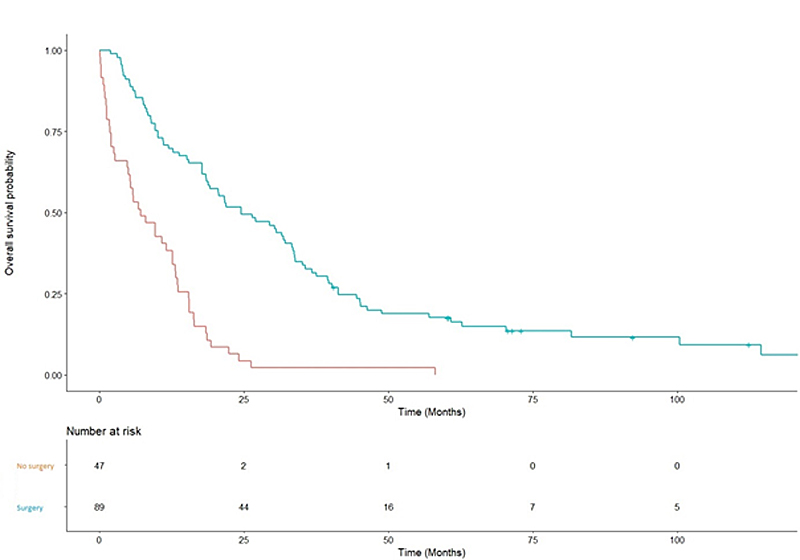
Figure 1 Overall survival when rectal surgery was performed or not.
DOI: https://doi.org/10.4414/SMW.2021.w30034
Nearly 20% of rectal cancer patients are initially diagnosed with metastases [1]. It was previously suggested that local treatment was a major independent predictive factor of increased progression-free survival and overall survival, even in metastatic settings [2–5]. However, series were mostly retrospective; they studied only the role of surgery and were designed to include mixed populations of colon and rectal cancer patients. The nature and consequences of local treatment in patients with metastases seemed to widely differ between colon and rectal cancer patients, however [6].
The optimal local management of the primary tumour in nonmetastatic rectal cancer is preoperative chemoradiation [7, 8]. The therapeutic index (efficacy/toxicity ratio) of such local management is particularly hard to assess in patients with metastases, since acute and late toxicities were never reported in literature. In daily routine, the place of local treatment (exclusively surgery, exclusively radiotherapy, preoperative chemoradiation) is often debated, since local rectal treatment complications may significantly decrease patients’ quality of life [9], with uncertain benefit on local and distant control.
Thus, it is of paramount importance to describe real-life local management in patients with metastatic rectal cancer, and report efficacy and toxicity data. These elements could help to define the therapeutic index of each local therapy and assess outcome predictors. Such predictors could play a part in deciding on the optimal treatment plan and might play a crucial role in achieving personalised anticancer treatments and follow-up in the future.
The aim of the present study was to describe outcome and treatment in a cohort of patients with metastatic rectal cancer undergoing local treatment with at least radiation therapy. The identification of prognostic factors of local failure, progression-free survival and overall survival was retrospectively performed in the whole set of patients.
A retrospective study was conducted at the Lucien Neuwirth comprehensive cancer centre (Saint Priest en Jarez, France). The institutional review board approved the study, which was conducted in accordance with the Helsinki declaration. Informed consent stating that patient’s medical data would be used to conduct retrospective studies was systematically obtained before radiation initiation. Therapeutic strategies were systematically discussed in tumour board meetings (including at least a medical oncologist, a radiation oncologist and a surgeon) before treatment initiation. Generally speaking, based on internal guidelines, the tumour board considered that those with 5 or fewer metastases could be treated with a curative intent. Conversely, patients with more than five metastases could be treated with a palliative intent.
The medical data of all consecutive patients undergoing radiotherapy for a metastatic rectal cancer between 2004 and 2015 were collected. Patients’ characteristics (age, sex, body mass index [BMI], World Health Organization [WHO] performance status), tumour histology, staging, chemotherapy characteristics (neoadjuvant, adjuvant, regimen of chemotherapy), radiotherapy characteristics (dose, fractionation, equivalent dose in 2 Gy per fraction [EQD2], rectal boost, association with chemotherapy), surgery characteristics (rectal surgery type, nodal staging, complete sterilisation of the operative specimen [ypCR], clear surgical margin [R0]) were recorded. Acute chemoradiotherapy toxicities and complications of surgery were also reported.
Chemo-radiation was defined as concurrent when chemotherapy overlapped radiotherapy.
Gross tumour volume, clinical tumour volume, planning tumour volume and organs at risk were delineated based on planning computed tomography (CT). Their definition evolved with the availability and development of CT scan and magnetic resonance imaging (MRI), and with the delineation guideline editions. 3D conventional radiotherapy was usually used. Intensity modulated radiotherapy was exceptionally performed in the case of special dose constraints (previous history of radiotherapy…). In each case, treatment plans were optimised according to dose limits for organs at risk and constraints for volume coverage, i.e., planning tumour volume should receive 95% to 107% of the prescribed dose. Rectal equivalent 2Gy (EQD2) dose was calculated using the EQD2 formula provided by Fowler [10] and α/β = 6.2 [11].
Surgery was defined as tumour resection. Defunctioning stoma was not considered as surgery. The rectal surgery type was cautiously reported (total mesorectal excision [TME] or other procedure).
Follow-up was calculated from the completion of radiotherapy. Patients were alternatively assessed for efficacy every 3 months by surgeons and clinical oncologists with clinical examination and chest/abdomen/pelvis-CT scan.
Toxicity was assessed weekly during the radiation course. Radiation-related toxicities were graded using the Common Terminology Criteria for Adverse Events v4.0 (CTCAEv4.0) [12]. Acute toxicity was defined as a toxicity occurring within 3 months from the beginning of radiotherapy. Chemotherapy-induced toxicities and postoperative complications were collected in medical oncology and in surgical files, respectively.
Progression-free survival was defined as the time from the date of radiotherapy completion to the date of clinical and/or radiological rectal cancer progression and/or distant metastases progression. Overall survival was defined as the time from the date of radiotherapy completion to the date of death or the last follow-up. Time to last follow-up was May 2018. Progression-free survival and overall survival were estimated with the Kaplan-Meier method. Survivals were then compared based on the log-rank test. Median values were given with the first and third quartiles (Q1–Q3) or with the range. Chi square test, Fisher test or Kruskal-Wallis test were performed to compare the distribution of patient characteristics. All p values were nominal without adjustment for multiple testing. Significance was defined by p <0·05. The multivariate analysis was performed using a Cox multivariate analysis based on the independent significant or close-to-significance (p <0.2)- factors in univariate analysis. Associated factors (chi-square tests: p <0.001) were excluded. The multivariate model was refined using the Akaike information criterion and corresponds to a step-by-step approach. Statistical analyses were processed with R 3.2.2 (R Core Team (2013, The R Foundation for Statistical Computing, Vienna, Austria).
Dataabout 148 consecutive rectal cancer patients with proven metastases undergoing radiotherapy between 2004 and 2015 were analysed. At the time of radiotherapy, median age was 65.2 (range 58–75), patients were mostly men (110 vs 38 women). Patients were in good condition: 134 patients (90.5%) had a 0–1 WHO performance status score. Malnutrition (BMI <18.5 kg/m2) was reported in six patients (4.1%). Twenty patients (13.5%) were diagnosed at a stage T4. Tumours were located in the lower (n = 62; 41.9%), middle (n = 68; 45.9%) or upper (n = 15; 10.1%) rectum. The most frequent histology was adenocarcinoma (n = 144; 97.3 %), with moderate differentiation (n = 69, 46.6%). Forty-three patients had 1–5 metastases, 8% had 6–10 metastases and 49% had more than 11 metastases. Liver metastases were the most frequent (75.7%) followed by lung metastases (32.4%); 9.5% of patients had symptoms in related to their metastases. Patient and tumour characteristics are reported in table 1.
Table 1Patient, tumour and treatment characteristics.
| Variables | Whole set of patients (n = 148, 100%) | Patients undergoingrectal surgery (n = 97, 65.5%) | Patients not undergoing rectal surgery (n = 51, 34.5%) | p value |
| Clinical data | ||||
| Median age, years (IQR) | 65.2 (58.5–75.2) | 63.6 (57.7–73.9) | 70.2 [59.1;79.8] | 0.012 a |
| – <70, n (%) | 87 (58.8) | 24 (47.1) | 63 (64.9) | 0.035 b |
| – ≥70, n (%) | 61 (41.2) | 27 (52.9) | 34 (35.1) | |
| Gender, n (%) | 0.10b | |||
| – Male | 110 (74.3) | 68 (70.1) | 42 (82.4) | |
| – Female | 38 (25.7) | 29 (29.9) | 9 (17.6) | |
| WHO performance status, n (%) | <0.001 b | |||
| – 0–1 | 134 (90.5) | 94 (96.9) | 40 (78.4) | |
| – 2–3 | 14 (9.5) | 3 (3.1) | 11 (21.6) | |
| T stage, n (%) | <0.001 a | |||
| – T2 | 2 (1.4) | 0 | 2 (3.9) | |
| – T3 | 107 (72.3) | 79 (81.4) | 28 (54.9) | |
| – T4 | 20 (13.5) | 8 (8.3) | 12 (23.6) | |
| – Missing data | 19 (12.8) | 10 (10.3) | 9 (17.6) | |
| N stage, n (%) | 0.60a | |||
| – N0 | 32 (21.6) | 21 (21.6) | 11 (21.6) | |
| – N1 | 85 (57.4) | 62 (63.9) | 23 (45.1) | |
| – N2 | 5 (3.4) | 3 (3.1) | 2 (3.9) | |
| – Missing data | 26 (17.6) | 11 (11.4) | 15 (29.4) | |
| Body mass index, n (%) | 0.40c | |||
| – <18.5 kg/m2 | 6 (4.1) | 3 (3.1) | 3 (5.8) | |
| – ≥18.5 kg/m2 | 111 (75.0) | 75 (77.3) | 36 (70.6) | |
| – Missing data | 31(20.9) | 19 (19.6) | 12 (23.5) | |
| Median follow-up, months (IQR) | 19.0 (7.3–40.5) | 29.4 (12.1–49.6) | 8.5 (2.3–17.4) | <0.001 a |
| Biological data, G/L (IQR)* | ||||
| Pre-radiation | ||||
| – Median neutrophil count | 4.44 (3.32–5.88) | 4.60 (3.48–6.0) | 5.06 (3.84–6.0) | 0.33a |
| – Median lymphocyte count | 1.52 (1.29–1.90) | 1.52 (1.33–1.88) | 1.4 (1.11–1.80) | 0.56a |
| Tumor data, n (%) | ||||
| Location | 0.15b | |||
| – Lower rectum | 62 (41.9) | 36 (37.1) | 26 (51.0) | |
| – Middle rectum | 68 (45.9) | 48 (49.5) | 20 (39.2) | |
| – Upper rectum | 15 (10.1) | 12 (12.4) | 3 (5.9) | |
| – Missing data | 3 (2.1) | 1 (1.0) | 2 (3.9) | |
| Tumor histology | 0.20b | |||
| – Adenocarcinoma | 144 (97.3) | 96 (99) | 48 (94.1) | |
| – Other | 4 (2.7) | 1 (1) | 3 (5.9) | |
| Tumor differentiation | 0.27c | |||
| – Poor | 19 (12.9) | 12 (12.4) | 7 (13.7) | |
| – Moderate | 69 (46.6) | 51 (52.6) | 18 (35.3) | |
| – Well | 45 (30.4) | 27 (27.8) | 18 (35.3) | |
| – Missing data | 15 (10.1) | 7 (7.2) | 8 (15.7) | |
| Metastases location** | 0.23b | |||
| – Liver | 112 (75.7) | 73 (75.3) | 39 (76.5) | |
| – Lung | 48 (32.4) | 29 (29.9) | 19 (37.3) | |
| – Bone | 7 (4.7) | 2 (2.1) | 5 (9.8) | |
| – Brain | 1 (0.6) | 1 (1.0) | 0 | |
| – Other | 9 (6.0) | 4 (4.1) | 5 (9.8) | |
| Rectal surgery type | ||||
| – Total mesorectal excision | 63 (42.6) | 63 (64.9) | ||
| – Other surgery | 34 (23.0) | 34 (35.1) | ||
| – No surgery | 51 (34.4) | 51 (100) | ||
| Radiation data | ||||
| Median rectal EQD2, Gy (IQR) | 47.7([43.9–50.0) | 49.2 (43.9–50.0) | 47.7 (43.9–50.0) | 0.20a |
| Median dose per fraction, Gy (IQR) | 2.0 (1.8–2.5) | 2.0 (1.8–2.0) | 2.0 (2.0–2.5) | 0.10a |
| Radiation technique, n (%) | 0.40b | |||
| – 3D-CRT | 135 (91.2) | 85 (87.6) | 50 (98.0) | |
| – IMRT | 3 (2.0) | 3 (3.1) | 0 | |
| – Missing data | 10 (6.8) | 9 (9.3) | 1 (2.0) | |
| Fractionation, n (%) | <0.001 b | |||
| – Normofractionated (1.8–2.4Gy/fr) | 107 (72.3) | 77 (79.4) | 30 (58.8) | |
| – Hypofractionated (≥2.5Gy/fr) | 41 (27.7) | 20 (20.6) | 21 (41.2) | |
| Rectal boost, n (%) | 0.006 b | |||
| – Yes | 69 (46.6) | 53 (54.6) | 16 (31.4) | |
| – No | 79 (53.4) | 44 (45.4) | 35 (68.6) | |
| Chemotherapy data, n (%) | ||||
| Concomitant chemotherapy | 0.01 c | |||
| – Capecitabin | 37 (25.0) | 28 (28.9) | 9 (17.6) | |
| – Intravenous 5-FU | 9 (6.1) | 6 (6.2) | 3 (5.9) | |
| – Folfox | 71 (48) | 51 (52.6) | 20 (39.2) | |
| – Other | 4 (2.7) | 1 (1) | 3 (5.9) | |
| – No chemotherapy | 27 (18.2) | 11 (11.3) | 16 (31.4) | |
Percentages were calculated based on the population of each column. T and N stages were assessed based on the UICC 7th edition. WHO: World Health Organization; IQR: interquartile range, EQD2: equivalent dose in 2 Gy per fraction; 3D-CRT: 3D conformational radiotherapy; IMRT: Intensity-modulated radiotherapy; 5-FU: 5-fluorouracil; Folfox: 5-FU, leucovorin, oxaliplatin
a Kruskal-Wallis test; b chi-square test; c Fischer test
*: Available in 58 patients (59.8%) experiencing surgery, and 22 patients (43%) not experiencing surgery
Treatment was given with a curative intent for 49% of patients (n = 73) and a palliative intent for 51% of patients (n = 75).
All patients received local radiotherapy, with a median equivalent 2 Gy per fraction dose of 47.7 Gy. Radiotherapy was delivered preopartively (n = 88, 59.4%) or postoperatively (n = 9, 6.1%) or exclusively (n = 51, 34.5%). In patients undergoing pre- or postoperative radiotherapy, median rectal EQD2 was 49.2 Gy (Q1–Q3 44–50). In the case of exclusive radiotherapy, median rectal EQD2 was 47.7 Gy (Q1–Q3 43.9–50). The median dose per fraction was 2 Gy for preoperative, postoperative and exclusive radiotherapy. The rectal boost was delivered in 53 patients in the surgery group (54.6%) and in 16 patients in the no-surgery group (31.4%). Radiotherapy characteristics are detailed in table 1.
Rectal surgery was performed in 97 patients (65.6 %). Sixty-three patients (42.6%) had a total mesorectal excision. Thirty-four (23.1%) had another surgical procedure. Twenty-eight percent of patients were considered tumour-free after one or more surgical interventions: some had a total mesorectal excision and local surgery on metastases.
Chemotherapy was combined with radiotherapy in most patients (n = 121, 81.8%). Concomitant chemoradiation was given significantly more often in patients undergoing surgery (n = 86, 88.7%) than in those without surgery (n = 35, 68.6%). Folfox and capecitabin regimens were mainly used in concomitant setting (n = 71, 48%; surgical group n = 51, 52.6%; non-surgical group n = 20, 39.2%) and n = 37, 25%; surgical group n = 28, 28.9%; non-surgical group (n = 9, 17.6%), respectively.
The Folfox regimen was the most frequently used (46% of patients), Folfiri+ bevacizumab or cetuximab was the second (15.5%), Folfiri alone was the third (6.2%) and Folfox and bevacizumab or cetuximab was the fourth (6%). Initial pre-plan chemotherapy regimens were not modified in frequency in 56.9% of patients and indose in 48.8% of patients. Frequency and dose were adapted to the patients’ characteristics and chemotherapy-induced toxicities. Chemotherapy characteristics are reported in table 1.
Grade 3–4 acute radiotherapy-related toxicities were observed in 6.1% of patients (table 2), mainly gastrointestinal toxicities (5.4%). Regarding grade 3–4 acute radiation therapy toxicities, no difference was reported between surgery and no-surgery groups. Grade 3–4 acute chemotherapy-related adverse effects were reported in 10.7% of patients, 7.4% were haematological toxicities. Three grade 5 toxicities were induced by concomitant chemotherapy. Finally, 22 out of 97 patients (22.8%) experienced significant postoperative complications such as fistulae (6.1%), sepsis (5.1%), abscess (3.1%), necrosis (3.1%), occlusion (2.2%), cutaneous scar disunion (2.2%) or cardiac disorder (1%). One patient died of postoperative occlusive syndrome 1 month after rectal surgery.
Table 2Treatment toxicities.
| Toxicity | Surgery group (n = 97) | No Surgery group (n = 51) | Whole set of patients (n = 148) | |||||||
| Grade 1–2 | Grade 3–4 | Grade 5 | Grade 1–2 | Grade 3–4 | Grade 5 | Grade 1–2 | Grade 3–4 | Grade 5 | ||
| Radiotherapy, (n, (%)) | Gastrointestinal | 38 (39.1) | 5 (5.1) | 0 | 16 (31.3) | 3 (5.9) | 0 | 54 (36.4) | 8 (5.4) | 0 |
| Genitourinary | 7 (7.2) | 1 (1) | 0 | 5 (9.8) | 0 | 0 | 12 (8.1) | 1 (0.7) | 0 | |
| Cutaneous | 10 (10.3) | 0 | 0 | 2 (3.9) | 0 | 0 | 12 (8.1) | 0 | 0 | |
| Chemotherapy (n, (%)) | Gastrointestinal | 35 (36.1) | 1 (1) | 0 | 5 (9.8) | 2 (3.9) | 2 (3.9) | 40 (27) | 3 (2) | 2 (1.3) |
| Neurotoxicity | 21 (21.6) | 2 (2.1) | 0 | 3 (5.9) | 0 | 0 | 24 (16.2) | 2 (1.3) | 0 | |
| Haematological | 2 (2.1) | 8 (8.2) | 1 (1) | 0 | 3 (5.9) | 0 | 2 (1.3) | 11 (7.4) | 1 (0.7) | |
| Cutaneous | 1 (1) | 0 | 0 | 0 | 0 | 0 | 1 (0.7) | 0 | 0 | |
Percentages were calculated based on the population of each column
At the time of the analysis, 85.8 % (n = 127) of patients had died, 63% (n = 80) of them had undergone rectal surgery and 37% (n = 47) had not. At the time of analysis, 96.6% (n = 143) of the 148 included patients had hadtumour progression (local relapse or metastasis).
Local relapse was reported in 23 patients (15.5%) at the end of follow-up, among whom 17 (17.5%) had had rectal surgery and 6 (11.8%) had not.
No independent prognostic factor for local failure was identified in multivariate analysis, in neither the whole set of patients (supplementary table S1 in the appendix) nor the subset of patients having surgery (supplementary table S2).
Histological subtypes other than adenocarcinoma were associated with poorer progression-free survival (hazard ratio [HR] 5.26, 95% confidence interval [CI] 1.815.26; p = 0.002) in multivariate analysis. Rectal surgery was an independent predictor of increased progression-free survival in multivariate analysis (HR 0.38, 95% CI 0.21–0.68; p <0.001).
Table 3Predictive factors for progression-free survival in the whole set of patients (n = 148).
| Variable | Tested vs adverse criteria | UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS | ||
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | ||
| Age | ≥70 vs <70 | 1.19 (0.85–1.67) | 0.30 | NS | |
| Gender | Female vs male | 0.75 (0.52–1.10) | 0.14 | ||
| WHO performance status score | 23 vs.01 | 1.98 (1.11–3.52) | 0.02 | NS | |
| T stage | T4 vs other | 2.14 (1.31–3.50) | 0.002 | NS | |
| N stage | N+ vs N0 | 1.11 (0.72–1.68) | 0.64 | ||
| Rectal tumour location | Upper vs middle | 1.45 (0.83–2.56) | 0.20 | ||
| Lower vs middle | 1.09 (0.76–1.54) | 0.67 | |||
| Tumour differentiation | Poor vs well | 1.27 (0.73–2.19) | 0.38 | ||
| Moderate vs well | 0.85 (0.57–1.28) | 0.42 | |||
| Metastasis location | Visceral vs bone | 1.75 (0.82–3.77) | 0.15 | NS | |
| Histology | Other vs adenocarcinoma | 5.88 (2.11–16.42) | <0.001 | 5.26 (1.81–5.26) | 0.002 |
| Rectal surgery type | Yes vs no | 0.27 (0.18–0.41) | <0.001 | 0.38 (0.21–0.68) | <0.001 |
| TME vs other | 0.55 (0.34–0.88) | 0.0135 | |||
| ypCR | Yes vs no | 1.09 (0.64–1.87) | 0.748 | ||
| Pre-radiation NLR | >2.8 vs ≤2.8 | 1.59 (1.00–2.52) | 0.0487 | ||
| Radiotherapy characteristics | Rectal EQD2 ≥45 Gy vs <45 Gy | 0.92 (0.65–1.32) | 0.68 | ||
| Hypofractionated vs normofractionated | 1.68 (1.14–2.48) | 0.008 | |||
| Concomitant chemotherapy | Yes vs no | 0.27 (0.11–0.68) | 0.005 | NS | |
95% CI: 95% confidence interval; WHO: World Health Organization; TME: total mesorectal excision; ypCR: pathological complete response (Mandard TRG1); NLR: neutrophil to lymphocyte ratio; EQD2: equivalent dose in 2 Gy per fraction; normofractionated: <2.5 Gy per fraction; hypofractionated: ≥2.5 Gy per fraction; NS: non-significant
T and N stages were assessed based on the UICC 7th edition.
All p-values ≤0.2 in univariate analysis have been tested in multivariate analysis, except correlated variables (correlation with p <0.001). Finally, only bold typed values were tested in multivariate analysis.
Median overall survival was 16 months. The most important independent predictive factor of increased overall survival was rectal surgery with a HR of 0.31 (95% CI 0.20–0.47; p <0.001) in multivariate analysis. Visceral metastasis location was an independent predictor of poor overall survival in multivariate analysis (HR 2.32, 95% CI 95% 1.06–5.09; p = 0.036).
Table 4Predictive factors for overall survival in the whole set of patients (n = 148).
| Variable | Tested vs. adverse criteria | UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS | ||
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | ||
| Age | ≥70 vs <70 | 1.61 (1.12-2.32) | 0.009 | 1.32 (0.90-1.93) | NS |
| Gender | Female vs male | 0.76 (0.51-1.13) | 0.18 | ||
| WHO performance status | 2–3 vs 0–1 | 2.51 (1.38-2.57) | 0.002 | NS | |
| T stage | T4 vs other | 2.02 (1.19-3.43) | 0.01 | NS | |
| N stage | N+ vs N0 | 1.14 (0.72-1.79) | 0.57 | ||
| Rectal tumor location | Upper vs middle | 0.99 (0.54-1.78) | 0.96 | ||
| Lower vs middle | 1.18 (0.81-1.72) | 0.39 | |||
| Tumor differentiation | Poor vs well | 1.25 (0.70-2.24) | 0.45 | ||
| Moderate vs well | 0.75 (0.50-1.13) | 0.17 | |||
| Metastasis location | Visceral vs bone | 3.12 (1.43-6.79) | 0.004 | 2.32 (1.06-5.09) | 0.036 |
| Histology | Other vs adenocarcinoma | 10.8 (3.22-36.0) | <0.001 | NS | |
| Rectal surgery type | Yes vs. no | 0.27 (0.18-0.40) | <0.001 | 0.31 (0.20-0.47) | <0.001 |
| TME vs. other | 0.83 (0.51-1.37) | 0.48 | |||
| ypCR | Yes vs no | 0.63 (0.15-2.60) | 0.52 | ||
| Pre-radiation NLR | >2.8 vs ≤2.8 | 1.81 (1.10-2.98) | 0.019 | ||
| Radiotherapy characteristics | Rectal EQD2 ≥45 Gy vs <45 Gy | 0.87 (0.60-1.27) | 0.48 | ||
| Hypofractionated vs normofractionated | 2.09 (1.40-3.12) | <0.001 | |||
| Concomitant chemotherapy | Yes vs no | 0.17 (0.06-0.47) | <0.001 | NS | |
95% CI: 95% confidence interval; WHO: World Health Organization; ypCR: pathological complete response (Mandard TRG1); NLR: neutrophil to lymphocyte ratio; EQD2: equivalent dose in 2 Gy per fraction; normofractionated: <2.5 Gy per fraction; hypofractionated: ≥2.5 Gy per fraction; TME: total mesorectal excision, NS: non-significant
T and N stages were assessed based on the UICC 7th edition.
All p-values ≤0.2 in univariate analysis have been tested in multivariate analysis, except correlated variables (correlation with p <0.001). Finally, only bold typed values were tested in multivariate analysis.
Median overall survival was 24.6 months for patients havingsurgery versus 7.1 months for patients who did not (p <0.001, fig. 1). Tumour-free status did not significantly impact the overall survival (19.7 vs 18.4 months ; p = 0.72).

Figure 1 Overall survival when rectal surgery was performed or not.
The present article retrospectively identified predictive factors of progression-free survival and overall survival in patients undergoing at least local radiotherapy for a metastatic rectal cancer. Tumour resection was the most important predictor of survival in the whole set of patients, highlighting a possible key role of surgery in the management of the metastatic rectal cancer. Overall survival was improved from 7.1 months for patients in the no-surgery group to 24.6 months for those in the surgery group. progression-free survival was improved from 4.5 months for patients in the no-surgery group to 11.4 months for those in the surgery group. Although the retrospective nature of the present study is a limitation, when surgery was performed in selected younger patients, with the best performance status and smaller tumours (table 1), a significant improvement of the median progression-free survival and overall survival was observed in the primary tumour resection group. This was also observed in most other published studies [2–4]. The largest study retrospectively analysed outcomes of 847 patients, with 547 patients undergoing rectal surgery and 300 not undergoing surgery [13]. Patients with rectal resection had longer overall survival (16.7–20.7 months for the surgery group vs 11.4–13.4 months for the no-surgery group) and longer progression-free survival (6.710.5 months for the surgery group vs 5.97.8 months for the no-surgery group). More recently, a study based on FFCD 9601 trial data compared 156 metastatic rectal cancer patients undergoing rectal resection with 60 patients not having surgery. overall survival (16.3 vs 9.6 months, p <0.001) and progression-free survival (5.1 vs 2.9 months, p <0.001) were significantly higher in the surgery group [14]. Finally, two recent studies using state-of-the-art propensity score analysis, and therefore controlling known biases of selection, also suggested a beneficial impact of rectal resection in metastatic setting (overall survival: 17.9 vs 7.9 months, p <0.001 [15] and 13.8 vs 6.3 months, p <0.001 [16]). Once again, all these studies were performed in colorectal cancer patients, whereas the present study was exclusively in rectal cancer patients. The biological rationale of the improvement of long-term outcomes induced by local treatment in the metastatic setting is still debated. Two interesting hypotheses have recently been proposed, with a possible reduction of tumour self-seeding thanks to primary tumour treatment. Tumour “self-seeding” is the continuous possibility for cancer cells to leave the primary tumour and seed metastases in distant organs [17]. This process can be led by aggressive cancer stem cells [18], and also by immune-mediated mechanisms, since the presence of primary tumour could suppress T-cell and antibody responses [19]. The removal of local tumour could therefore reduce metastatic progression and explain the benefit in overall survival.
In our series, most patients (86/97, 88.7%) had preoperative chemoradiation, 63 patients (42.6%) underwent gold standard surgery (total mesorectal excision). All patients received local radiotherapy, with a median equivalent 2 Gy per fraction dose of 47.7 Gy. Such extensive local treatments probably explain the low rate of local failure (15.5%), leading to weak statistical power of the univariate and multivariate analyses of local failure, and perhaps resulting in the failure to e identify predictors of local relapse.
The present study revealed that 22.8% of patients undergoing surgical treatment presented with significant complications, necessitating a delay of systemic treatment. These data supported previously reported morbidity after primary tumour rectal resection, both in the non-metastatic setting (20.1% to 26.8% in two major phase III trials) [8, 20] and in the metastatic setting (23% in a recent meta-analysis) [21]. Only one death was related to surgery, which is far below the usual rates reported with primary tumour rectal resection in the setting of metastasis (11.728%) [22]. Regarding radiotherapy-related toxicities, only 5.4% of acute grade 34 gastrointestinal toxicities were observed. This was consistent with rates reported in non-metastatic patients (from 7.4% [8] to 13.5% [20]). Altogether, these data suggest that real-life metastatic patients seem to have a similar radiotherapy and surgery toxicity profiles as non-metastatic phase-III trial patients.
The present study reports real-life data about efficacy and toxicity of local treatment in metastatic rectal cancer patients. Rectal surgery appeared as a key predictor of increased progression-free survival and overall survival in patients undergoing at least local radiotherapy. Local surgery and radiation appeared to be as well tolerated in our series of real-life patients as in selected phase III non-metastatic rectal cancer patients. These data suggested that local management could be beneficial for metastatic rectal cancer patients. However, these findings should be taken with caution and need to be prospectively confirmed with randomised treatment. A prospective phase III study (GRECCAR 8 trial, NCT02314182) is currently recruiting in order to determine the place of local surgery in this population.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest was disclosed.
1. Verberne CJ , de Bock GH , Pijl ME , Baas PC , Siesling S , Wiggers T . Palliative resection of the primary tumour in stage IV rectal cancer. Colorectal Dis. 2012 Mar;14(3):314–9. https://doi.org/10.1111/j.1463-1318.2011.02618.x
2. Bajwa A , Blunt N , Vyas S , Suliman I , Bridgewater J , Hochhauser D , et al. Primary tumour resection and survival in the palliative management of metastatic colorectal cancer. Eur J Surg Oncol. 2009 Feb;35(2):164–7. https://doi.org/10.1016/j.ejso.2008.06.005
3. Benoist S , Pautrat K , Mitry E , Rougier P , Penna C , Nordlinger B . Treatment strategy for patients with colorectal cancer and synchronous irresectable liver metastases. Br J Surg. 2005 Sep;92(9):1155–60. https://doi.org/10.1002/bjs.5060
4. Ruo L , Gougoutas C , Paty PB , Guillem JG , Cohen AM , Wong WD . Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg. 2003 May;196(5):722–8. https://doi.org/10.1016/S1072-7515(03)00136-4
5. Scoggins CR , Meszoely IM , Blanke CD , Beauchamp RD , Leach SD . Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol. 1999 Oct-Nov;6(7):651–7. https://doi.org/10.1007/s10434-999-0651-x
6. Cotte E , Villeneuve L , Passot G , Boschetti G , Bin-Dorel S , Francois Y , et al. French Research Group of Rectal Cancer Surgery (GRECCAR). GRECCAR 8: impact on survival of the primary tumour resection in rectal cancer with unresectable synchronous metastasis: a randomized multicentre study. BMC Cancer. 2015;15:47. https://doi.org/10.1186/s12885-015-1060-0
7. Gérard JP , Conroy T , Bonnetain F , Bouché O , Chapet O , Closon-Dejardin MT , et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006 Oct;24(28):4620–5. https://doi.org/10.1200/JCO.2006.06.7629
8. Bosset JF , Collette L , Calais G , Mineur L , Maingon P , Radosevic-Jelic L , et al.; EORTC Radiotherapy Group Trial 22921 . Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006 Sep;355(11):1114–23. https://doi.org/10.1056/NEJMoa060829
9. Stillwell AP , Buettner PG , Ho YH . Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg. 2010 Apr;34(4):797–807. https://doi.org/10.1007/s00268-009-0366-y
10. Fowler JF . Sensitivity analysis of parameters in linear-quadratic radiobiologic modeling. Int J Radiat Oncol Biol Phys. 2009 Apr;73(5):1532–7. https://doi.org/10.1016/j.ijrobp.2008.11.039
11. Nahum AE . The radiobiology of hypofractionation. Clin Oncol (R Coll Radiol). 2015 May;27(5):260–9. https://doi.org/10.1016/j.clon.2015.02.001
12. Common Terminology Criteria for Adverse Events (CTCAE) - CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf [Internet]. [cited 2017 Feb 28]. Available 2017 Feb 28, from https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
13. Venderbosch S , de Wilt JH , Teerenstra S , Loosveld OJ , van Bochove A , Sinnige HA , et al. Prognostic value of resection of primary tumor in patients with stage IV colorectal cancer: retrospective analysis of two randomized studies and a review of the literature. Ann Surg Oncol. 2011 Nov;18(12):3252–60. https://doi.org/10.1245/s10434-011-1951-5
14. Ferrand F , Malka D , Bourredjem A , Allonier C , Bouché O , Louafi S , et al. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: results from the multicenter, randomised trial Fédération Francophone de Cancérologie Digestive 9601. Eur J Cancer. 2013 Jan;49(1):90–7. https://doi.org/10.1016/j.ejca.2012.07.006
15. Gresham G , Renouf DJ , Chan M , Kennecke HF , Lim HJ , Brown C , et al. Association between palliative resection of the primary tumor and overall survival in a population-based cohort of metastatic colorectal cancer patients. Ann Surg Oncol. 2014 Nov;21(12):3917–23. https://doi.org/10.1245/s10434-014-3797-0
16. Ishihara S , Hayama T , Yamada H , Nozawa K , Matsuda K , Miyata H , et al. Prognostic impact of primary tumor resection and lymph node dissection in stage IV colorectal cancer with unresectable metastasis: a propensity score analysis in a multicenter retrospective study. Ann Surg Oncol. 2014 Sep;21(9):2949–55. https://doi.org/10.1245/s10434-014-3719-1
17. Norton L , Massagué J . Is cancer a disease of self-seeding? Nat Med. 2006 Aug;12(8):875–8. https://doi.org/10.1038/nm0806-875
18. Kim MY , Oskarsson T , Acharyya S , Nguyen DX , Zhang XH , Norton L , et al. Tumor self-seeding by circulating cancer cells. Cell. 2009 Dec;139(7):1315–26. https://doi.org/10.1016/j.cell.2009.11.025
19. Danna EA , Sinha P , Gilbert M , Clements VK , Pulaski BA , Ostrand-Rosenberg S . Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004 Mar;64(6):2205–11. https://doi.org/10.1158/0008-5472.CAN-03-2646
20. Gérard JP , Conroy T , Bonnetain F , Bouché O , Chapet O , Closon-Dejardin MT , et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006 Oct;24(28):4620–5. https://doi.org/10.1200/JCO.2006.06.7629
21. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. - PubMed - NCBI [Internet]. [Cited 2018 Jul 13]. Available in 2018 Jul 13, from: https://www.ncbi.nlm.nih.gov/pubmed/?term=Stillwell+AP%2C+Buettner+PG%2C+Ho+YH.+Meta-analysis+of+survival+of+patients+with
22. Stelzner S , Hellmich G , Koch R , Ludwig K . Factors predicting survival in stage IV colorectal carcinoma patients after palliative treatment: a multivariate analysis. J Surg Oncol. 2005 Mar;89(4):211–7. https://doi.org/10.1002/jso.20196
Table S1Predictive factors for local relapse in the whole set of patients (n = 148).
| Variable | Univariate analysis | Multivariate analysis | |||
| Tested vs adverse criteria | Logistic regression coefficient (95% CI) | p-value | Logistic regression coefficient (95% CI) | p-value | |
| Age | ≥70 vs <70 | 1.22 (0.48–3.02) | 0.66 | ||
| Gender | Female vs male | 1.28 (0.45–3.33) | 0.62 | ||
| WHO performance status | 2–3 vs 0–1 | 1.26 (0.18–5.49) | 0.78 | ||
| T stage | T4 vs other | 3.44 (0.63–50) | 0.25 | ||
| N stage | N+ vs N0 | 1.96 (0.66–5.56) | 0.21 | ||
| Rectal tumour location | Upper vs middle | 3.0 (0.79–10.8) | 0.09 | NS | |
| Lower vs middle | 1.0 (0.35–2.81) | 1 | |||
| Tumour differentiation | Poor vs well | 2.76 (0.67–11.5) | 0.15 | NS | |
| Moderate vs well | 1.69 (0.57–5.71) | 0.36 | |||
| Metastasis location | Visceral vs bone | 1.28 (0.20–25) | 0.82 | ||
| Histology | Other vs adenocarcinoma | 1.69 (0.08–14.3) | 0.66 | ||
| Rectal surgery | Yes vs no | 0.71 (0.19–1.85) | 0.5 | ||
| Rectal surgery type | TME vs other | 0.74 (0.19–2.38) | 0.63 | ||
| Resection in sano | Yes vs no | 1.00 (0.27–4.84) | 0.99 | ||
| Pre-radiation NLR | >2.8 vs ≤2.8 | 0.61 (0.18–2.06) | 0.42 | ||
| Radiotherapy characteristics | Rectal EQD2 ≥45 Gy vs <45 Gy | 0.58 (0.20–1.65) | 0.30 | ||
| Hypofractionated vs normofractionated | 1.56 (0.47–7.14) | 0.51 | |||
| Concomitant chemotherapy | Yes vs no | 0.60 (0.22–1.84) | 0.34 | ||
95% CI: 95% confidence interval; WHO: World Health Organization; NLR: neutrophil to lymphocyte ratio; EQD2: equivalent dose in 2 Gy per fraction; normofractionated: <2.5 Gy per fraction; hypofractionated: ≥2.5 Gy per fraction; TME: total mesorectal excision, NS: non-significant.
T and N stages were assessed based on the UICC 7th edition.
All p-values ≤0.2 in univariate analysis have been tested in multivariate analysis, except correlated variables (correlation with p <0.001). Finally, only bold typed values were tested in multivariate analysis.
Table S2Predictive factors for local relapse in patients undergoing primary tumour resection (n = 97).
| Variable | UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS | |||
| Tested vs adverse criteria | Logistic regression coefficient (95% CI) | p-value | Logistic regression coefficient (95% CI) | p-value | |
| Age | ≥70 vs <70 | 1.93 (0.65–5.65) | 0.23 | ||
| Gender | Female vs male | 1.83 (0.60–5.42) | 0.28 | ||
| WHO performance status | 2–3 vs 0–1 | 4.48 (0.18–122) | 0.28 | ||
| T stage | T4 vs other | 1.33 (0.20–25) | 0.80 | ||
| N stage | N+ vs N0 | 2.56 (0.75–8.33) | 0.12 | NS | |
| Rectal tumour location | Upper vs middle | 4.64 (1.09–20.1) | 0.04 | NS | |
| Lower vs middle | 10.8 (0.28–39.3) | 0.90 | |||
| Tumour differentiation | Poor vs well | 2.06 (0.34–11.52) | 0.40 | ||
| Moderate vs. well | 1.41 (0.41–5.62) | 0.59 | |||
| Metastasis location | Visceral vs bone | 4.44 (0.17–116) | 0.30 | ||
| Histology | Other vs adenocarcinoma | NA | |||
| Type of rectal surgery | TME vs other | 0.78 (0.20–2.56) | 0.69 | ||
| ypCR | Yes vs No | NA | |||
| Clear surgical margin | Yes vs No | 1.00 (0.27–4.84) | 0.99 | ||
| Preradiation NLR | >2.8 vs ≤2.8 | 0.91 (0.23–3.46) | 0.89 | ||
| Radiotherapy characteristics | Rectal EQD2 ≥45 Gy vs <45 Gy | 0.40 (0.10–1.45) | 0.16 | NS | |
| Hypofractionated vs normofractionated | 2.40 (0.47–10.0) | 0.25 | |||
| Concomitant chemotherapy | Yes vs No | 0.19 (0.05–0.75) | 0.015 | NS | |
95% CI: 95% confidence interval; WHO: World Health Organization; TME: total mesorectal excision; ypCR: pathological complete response (Mandard TRG1); NLR: neutrophil to lymphocyte ratio; NA: not assessable (no event), typically giving infinity values. EQD2: equivalent dose in 2 Gy per fraction; normofractionated: <2.5 Gy per fraction; hypofractionated: ≥2.5 Gy per fraction; NS: non-significant.
T and N stages were assessed based on the UICC 7th edition.
All p-values ≤0.2 in univariate analysis have been tested in multivariate analysis, except correlated variables (correlation with p <0.001). Finally, only bold typed values were tested in multivariate analysis.