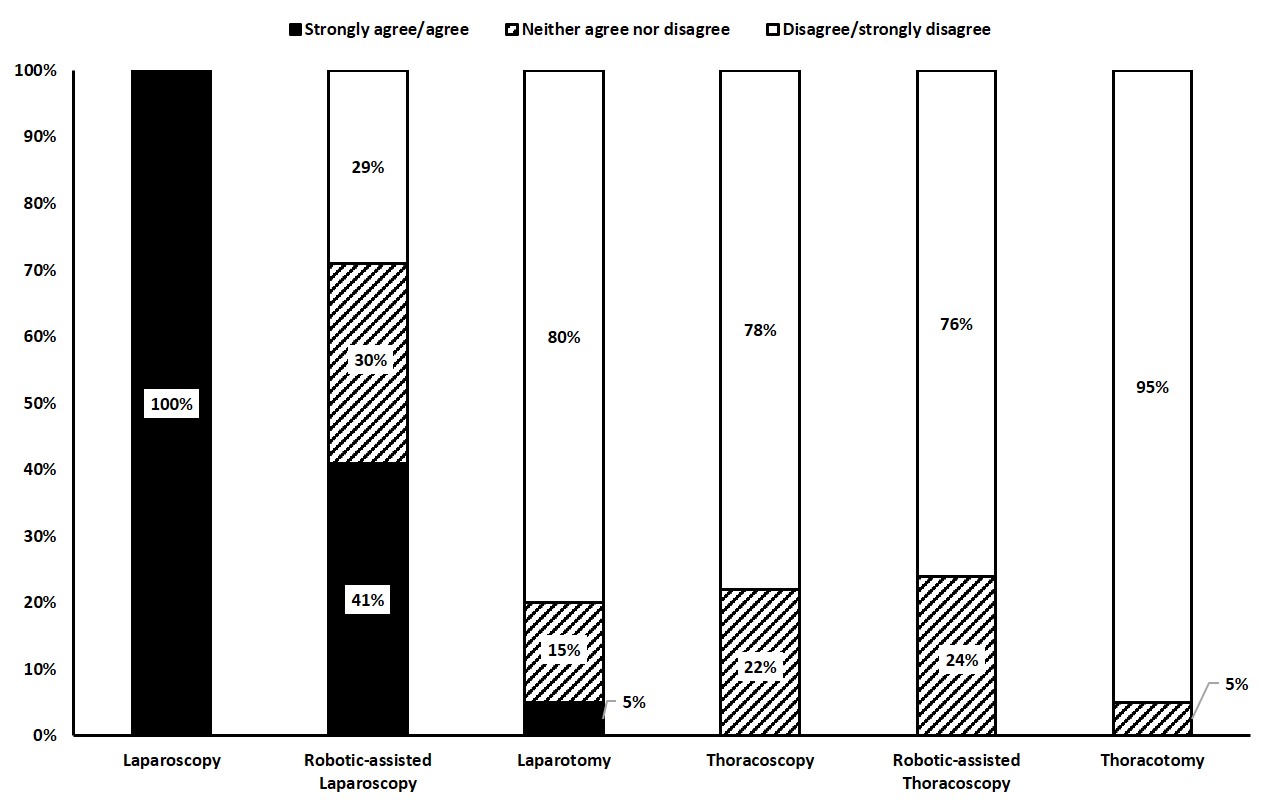
Figure 1 Surgical access routes in type III hiatal hernia repair (% of agreement).
DOI: https://doi.org/10.4414/SMW.2021.w30052
Optimal treatment of large hiatal hernias remains a hotly contested topic in upper gastrointestinal surgery. Numerous aspects of the surgical management of this clinical entity are not broadly accepted, and even experts disagree on critical components including the application of surgical meshes for hiatal reinforcement, the indication for complementary antireflux repair and the diagnosis and treatment of short oesophagus.
Based on our own clinical experience, we hypothesised that current surgical practice in Switzerland mirrors the aforementioned uncertainties. Since there are no official national recommendations or guidelines on this topic, we found it pertinent to perform a snapshot survey of members of the Swiss Society of Visceral Surgeons (SGVC) to ascertain potential variation in current surgical management of type III hiatal hernia.
In April 2020, we invited all members of the SGVC via email to participate in an anonymous online survey regarding current surgical strategies for hiatal hernia. In order to minimise bias, the focus was strictly on type III (mixed axial and paraoesophageal) hiatal hernia; other hernia types, emergencies and recurrences were considered outside the scope of this study. We designed a 25-question survey to elicit respondent feedback on the following points: personal and institutional experience of participants, diagnostic work-up, indications and technical details of hiatal repair (surgical access routes, crural dissection and reconstruction phase). An online survey tool (SurveyMonkey, Palo Alto, CA, USA) was employed to disseminate the survey and collect answers. Participants were asked to rank their agreement on predefined answers to each question using a five-point Likert scale. Two scale variations were employed. The first indicated the level of agreement with a certain technique (1 = strongly disagree, 2 = disagree, 3 = neither agree nor disagree, 4 = agree, 5 = strongly agree). The second concerned the frequency with which technical steps are performed by the participant (1 = always, 2 = very often, 3 = sometimes, 4 = rarely, 5 = never). The attendees were also invited to leave comments on each question. A reminder was sent via email after 2 weeks. Details of the questionnaire are shown in the appendix.
Data were analysed using descriptive statistics and expressed as percentage of agreement and median (interquartile range; IQR) using SPSS version 26.0 (IBM Inc., Chicago, Ill, USA). Consensus was defined as ≥75% of experts agreeing (strongly agree or agree and always or very often) on a given question.
Three-hundred-and-ten members of the SGVC were invited, and 47 surgeons (response rate 15%) across 12 cantons participated in the survey. All respondents were specialist visceral surgeons with a median experience of 15.7 years (IQR 7.3–23) after board examination in surgery. The median personal and institutional annual caseload of type III hiatal hernia was 10 (IQR 5–17) and 15 (10–30), respectively. The hospitals' levels of care and participants' positions within each hospital hierarchy are summarised in table 1. Standardised treatment algorithms for hiatal hernia had been established by 76%.
Table 1Participating experts and institutions.
| Participating expert characteristics | n (%) | |
| Per region | German-speaking canton | 38 (81) |
| French-speaking canton | 5 (11) | |
| Italian-speaking canton | 4 (8) | |
| Per institution | Private hospital | 11 (23) |
| General hospital | 11 (23) | |
| Teaching hospital | 16 (34) | |
| Maximum care hospital | 9 (19) | |
| Clinical position of participants | Department head | 15 (32) |
| Senior consultant | 21 (45) | |
| Consultant | 1 (2) | |
| Attending surgeon | 5 (11) | |
| Other position | 5 (11) | |
Most surgeons agreed that the preoperative diagnostic work-up for patients with type III hiatal hernia should entail upper gastrointestinal tract endoscopy (100%) and computed tomography (CT) (78%). Conversely, there was no consensus regarding contrast radiography, oesophageal manometry, oesophageal pH-metry, plain chest radiography, magnetic resonance imaging (MRI) or endoscopic ultrasound prior to surgery (table 2).
Table 2Preoperative work-up
| Strongly agree/agree | Neither agree nor disagree | Disagree/strongly disagree | |
| Upper gastrointestinal tract endoscopy | 100.0% | 0.0% | 0.0% |
| Contrast radiography | 42.6% | 34.0% | 23.4% |
| CT scan | 78.7% | 17.0% | 4.3% |
| Oesophageal manometry | 59.6% | 23.4% | 17.0% |
| Oesophageal pH-metry / impedance pH-metry | 40.4% | 34.0% | 25.5% |
| Chest X-ray | 17.0% | 34.0% | 48.9% |
| Other diagnostic modalities (MRI, endosonography, oesophageal scintigraphy) | 0.0% | 25.5% | 74.5% |
CT: computed tomography; MRI: magnetic resonance imaging
There was strong agreement amongst respondents that both older and younger patients with relevant symptoms (98% and 100%, respectively) or chronic anaemia with Cameron lesions (96% and 100%, respectively) should undergo surgery. Likewise, there was clear consensus on operating on younger (<70 years, physically fit) asymptomatic patients (64% agreement). In contrast, no agreement was found regarding older (>70 years, physically fit) asymptomatic patients without Cameron lesions (36%).
Surgical access via laparoscopy was preferred by all participants (100% agreement). In contrast, laparotomy or robot-assisted techniques were rarely used, and none of the participants reported a preference for the transthoracic route (fig. 1).

Figure 1 Surgical access routes in type III hiatal hernia repair (% of agreement).
Technical steps during dissection of the hiatus are summarised in Table 3. Most participants agreed upon the necessity of division of the phreno-oesophageal ligament, the mobilisation and resection of the hernia sac, and an extensive mediastinal mobilisation to obtain sufficient oesophageal length. Furthermore, there was consensus in favour of visualising both vagus nerves and ensuring preservation of an aberrant left hepatic artery. Conversely, no consensus ≥75% was achieved pertaining to preservation of the hepatic branches of the vagus, mobilisation of the gastric fundus, and resection of a posterior retro-cardiac lipoma or pre-cardiac fat pad. Similarly, intraoperative endoscopy aimed at determining the length of the oesophagus in the case of suspected short oesophagus was deemed necessary by just a minority of respondents.
Table 3Technical details of hiatal dissection.
| Strongly agree/agree | Neither agree nor disagree | Disagree / strongly disagree | |
| Repositioning of hernia sac contents into the abdominal cavity as initial surgical step | 70.2% | 8.5% | 21.3% |
| Dissection/transsection of the phreno-oesophageal ligament | 80.9% | 17.0% | 2.1% |
| Resection of hernia sac | 78.7% | 12.8% | 8.5% |
| Wide mediastinal dissection to achieve sufficient oesophageal length | 95.8% | 4.3% | 0.0% |
| Visualisation and dissection of both vagus nerves | 83.0% | 8.5% | 8.5% |
| Mobilisation of gastric fundus / division of gastro-splenic ligament including short gastric vessels | 66.0% | 21.3% | 12.8% |
| Resection or dissection of posterior retro-cardiac lipoma (if present) | 68.1% | 27.7% | 4.3% |
| Resection or dissection of the pre-cardiac fat pad | 31.9% | 38.3% | 29.8% |
| Intraoperative endoscopy to determine oesophageal length (in the case of suspected oesophageal shortening) | 21.3% | 42.6% | 36.2% |
| Preservation of the crural fascia | 68.1% | 29.8% | 2.1% |
| Preservation of aberrant left hepatic artery | 80.9% | 14.9% | 4.3% |
| Preservation of hepatic branches of vagus nerves | 51.1% | 31.9% | 17.0% |
| Preservation of pulmonary branches of vagus nerves | 48.9% | 38.3% | 12.8% |
There was a clear consensus for crurorraphy of the posterior aspect of the hiatus with single stitches using braided, non-resorbable suture material (size 0 or 2-0) (table 4). Lower agreement scores were reported for combined anterior and posterior or exclusive anterior crurorraphy, the use of pledgets, running sutures, or single form-8 sutures. Very few surgeons reported performing relaxing incisions on the diaphragm to reduce tension (table 4).
Table 4Technical details of hiatal reconstruction.
| Alway/very often | Sometimes | Rarely/never | |
| Suture repair of hiatus | 100.0% | 0.0% | 0.0% |
| Use of mesh to reinforce hiatal repair | 57.5% | 21.3% | 21.3% |
| Other options to reinforce hiatal repair | 0.0% | 6.4% | 93.6% |
| Use of relaxing diaphragmatic incisions | 2.1% | 4.3% | 93.6% |
| Gastropexy / fundo-phrenicopexy | 61.7% | 10.6% | 27.7% |
| Antireflux procedure | 76.6% | 14.9% | 8.5% |
| Oesophageal lengthening procedure | 10.6% | 12.8% | 76.6% |
Although a relevant percentage of participants reported having encountered mesh-related complications in own or assigned patients (table 5), most surgeons indicated regular (in all or most cases) use of surgical mesh for hiatal reinforcement (always 28%, in most cases 30%, in selected cases 34%). The most common indications for mesh use included fragile texture of the diaphragmatic musculature (79%) and large hiatal defects (85%). In contrast, consensus was not achieved for other potentially predisposing factors such as biologically young (40%) or old (45%) age, history of other abdominal hernia (34%) and obesity (51%). Likewise, agreement was limited regarding the choice of mesh types (fig. 2) and mesh placement (exclusively on posterior hiatoplasty 26%, on posterior hiatoplasty and crura 79%, on anterior hiatoplasty and crura 29%, circular around the oesophagus 34%, individually adapted to the specific patho-anatomy 50%, and avoiding contact with the oesophagus 61%). Most surgeons agreed on fixing the mesh with sutures (71%), whereas other techniques of mesh fixation (tacks 22%, fibrin glue 32%) did not achieve consensus.
Table 5Mesh-related complications.
| Yes, in own patients | Yes, in referred patients | Never | |
| Mesh erosion to oesophagus | 8.5% | 36.2% | 59.6% |
| Mesh erosion to stomacha | 2.2% | 21.7% | 76.1% |
| Mesh erosion to oesophago-gastric junctiona | 6.5% | 30.4% | 65.2% |
| Mesh erosion to other organs (aorta, lung)b | 0.0% | 6.7% | 93.3% |
| Stenosis distal oesophagus / oesophago-gastric junctiona | 26.1% | 23.9% | 56.5% |
| Mesh migrationa | 10.9% | 32.6% | 56.5% |
| Mesh infectiona | 6.5% | 10.9% | 82.6% |
| Pericardial haemorrhage/effusiona | 8.7% | 15.2% | 78.3% |
| Pleural haemorrhage/effusionb | 15.6% | 11.1% | 77.8% |
| Perioperative haemorrhage caused by tacks during mesh fixationa | 4.4% | 10.9% | 84.8% |
| Perioperative haemorrhage caused by sutures during mesh fixationa | 6.5% | 8.7% | 84.8% |
| Pneumothoraxa | 37.0% | 13.0% | 54.4% |
| Chronic paina | 21.7% | 26.1% | 58.7% |
| Seroma formationa | 21.7% | 15.2% | 67.4% |
a Question answered by 46 respondents.
b Question answered by 45 respondents.
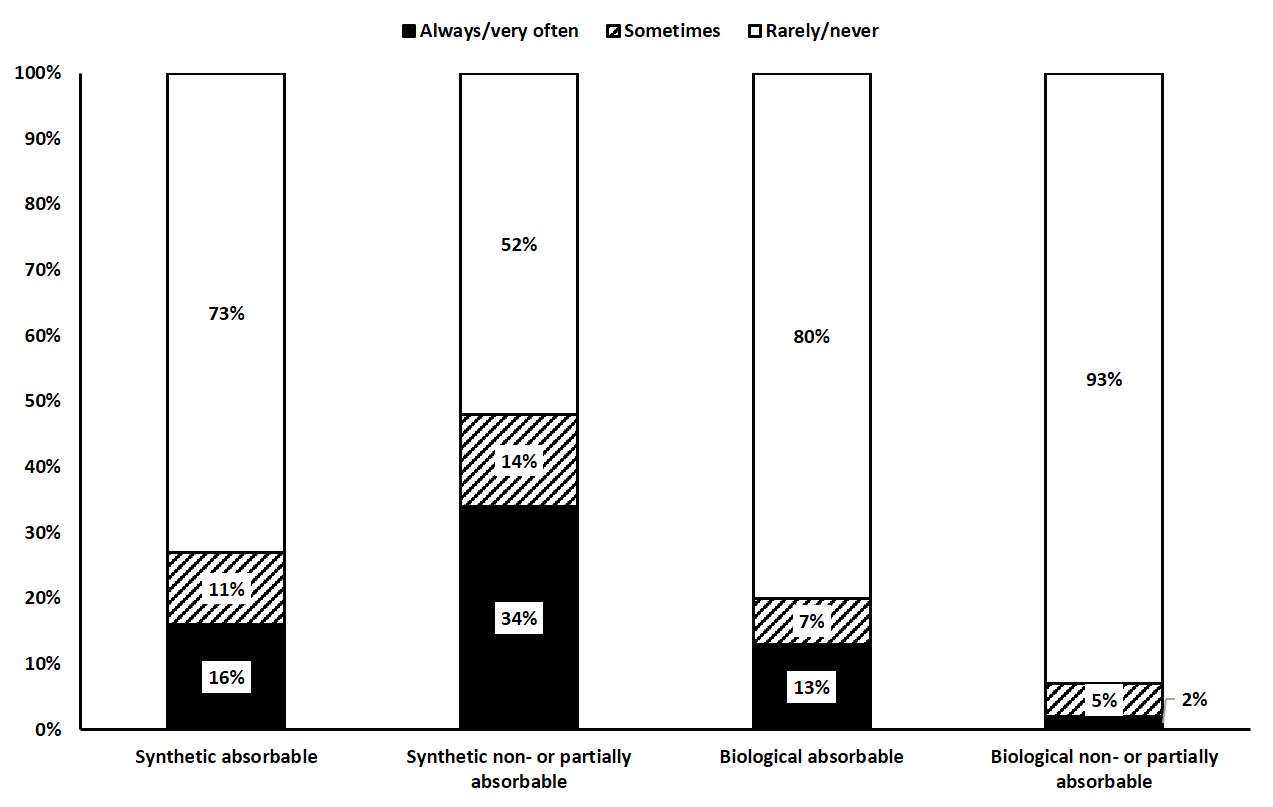
Figure 2 Mesh types used in type III hiatal hernia repair (% of frequency).
Most participants (77%) reported regularly performing an additional antireflux procedure in combination with crurorraphy. The majority of participants (64%) employed braided, non-absorbable suture material (size 2-0). In particular, biologically younger (<70 years) or physically fit patients (70%) and individuals with reflux symptoms (89%), oesophagitis or Barrett’s metaplasia (83%), or positive functional reflux tests (impedance/pH-metry) (87%) were considered ideal candidates for an additional antireflux repair. However, there was no clear preference regarding specific surgical techniques (table 6). Gastro- or fundo-phrenicopexy was regularly performed by the majority of surgeons (62%) despite not reaching consensus.
Table 6Antireflux procedures.
| Always/very often | Sometimes | Rarely/Never | |
| Floppy 360° (Nissen type) fundoplication | 29.8% | 14.9% | 55.3% |
| Anterior partial 90–200° (Dor/Thal type) fundoplicationa | 13.1% | 10.9% | 76.1% |
| Posterior partial 180–270° (Toupet type) fundoplication | 44.7% | 31.9% | 23.4% |
| His-angle reconstruction techniques (Hill gastropexy, Lortat-Jacob or similar)a | 10.9% | 8.7% | 80.4% |
| Transthoracic antireflux techniques (i.e., Belsey Mark IV)a | 0.0% | 0.0% | 100.0% |
| Tailored approach: No 360° fundoplication in patients with signs of oesophageal motility disorder | 17.0% | 12.8% | 70.2% |
| Other antireflux procedures (magnetic sphincter augmentation [LINX], EndoStim, RefluxStop or similar) | 2.1% | 2.1% | 95.8% |
| Endoscopic interventional (via gastroscopy) antireflux proceduresa | 0.0% | 0.0% | 100.0% |
a Question answered by 46 respondents.
Short oesophagus has been defined as a tension-free intra-abdominal oesophageal segment <2–2.5cm after extensive mediastinal (type II) dissection [1]. No consensus was achieved (agreement 38%) amongst respondents on whether short oesophagus represents a relevant clinical finding during type III hiatal hernia repair. However, oesophageal lengthening (Collis procedure) in combination with fundoplication (agreement 62%) or with fundo-phrenicopexy (agreement 26%) was the most popular surgical strategy amongst those who agreed on the clinical relevance of oesophageal shortening.
This comprehensive survey amongst SGVC members demonstrates that presently, there is very limited standardisation or consensus on elementary steps of type III hiatal hernia treatment in Switzerland, reflecting the results of other surveys on hiatal hernia surgery from the US and Europe [2–5].
In this context, we observed consensus (agreement ≥75%) for indications for surgery (symptoms, chronic anaemia with Cameron lesions), preoperative work-up (endoscopy and CT scan), and surgical access routes (laparoscopy). Furthermore, several basic technical steps during surgical dissection (resection of the hernia sac, wide mediastinal dissection, preservation of vagus nerves and aberrant left hepatic arteries) and of the reconstruction phase (single-stitch posterior crurorraphy and complementary antireflux procedure) achieved high rates of agreement. However, this survey also revealed considerable inconsistencies in many important technical details such as the use of meshes, gastro- or fundo-phrenicopexy, type of antireflux procedure and the management of short oesophagus.
The strengths of our survey include a high rate of experienced participants and a well-defined index procedure (type III hiatal hernia). The questionnaires were exclusively targeted at visceral surgeons, the majority of whom were experienced specialists holding appointments as chiefs or senior consultants. Most participants had an annual case load >10 type III hiatal hernia, and 76% followed standardised work-up and surgical procedures for this entity. We selected type III hiatal hernia as index procedure because this entity is by far the most prevalent paraoesophageal hernia type (about 15% of all hiatal hernia cases) and excluded other hiatal hernia types to allow for a more precise interpretation of results and conclusions. Our focus on type III hiatal hernia is in contrast to the available published literature, which comprises four surveys from the last decade, addressed to either members of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) [2, 3] or the European Association for Endoscopic Surgery (EAES) [4, 5]. Of note, except for the European study, which focussed on “large” type II–IV hiatal hernia [4], the other surveys were designed to gather data on all types of hiatal hernia including gastro-oesophageal reflux disease. Therefore, comparison with our results remains partly elusive. In addition, two retrospective population-based analyses on outcomes of mesh use in paraoesophageal (type II–IV) hiatal hernia repair using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database have been recently reported [6, 7]. The prospective multi-national HERNIAMED data collection included 5462 paraoesophageal hernia repairs and remains another important source of information on the subject [8].
Laparoscopy was the preferred surgical access route amongst Swiss visceral surgeons (100% agreement), which compares favourably with data from the NSQIP and HERNIAMED databases [6, 8], and the SAGES surveys [2, 3]. In accordance with existing literature, this study confirmed that transthoracic access has been largely abandoned by most surgeons. Of note, robot-assisted procedures appear to be gaining popularity with 41% agreement among our participants.
The use of mesh to reinforce hiatal repairs remains a controversial subject. Current scientific evidence is extremely fragmented owing to different mesh types, shapes, fixation techniques and follow-up periods. In addition, the incidence of the much-feared mesh-related complications, such as erosion, stenosis and infection is not precisely known [9]. Although most randomised controlled trials have demonstrated reduced recurrence rates after mesh reinforcement at short- and mid-term follow-up [10–15]., long-term data beyond 3 years of follow-up show no clear benefit regarding clinical results or objective recurrence rates [13, 15], or even inferior symptomatic outcomes [15]. However, in a recent meta-analysis of randomised controlled trials, mesh reinforcement was associated with fewer reoperations but similar recurrence rates [16]. Data from the HERNIAMED registry suggests a rather constant utilisation of mesh in paraoesophageal hernia repair in Austria, Germany and Switzerland (33.0% and 38.9% in 2013 and 2019, respectively) [8], whereas in the US, this rate decreased from 45% in 2010 to 36% in 2017 [7]. With this in mind, we were surprised that more than 90% of respondents reported performing mesh reinforcement in all or selected cases. Likewise, 28% of our participants reported routine mesh use in all type III hiatal hernia repairs, which corresponds to a significantly higher rate than in previous surveys performed in Europe [4, 5] and the US [2, 3]. In contrast to earlier research, biological meshes play a minor role in the current surgical armamentarium, probably owing to the disappointing long-term results from two randomised controlled trials [13, 15]. Thus, most of our participants chose synthetic non-absorbable mesh, which is in line with other recent surveys [4, 5]. In this context, the significance of synthetic long-term absorbable materials remains unclear. Recent retrospective cohort studies have shown promising results, but long-term follow-up is currently not available [17, 18].
Antireflux procedures are frequently performed adjuncts to type III hiatal hernia repair with a high acceptance rate among our participants. Our results confirm recent data from the multi-institutional HERNIAMED registry reporting additional fundoplication in paraoesophageal hernia repair in 60–70% [8, 19]. However, routine and selective antireflux procedures are performed by 55% and 36% of our participants, respectively, which contrasts with the 84% (routine) and 9% (selective) fundoplications in the EAES survey [4]. We assume that these differences reflect the rather weak scientific evidence for additional antireflux surgery in the literature, which is mainly based on a single randomised controlled trial [20], and a number of case series and small cohort studies [21]. Consistent with other studies [4, 19], Toupet and Nissen fundoplication were the dominant antireflux techniques in our survey.
As observed in other publications, the present survey did not establish any clear pattern regarding gastro- or fundo-phrenicopexy. The high agreement rate in the survey (62%) suggests that this surgical adjunct may be performed both in combination with antireflux surgery (as part of a Toupet fundoplication) or as a stand-alone procedure. However, evidence supporting fundo-phrenicopexy in paraoesophageal hiatal hernia is conflicting and limited to just a few retrospective case series [21–24]. Similarly, we found a mixed attitude towards short oesophagus, which was defined as a tension-free intra-abdominal oesophageal segment <2–2.5cm after extensive mediastinal dissection: only 38% of participants acknowledged that oesophageal shortening represents a relevant finding during type III hiatal hernia repair. Of these, 62% agreed that an oesophageal lengthening (Collis) procedure and fundoplication around the neo-oesophagus should be performed in this situation, which is in line with current expert recommendations [25].
There are certain limitations associated with our study. First, similar to other surveys on the subject [2–5], our questionnaire did not go through a formal validation process before dissemination. Second, despite a response rate in the upper range of similar surveys, only 47 experts completed the full questionnaire, potentially limiting the relevance of our results. However, as stated above, this work had a clear focus on national specialists in the field, which represents a rather confined target group in a small country like Switzerland. Other limitations include the definition of the index procedure. Although classification of hiatal hernia into four types according to Skinner and Belsey [26–27] is accepted by most surgeons, major uncertainties remain, particularly regarding an inconsistent and synonymous use of the terms “type III hiatal hernia”, “mixed hiatal hernia”, “large hiatal hernia”, “paraoesophageal hernia”, “upside-down stomach”, and “(intra)thoracic stomach”. Thus, in the US, the term “paraoesophageal hiatal hernia” generally refers to all large hiatal hernia (types I–IV) with migration of the fundus into the mediastinum, whereas many European surgeons strictly reserve this term for paraoesophageal hernia type II (without any sliding component) independent of its size [28–32]. Therefore, despite our effort to adequately define the index procedure of our survey, we cannot guarantee that all participants share a similar understanding of type III hiatal hernia.
In conclusion, consensus amongst Swiss visceral surgeons is limited to just a few basic components of type III hiatal hernia surgical management. Although the observed therapeutic polypragmatism may simply manifest the necessity to adapt to the clinical variability and to the complexity of type III hiatal hernia, it may also reflect a lack of standardisation of care. Therefore, as a next step, our group intends to follow-up with a multinational expert Delphi survey aimed at establishing treatment algorithms and guidelines for paraoesophageal hiatal hernia.
SG, PCM: study design, performing the experiments, drafting the manuscript; DV, JRK: performing the experiments, interpretation of data, critical revision of the manuscript; SG, CAG: statistical analysis, interpretation of data, critical revision of the manuscript; CAG: study design, performing the experiments, interpretation of data, drafted the manuscript. All authors gave their final approval.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
No funding was received.
1. Volonté F , Collard JM , Goncette L , Gutschow C , Strignano P . Intrathoracic periesophageal fundoplication for short esophagus: a 20-year experience. Ann Thorac Surg. 2007 Jan;83(1):265–71. https://doi.org/10.1016/j.athoracsur.2006.07.056
2. Frantzides CT , Carlson MA , Loizides S , Papafili A , Luu M , Roberts J , et al. Hiatal hernia repair with mesh: a survey of SAGES members. Surg Endosc. 2010 May;24(5):1017–24. https://doi.org/10.1007/s00464-009-0718-6
3. Pfluke JM , Parker M , Bowers SP , Asbun HJ , Daniel Smith C . Use of mesh for hiatal hernia repair: a survey of SAGES members. Surg Endosc. 2012 Jul;26(7):1843–8. https://doi.org/10.1007/s00464-012-2150-6
4. Furnée EJ , Smith CD , Hazebroek EJ . The Use of Mesh in Laparoscopic Large Hiatal Hernia Repair: A Survey of European Surgeons. Surg Laparosc Endosc Percutan Tech. 2015 Aug;25(4):307–11. https://doi.org/10.1097/SLE.0000000000000162
5. Huddy JR , Markar SR , Ni MZ , Morino M , Targarona EM , Zaninotto G , et al. Laparoscopic repair of hiatus hernia: does mesh type influence outcome? A meta-analysis and European survey study. Surg Endosc. 2016 Dec;30(12):5209–21. https://doi.org/10.1007/s00464-016-4900-3
6. Schlosser KA , Maloney SR , Prasad T , Augenstein VA , Heniford BT , Colavita PD . Mesh reinforcement of paraesophageal hernia repair: trends and outcomes from a national database. Surgery. 2019 Nov;166(5):879–85. https://doi.org/10.1016/j.surg.2019.05.014
7. Schlottmann F , Strassle PD , Patti MG . Laparoscopic Paraesophageal Hernia Repair: Utilization Rates of Mesh in the USA and Short-Term Outcome Analysis. J Gastrointest Surg. 2017 Oct;21(10):1571–6. https://doi.org/10.1007/s11605-017-3452-8
8. Köckerling F , Simon T , Hukauf M , Hellinger A , Fortelny R , Reinpold W , et al. The Importance of Registries in the Postmarketing Surveillance of Surgical Meshes. Ann Surg. 2018 Dec;268(6):1097–104. https://doi.org/10.1097/SLA.0000000000002326
9. Li J , Cheng T . Mesh erosion after hiatal hernia repair: the tip of the iceberg? Hernia. 2019 Dec;23(6):1243–52. https://doi.org/10.1007/s10029-019-02011-w
10. Frantzides CT , Madan AK , Carlson MA , Stavropoulos GP . A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg. 2002 Jun;137(6):649–52. https://doi.org/10.1001/archsurg.137.6.649
11. Granderath FA , Schweiger UM , Kamolz T , Asche KU , Pointner R . Laparoscopic Nissen fundoplication with prosthetic hiatal closure reduces postoperative intrathoracic wrap herniation: preliminary results of a prospective randomized functional and clinical study. Arch Surg. 2005 Jan;140(1):40–8. https://doi.org/10.1001/archsurg.140.1.40
12. Ilyashenko VV , Grubnyk VV , Grubnik VV . Laparoscopic management of large hiatal hernia: mesh method with the use of ProGrip mesh versus standard crural repair. Surg Endosc. 2018 Aug;32(8):3592–8. https://doi.org/10.1007/s00464-018-6087-2
13. Oelschlager BK , Pellegrini CA , Hunter JG , Brunt ML , Soper NJ , Sheppard BC , et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg. 2011 Oct;213(4):461–8. https://doi.org/10.1016/j.jamcollsurg.2011.05.017
14. Oor JE , Roks DJ , Koetje JH , Broeders JA , van Westreenen HL , Nieuwenhuijs VB , et al. Randomized clinical trial comparing laparoscopic hiatal hernia repair using sutures versus sutures reinforced with non-absorbable mesh. Surg Endosc. 2018 Nov;32(11):4579–89. https://doi.org/10.1007/s00464-018-6211-3
15. Watson DI , Thompson SK , Devitt PG , Aly A , Irvine T , Woods SD , et al. Five Year Follow-up of a Randomized Controlled Trial of Laparoscopic Repair of Very Large Hiatus Hernia With Sutures Versus Absorbable Versus Nonabsorbable Mesh. Ann Surg. 2020 Aug;272(2):241–7. https://doi.org/10.1097/SLA.0000000000003734
16. Memon MA , Siddaiah-Subramanya M , Yunus RM , Memon B , Khan S . Suture Cruroplasty Versus Mesh Hiatal Herniorrhaphy for Large Hiatal Hernias (HHs): An Updated Meta-Analysis and Systematic Review of Randomized Controlled Trials. Surg Laparosc Endosc Percutan Tech. 2019 Aug;29(4):221–32. https://doi.org/10.1097/SLE.0000000000000655
17. Abdelmoaty WF , Dunst CM , Filicori F , Zihni AM , Davila-Bradley D , Reavis KM , et al. Combination of Surgical Technique and Bioresorbable Mesh Reinforcement of the Crural Repair Leads to Low Early Hernia Recurrence Rates with Laparoscopic Paraesophageal Hernia Repair. J Gastrointest Surg. 2020 Jul;24(7):1477–81. https://doi.org/10.1007/s11605-019-04358-y
18. Panici Tonucci T , Asti E , Sironi A , Ferrari D , Bonavina L . Safety and Efficacy of Crura Augmentation with Phasix ST Mesh for Large Hiatal Hernia: 3-Year Single-Center Experience. J Laparoendosc Adv Surg Tech A. 2020 Apr;30(4):369–72. https://doi.org/10.1089/lap.2019.0726
19. Köckerling F , Zarras K , Adolf D , Kraft B , Jacob D , Weyhe D , et al. What Is the Reality of Hiatal Hernia Management?-A Registry Analysis. Front Surg. 2020 Oct;7:584196. https://doi.org/10.3389/fsurg.2020.584196
20. Müller-Stich BP , Kenngott HG , Gondan M , Stock C , Linke GR , Fritz F , et al. Use of Mesh in Laparoscopic Paraesophageal Hernia Repair: A Meta-Analysis and Risk-Benefit Analysis. PLoS One. 2015 Oct;10(10):e0139547. https://doi.org/10.1371/journal.pone.0139547
21. Kohn GP , Price RR , DeMeester SR , Zehetner J , Muensterer OJ , Awad Z , et al.; SAGES Guidelines Committee . Guidelines for the management of hiatal hernia. Surg Endosc. 2013 Dec;27(12):4409–28. https://doi.org/10.1007/s00464-013-3173-3
22. Diaz S , Brunt LM , Klingensmith ME , Frisella PM , Soper NJ . Laparoscopic paraesophageal hernia repair, a challenging operation: medium-term outcome of 116 patients. J Gastrointest Surg. 2003 Jan;7(1):59–67. https://doi.org/10.1016/S1091-255X(02)00151-8
23. Ponsky J , Rosen M , Fanning A , Malm J . Anterior gastropexy may reduce the recurrence rate after laparoscopic paraesophageal hernia repair. Surg Endosc. 2003 Jul;17(7):1036–41. https://doi.org/10.1007/s00464-002-8765-2
24. Poncet G , Robert M , Roman S , Boulez JC . Laparoscopic repair of large hiatal hernia without prosthetic reinforcement: late results and relevance of anterior gastropexy. J Gastrointest Surg. 2010 Dec;14(12):1910–6. https://doi.org/10.1007/s11605-010-1308-6
25. Durand L , De Antón R , Caracoche M , Covián E , Gimenez M , Ferraina P , et al. Short esophagus: selection of patients for surgery and long-term results. Surg Endosc. 2012 Mar;26(3):704–13. https://doi.org/10.1007/s00464-011-1940-6
26. Altorki NK , Yankelevitz D , Skinner DB . Massive hiatal hernias: the anatomic basis of repair. J Thorac Cardiovasc Surg. 1998 Apr;115(4):828–35. https://doi.org/10.1016/S0022-5223(98)70363-0
27. Skinner DB , Belsey RH , Russell PS . Surgical management of esophageal reflux and hiatus hernia. Long-term results with 1,030 patients. J Thorac Cardiovasc Surg. 1967 Jan;53(1):33–54. https://doi.org/10.1016/S0022-5223(19)43239-X
28. Cheverie JN , Lam J , Neki K , Broderick RC , Lee AM , Matsuzaki T , et al. Paraesophageal hernia repair: a curative consideration for chronic anemia? Surg Endosc. 2020 May;34(5):2243–7. https://doi.org/10.1007/s00464-019-07014-3
29. Kahrilas PJ , Kim HC , Pandolfino JE . Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008;22(4):601–16. https://doi.org/10.1016/j.bpg.2007.12.007
30. Stefanidis D , Hope WW , Kohn GP , Reardon PR , Richardson WS , Fanelli RD ; SAGES Guidelines Committee . Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010 Nov;24(11):2647–69. https://doi.org/10.1007/s00464-010-1267-8
31. Vakil N , van Zanten SV , Kahrilas P , Dent J , Jones R ; Global Consensus Group . The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006 Aug;101(8):1900–20. https://doi.org/10.1111/j.1572-0241.2006.00630.x
32. DeMeester TR . “Etiology and Natural History of Gastroesophageal Reflux Disease and Predictors of Progressive Disease,” in Shackelford’s Surgery of the Alimentary Tract, 2 Volume Set, Elsevier, 2019, pp. 204–220.
The questionnaire is added as appendix in the PDF version of this article.