between the three most used eradication regimens in our study (PPI-amoxicillin-metronidazole 71%; PPI-amoxicillin-clarithromycin 21%; PPI-metronidazole-clarithromycin 6%), no statistically significant difference in eradication rates (70%, 71% and 72%) was found. Ergo, despite expected clarithromycin resistance the H. pylori eradication rate in the two clarithromycin-containing regimens did not significantly differ from the non-clarithromycin-containing therapies in the examined region (Zurich, Switzerland). Hence, in order to achieve higher eradication success rates (>90%), the choice of triple therapy does not seem to matter but rather other primary therapy schemes such as quadruple therapies, as also suggested by Nyssen at al. [37], should be pursued.
The data that support the findings of this study are not publicly available because they contain information that could compromise the privacy of research participants but are available from Tobias Braendli (corresponding author) in consultation with the Zurich Ethic committee’s rules and regulations.
Tobias Braendli: first author, did the main part of the research and mainly wrote the paper. He made substantial contributions to the conception and design as well as the analysis and interpretation of data for the work. He furthermore was critically involved in drafting and revising the work.
Valeria Schindler: Substantial contributions to the conception and design of the work as well as to the analysis and interpretation of data for the work. Furthermore, she was crucially involved in drafting the work and revising it critically.
Dominique Laurent Braun: Substantial contributions to the conception, analysis and interpretation of data for the work. He furthermore revised it critically several times for important intellectual content.
Fritz Ruprecht Murray: Revised the work critically for important intellectual content and made substantial contributions to the interpretation of data for the work.
Juliane Hente: Revised the work critically for important intellectual content and made substantial contributions to the interpretation of data for the work.
Daniel Pohl: Was leading and responsible for the project, made substantial contributions to the conception and design as well as the analysis and interpretation of data for the work. He furthermore was critically involved in drafting and revising the work. Finally, he gave his final approval of the current version to be published.
There was no outside funding necessary for this data analysis and manuscript preparation.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun;1(8390):1311–5. https://doi.org/10.1016/S0140-6736(84)91816-6
2. Yokota S, Konno M, Fujiwara S, Toita N, Takahashi M, Yamamoto S, et al. Intrafamilial, Preferentially Mother-to-Child and Intraspousal, Helicobacter pylori Infection in Japan Determined by Mutilocus Sequence Typing and Random Amplified Polymorphic DNA Fingerprinting. Helicobacter. 2015 Oct;20(5):334–42. https://doi.org/10.1111/hel.12217
3. Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al.; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017 Jan;66(1):6–30. https://doi.org/10.1136/gutjnl-2016-312288
4. Krumbiegel P, Lehmann I, Alfreider A, Fritz GJ, Boeckler D, Rolle-Kampczyk U, et al. Helicobacter pylori determination in non-municipal drinking water and epidemiological findings. Isotopes Environ Health Stud. 2004 Mar;40(1):75–80. https://doi.org/10.1080/10256010310001639868
5. McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010 Apr;362(17):1597–604. https://doi.org/10.1056/NEJMcp1001110
6. Hooi JK, Lai WY, Ng WK, Suen MM, Underwood FE, Tanyingoh D, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017 Aug;153(2):420–9. https://doi.org/10.1053/j.gastro.2017.04.022
7. Malfertheiner P, Selgrad M, Bornschein J. Helicobacter pylori: clinical management. Curr Opin Gastroenterol. 2012 Nov;28(6):608–14. https://doi.org/10.1097/MOG.0b013e32835918a7
8. Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, et al.; Study Group participants. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013 Jan;62(1):34–42. https://doi.org/10.1136/gutjnl-2012-302254
9. Soltermann A, Perren A, Schmid S, Eigenmann F, Güller R, Weber KB, et al. Assessment of Helicobacter pylori clarithromycin resistance mutations in archival gastric biopsy samples. Swiss Med Wkly. 2005 May;135(21-22):327–32. https://doi.org/10.4414/smw.2005.10866
10. Zhou Q, Li L, Ai Y, Pan Z, Guo M, Han J. Diagnostic accuracy of the. Wien. Klin Wochenschr. 2017;129(1-2):38–45. https://doi.org/10.1007/s00508-016-1117-3
11. Fischbach W, Malfertheiner P, Lynen Jansen P, Bolten W, Bornschein J, Buderus S, et al.; Verantwortlich für die DGVS. [S2k-guideline Helicobacter pylori and gastroduodenal ulcer disease]. Z Gastroenterol. 2016 Apr;54(4):327–63. https://doi.org/10.1055/s-0042-102967
12. Suzuki T, Matsuo K, Ito H, Sawaki A, Hirose K, Wakai K, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006 Mar;119(3):217–24. https://doi.org/10.1016/j.amjmed.2005.10.003
13. Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007 Aug;102(8):1808–25. https://doi.org/10.1111/j.1572-0241.2007.01393.x
14. Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008 Jun;148(12):923–31. https://doi.org/10.7326/0003-4819-148-12-200806170-00226
15. Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88(1):33–45. https://doi.org/10.1159/000350719
16. Cuadrado-Lavín A, Salcines-Caviedes JR, Diaz-Perez A, Carrascosa MF, Ochagavía M, Fernandez-Forcelledo JL, et al. First-line eradication rates comparing two shortened non-bismuth quadruple regimens against Helicobacter pylori: an open-label, randomized, multicentre clinical trial. J Antimicrob Chemother. 2015 Aug;70(8):2376–81. https://doi.org/10.1093/jac/dkv089
17. Molina-Infante J, Lucendo AJ, Angueira T, Rodriguez-Tellez M, Perez-Aisa A, Balboa A, et al.; European Registry on H. pylori management (Hp‐EuReg). Optimised empiric triple and concomitant therapy for Helicobacter pylori eradication in clinical practice: the OPTRICON study. Aliment Pharmacol Ther. 2015 Mar;41(6):581–9. https://doi.org/10.1111/apt.13069
18. Tepes B, Brglez Jurecic N, Tepes K, Espada Sanchez M, Perez Nyssen O, Smith S, et al. Helicobacter pylori eradication rates in Slovenia in the period from 2017 to 2019 - data from the European Registry on Helicobacter pylori Management (Hp-EuReg). Dig Dis. 2020.
19. Sánchez-Delgado J, García-Iglesias P, Castro-Fernández M, Bory F, Barenys M, Bujanda L, et al. High-dose, ten-day esomeprazole, amoxicillin and metronidazole triple therapy achieves high Helicobacter pylori eradication rates. Aliment Pharmacol Ther. 2012 Jul;36(2):190–6. https://doi.org/10.1111/j.1365-2036.2012.05137.x
20. Budzyński J. Helicobacter pylori eradication: not only early consequences. EBioMedicine. 2018 Sep;35:8–9. https://doi.org/10.1016/j.ebiom.2018.08.051
21. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017 Feb;112(2):212–39. https://doi.org/10.1038/ajg.2016.563
22. Park JY, Dunbar KB, Mitui M, Arnold CA, Lam-Himlin DM, Valasek MA, et al. Helicobacter pylori Clarithromycin Resistance and Treatment Failure Are Common in the USA. Dig Dis Sci. 2016 Aug;61(8):2373–80. https://doi.org/10.1007/s10620-016-4091-8
23. Shiota S, Reddy R, Alsarraj A, El-Serag HB, Graham DY. Antibiotic Resistance of Helicobacter pylori Among Male United States Veterans. Clin Gastroenterol Hepatol. 2015 Sep;13(9):1616–24. https://doi.org/10.1016/j.cgh.2015.02.005
24. Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy failure (for Helicobacter pylori eradication). Expert Opin Pharmacother. 2013 May;14(7):843–61. https://doi.org/10.1517/14656566.2013.782286
25. Dore MP, Leandro G, Realdi G, Sepulveda AR, Graham DY. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta-analytical approach. Dig Dis Sci. 2000 Jan;45(1):68–76. https://doi.org/10.1023/A:1005457226341
26. Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007 Aug;26(3):343–57. https://doi.org/10.1111/j.1365-2036.2007.03386.x
27. Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014 Feb;12(2):177–86.e3. https://doi.org/10.1016/j.cgh.2013.05.028
28. Wu DC, Hsu PI, Tseng HH, Tsay FW, Lai KH, Kuo CH, et al. Helicobacter pylori infection: a randomized, controlled study comparing 2 rescue therapies after failure of standard triple therapies. Medicine (Baltimore). 2011 May;90(3):180–5. https://doi.org/10.1097/MD.0b013e31821c9d1c
29. de Martel C, Parsonnet J. Helicobacter pylori infection and gender: a meta-analysis of population-based prevalence surveys. Dig Dis Sci. 2006 Dec;51(12):2292–301. https://doi.org/10.1007/s10620-006-9210-5
30. Naja F, Kreiger N, Sullivan T. Helicobacter pylori infection in Ontario: prevalence and risk factors. Can J Gastroenterol. 2007 Aug;21(8):501–6. https://doi.org/10.1155/2007/462804
31. Perez-Perez GI, Rothenbacher D, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2004;9(s1 Suppl 1):1–6. https://doi.org/10.1111/j.1083-4389.2004.00248.x
32. Bruce MG, Maaroos HI. Epidemiology of Helicobacter pylori infection. Helicobacter. 2008 Oct;13 Suppl 1:1–6. https://doi.org/10.1111/j.1523-5378.2008.00631.x
33. Malaty HM, Nyren O. Epidemiology of Helicobacter pylori infection. Helicobacter. 2003;8(s1 Suppl 1):8–12. https://doi.org/10.1046/j.1523-5378.2003.00163.x
34. Suki M, Leibovici Weissman Y, Boltin D, Itskoviz D, Tsadok Perets T, Comaneshter D, et al. Helicobacter pylori infection is positively associated with an increased BMI, irrespective of socioeconomic status and other confounders: a cohort study. Eur J Gastroenterol Hepatol. 2018 Feb;30(2):143–8. https://doi.org/10.1097/MEG.0000000000001014
35. Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007 Oct;147(8):553–62. https://doi.org/10.7326/0003-4819-147-8-200710160-00008
36. Graham DY. Transitioning of. Antibiotics (Basel). 2020 Oct;9(10):671. https://doi.org/10.3390/antibiotics9100671
37. Nyssen OP, Bordin D, Tepes B, Pérez-Aisa Á, Vaira D, Caldas M, et al.; Hp-EuReg Investigators. European Registry on Helicobacter pylori management (Hp-EuReg): patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut. 2021 Jan;70(1):40–54. https://doi.org/10.1136/gutjnl-2020-321372
38. Li M, Oshima T, Horikawa T, Tozawa K, Tomita T, Fukui H, et al. Systematic review with meta-analysis: Vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori. Helicobacter. 2018 Aug;23(4):e12495. https://doi.org/10.1111/hel.12495
39. Suzuki S, Gotoda T, Takano C, Horii T, Sugita T, Ogura K, et al. Long term impact of vonoprazan-based Helicobacter pylori treatment on gut microbiota and its relation to post-treatment body weight changes. Gut. 2021;69:1019–26. https://doi.org/10.1136/gutjnl-2019-319954
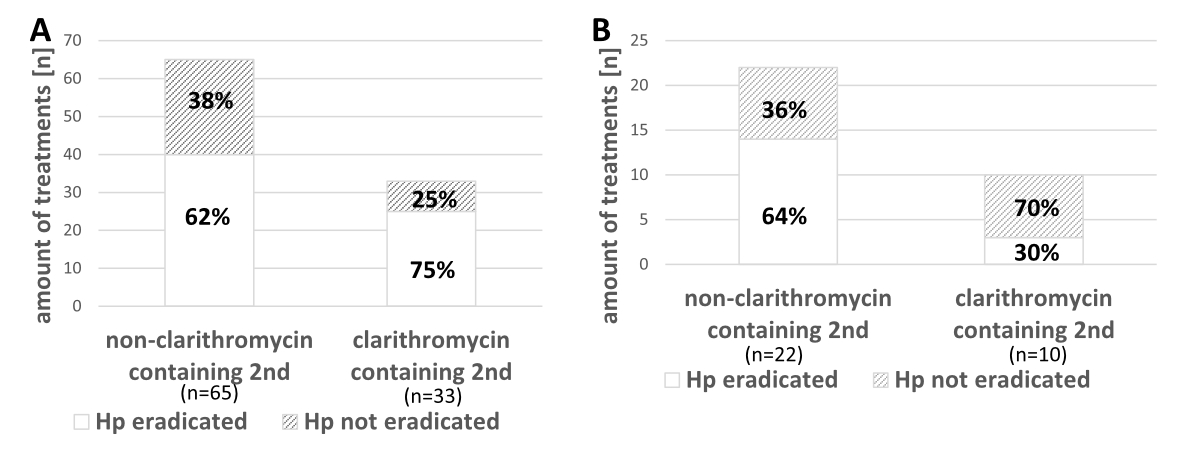
Figure S1 Second-line Helicobacter pylori eradication therapies and their success (between the years 2004 and 2018). A: Different second-line therapies and their successfulness after failed non-clarithromycin containing first-line therapies. B: Different second-line therapies and their successfulness after failed first-line clarithromycin containing therapies.
Hp: Helicobacter pylori