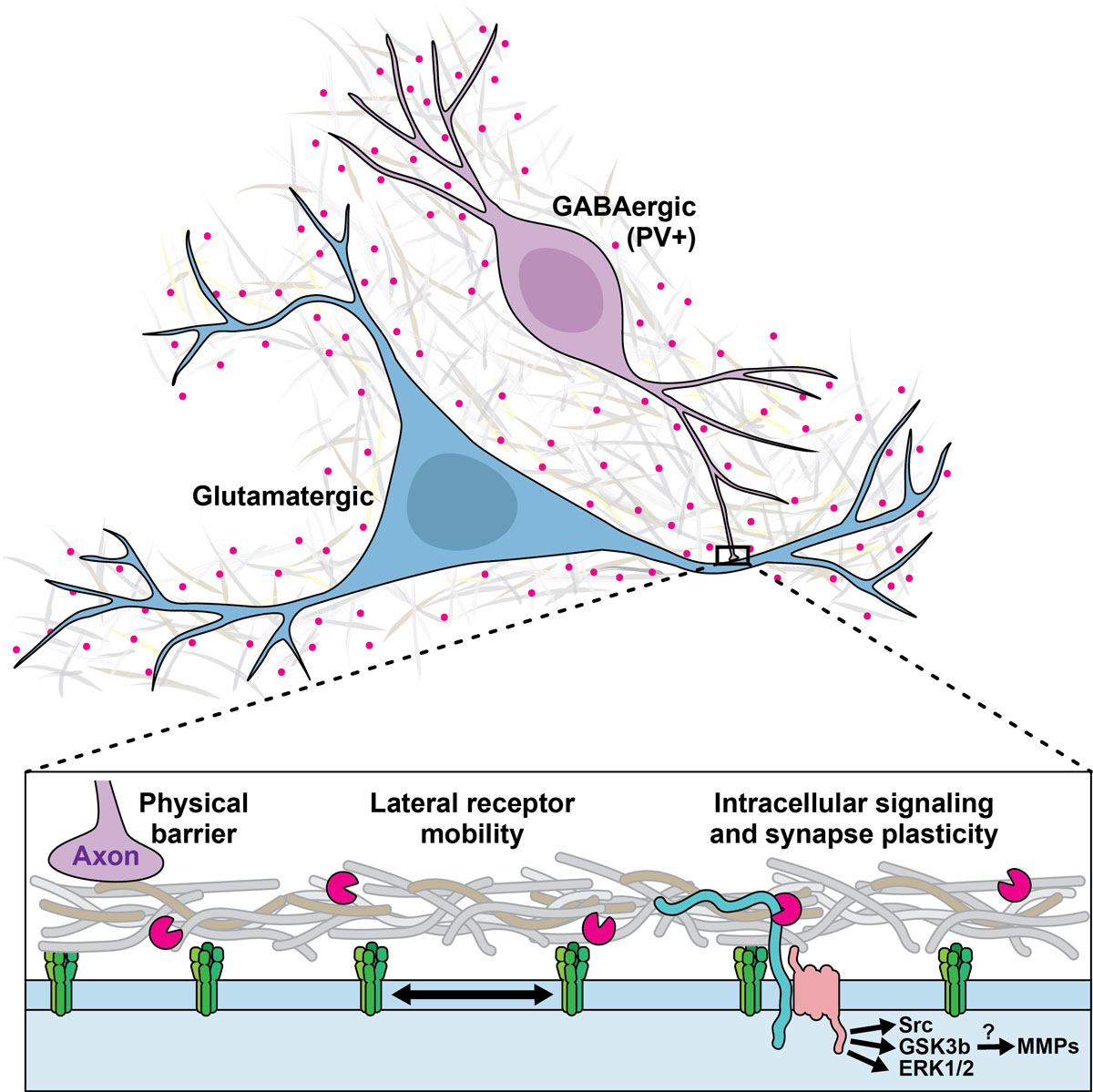
Figure 1 ECM and synaptic plasticity regulation. Different mechanisms underlying the impact of ECM components on synaptic plasticity are suggested.
DOI: https://doi.org/10.4414/smw.2019.20060
The extracellular matrix (ECM) is the structure that surrounds all cells in the body, including neurons and glia in the brain. It makes up about 20% of the brain's entire mass [1]. The ECM is tightly coupled to the cells it surrounds: its components are produced and secreted by the cells, and conversely, they impact back on those cells. The ECM serves various functions across the body: determining mechanical tissue properties, guiding cell migration, providing adhesion loci for individual cells, and enabling intercellular communication [2]. The ECM fills the intercellular space across the brain and spinal cord, and its composition is variable [1]. Here, we focus on two specific components of the ECM in the brain that are involved in higher cognitive functions. Matrix metalloproteinases (MMPs) are enzymes that sculpt the proteoglycan structures of the ECM, but they also play a role in the extracellular signalling pathways involved in the remodelling of excitatory synapses during learning [3, 4]. Perineuronal nets (PNNs) are lattice-like protein structures that scaffold specific neurons, mostly inhibitory interneurons [3]. Their integrity appears to be important for synaptic plasticity and learning [5]. Both these ECM components could, in different parts of neural microcircuits, be key players in the development of disorders based on memory and learning (such as anxiety disorders, post-traumatic stress disorder or addiction), as well as during the plasticity-dependent psychotherapeutic modulation of mental disorders.
This review has three aims. To provide a cognitive background, we first discuss the psychiatric conditions for which synaptic plasticity may be relevant, important research paradigms used to investigate the synaptic basis of memory, and potential strategies for memory modification. We then review the molecular evidence that ECM components are involved in the formation and maintenance of memory, and how this could be leveraged for treatment. Finally, we summarise initial clinical evidence that ECM pathology, such as PNN dysfunction, may be causally involved in some psychiatric conditions.
A range of psychiatric disorders are caused or shaped by previous adverse experiences or potentially traumatic stressors. Prime exemplars are post-traumatic stress disorder (PTSD) and acute stress disorder, which are by definition responses to a specific event. Memories of this event are key to the development of symptoms and thus synaptic changes [6]. Another obvious example is addiction, which requires exposure to a drug. It appears plausible that some form of synaptic plasticity plays a role in the development of addiction [7]. Other disorders may develop with or without the presence of a particular, defined experience, but if present this experience can contribute to the development, maintenance or exacerbation of symptoms. For example, early adverse experience is considered a risk factor for a broad range of psychiatric conditions [8]. A meta-analysis of prospective studies showed that childhood maltreatment increases the risk of later depression by a factor of 2, and of anxiety disorders by a factor of 2.8 [9]. Another type of experience is parental loss due to death or separation, and a retrospective study showed that parental loss before the age of 9 years is about 10 times more frequent in individuals who later develop unipolar depression than in the healthy population [10]. Even disorders with a strong genetic determination, such as schizophrenic psychosis, appear to be exacerbated or worsened by an early adverse experience [11].
These striking correlations motivate us to question how synaptic plasticity mechanisms contribute to the development and maintenance of psychiatric disorders. Because not all individuals exposed to a particular experience (such as trauma) develop a condition (such as PTSD), pre-experience synaptic pathology may be causally involved. Furthermore, when particular memories are important for the maintenance of a disorder, targeting synaptic plasticity mechanisms may provide a way to treat the condition, since synaptic weights form the biological basis of memory [12].
Moreover, synaptic plasticity changes are the biological basis for psychotherapy, a standard first-line or adjunct treatment for most psychiatric conditions. Some forms of psychotherapy (for example, cognitive behavioural therapy) specifically build on learning principles, modulating specific emotional memories or dysfunctional beliefs and cognitions. Learning processes are known to involve synaptic plasticity [6]. Other therapies are not founded primarily on such principles, but nevertheless modify subjective experience and behaviour, which are likely to rely on learning and plasticity in some form.
Therefore, synaptic plasticity provides a way to induce change, independent of the aetiology of a psychiatric condition. This is not unlike the plastic changes generated by physiotherapy or speech therapy after a stroke or brain injury, whose success does not depend on the aetiology of the lesion. Although the precise microscopic and macroscopic mechanisms underlying psychotherapy remain elusive, attempts have been made to integrate psychotherapy with pharmacological interventions aimed at plasticity facilitation [13]. We will review some of these after presenting the laboratory models used to study the synaptic basis of memory.
Memory is the storage of information acquired during an individual's lifetime [14]. Many different forms of memory exist in mammals, including declarative memory, or explicit memory, referring to the conscious, intentional recollection of factual information and previous experiences, thus corresponding to the colloquial meaning of the word "memory". There are also other ways in which information is retained, including partly or entirely non-conscious memories (e.g. motor memory). At least some of them involve plasticity in macroscopically distinct brain areas [15]. Some of the experience-dependent mechanisms discussed in the context of psychiatric disorders involve memories for reward prediction or memories for particular behavioural patterns. However, adverse experience is arguably the most widely relevant type of experience in the causation and development of psychiatric conditions. This is why laboratory research has largely focused on aversive memories. A memory test used particularly widely in humans and non-humans is Pavlovian fear conditioning, also termed threat conditioning [16]. This paradigm, in which an individual is passively exposed to predictors of an aversive event, is experimentally simple and serves as a basic mechanistic model of psychiatric conditions such as post-traumatic stress disorder [17]. In this paradigm an initially neutral cue (e.g. a coloured light, termed the conditioned stimulus, CS+) is contingently coupled with an aversive event (e.g. an electric shock, termed the unconditioned stimulus, US). Many animal species, including humans, show responses to the CS+ after a few pairings, for example freezing behaviour in rodent species, or bradycardia in many mammals including humans [18]. This type of memory is usually retained for a prolonged period of time. In animals, it can be retained for a lifetime [19]. Crucially, and with relevance to psychotherapy, such memories may persist even if the animal (or human) is exposed to contrary experiences. In an experimental paradigm termed extinction learning, the individual is exposed to the same CS+, but this time no longer followed by the US, and over time they will reduce their conditioned response to the CS+. Crucially, the initial fear memory remains intact during extinction, but the organism acquires an additional, inhibitory memory about the safety of the CS+ [20]. The persistence of the initial fear memory can be probed in tests in which the context changes from the extinction context. For example, conditioned responses re-emerge after a passage of time (spontaneous recovery, change of temporal context), after the delivery of an unsignalled US (reinstatement, change of threat context), or when exposed to a novel environment (renewal, change of spatial context) [21]. This may resemble the clinical phenomenon of relapse in the years after, for example, cognitive-behavioural psychotherapy for phobia [21].
Learning theory regards extinction as a form of meta-learning. Memories do not get “erased” and the individual continues to remember that the CS+ may indicate danger, but it also learns that this association is currently not applicable [22]. According to this theory, during fear acquisition the agent learns that there exists a 'latent cause' that generates a US contingent on CS+ presentation. During extinction, they learn that this 'latent cause' is currently absent – but that it may still exist somewhere. Such a model is supported by recent studies of neural ensemble coding during the formation and extinction of rodent fear memories in which extinction does not return the relevant neural populations to the default state [23]. In novel spatiotemporal environments (recovery/renewal), or in contexts where the threat was recently encountered (reinstatement), the agent will infer that the initial cause of the US is likely to be present again, and thus respond to the CS+. This is why it appears to be very difficult to entirely “erase” a fear memory with behavioural (non-pharmacological) procedures once it has been acquired [21], although it may not be impossible [24]. More recent work has therefore looked into the possibility of pharmacologically altering fear memory.
Importantly, cued Pavlovian fear conditioning is only one model of aversive memory, and mainly based on synaptic plasticity in basolateral and central amygdala. Other, closely related memory models, such as contextual conditioning or trace fear conditioning (where the predictor and outcome are separated by a temporal gap), require synaptic plasticity in macroscopically distinct brain structures such as the hippocampus [25, 26], and fear extinction crucially relies on prefrontal cortex plasticity [20]. Close scrutiny is therefore required to determine whether results from fear conditioning paradigms can be extrapolated to other experimental paradigms or to clinical application.
After a memory is consolidated in a protein synthesis-dependent process, retrieval of that memory renders it labile [20]. Under physiological conditions, a subsequent spontaneous reconsolidation process stabilises the memory. Chemically blocking this process erases fear memories in rodents [27]. In this reconsolidation blockade paradigm, an animal is exposed to a CS+/US coupling on day one. After this memory is consolidated on day two, the memory is re-activated by the presentation of just one CS+. Immediately afterwards, the protein synthesis inhibitor anisomycin is injected into the amygdala, the structure in which synaptic re-modelling to store the CS+/US association occurs [20]. On day three, the animal is exposed to the CS+ again. Unlike on day one, the animal shows no conditioned freezing response to the CS+. However, if anisomycin is injected six hours after re-activation rather than immediately after it, the fear memory is left intact [27]. Cued fear memory can be erased not only on the day after training, but also two weeks later [27]. Although contextual memories appear to become more stable after systems-level consolidation, they can still be erased after more than five weeks [28], and a clear temporal boundary when they become inerasable has not yet been found [29]. Reconsolidation appears to truly erase fear memories, i.e. they don't re-appear after reinstatement or recovery [30]. A pre-condition for retrieval to make a memory labile appears to be that the retrieval experience is not entirely predictable [31]. In the initial study by Nader et al., for example, the CS+ was not followed by the US, and this omission of the US constituted an unexpected event.
The precise boundary conditions for reconsolidation blockade are still unknown [29]. Nevertheless, the procedure provides a potential way of modifying memory by re-activating a particular memory and then interfering with its reconsolidation. Anisomycin is toxic for humans, but other interventions have been repeatedly demonstrated to block reconsolidation in rodents as well: electroconvulsive shock [32], amnesic agents such as benzodiazepines [33] and the noradrenalin antagonist propranolol [34]. In humans, electroconvulsive shock [35] and propranolol [36–38] have been shown to impair reconsolidation. Purely behavioural manipulations (stress, information interference, retrieval-extinction) have also been suggested as ways to disrupt reconsolidation, but were harder to replicate across studies [39, 40].
Interestingly, post-retrieval propranolol appeared to block the memory for involuntary conditioned responses to the CS+, but not the declarative recollection of CS+/US associations [36]. Several studies have demonstrated the potential of the reconsolidation blockade approach for translational neuroscience and its potential clinical relevance [39, 41]. Since ECM components play a role in the synaptic remodelling that underlies memory formation and maintenance, this encourages investigations into their contribution to synaptic plasticity during reconsolidation.
MMPs are a group of enzymes (23 in humans) that are involved in the structural remodelling of the ECM inside and outside the brain [4]. The most widely researched MMP is arguably MMP-9, for which marker reagents exist [4]. MMPs’ contribution to structural ECM pathology outside the brain has long been recognised, and MMP inhibitors are clinically tested or approved to treat conditions such as breast cancer [42], corneal erosion [43], rosacea [44] and periodontitis [44]. Similarly, MMPs are involved in the pathological proteolysis which occurs in brain pathologies such as hypoxia and ischaemia, traumatic injury and neuroinflammation [4]. More recently, it has emerged that MMPs are also involved in an extracellular signalling pathway that drives learning on a short timescale of about 30 minutes (figure 1). The level of activity of several MMPs is increased in behavioural tasks involving learning and memory [45, 46]. More specifically, the electrophysiological phenomenon of long-term potentiation (LTP) is based on structural synaptic changes that causally underlie learning and memory [6, 47]. MMP-9 is a critical component in the signalling pathway that leads to some forms of LTP, such as late-phase LTP. Pharmacological MMP-9 inhibition, applying an endogenous tissue inhibitor of metalloproteinases (TIMP), or genetic knock-out of MMP-9 reduces LTP [48–52]. MMP-9 is transported to synapses at times of neural activity [53], and co-localises with the NMDA- and AMPA-receptors involved in LTP [54]. Crucially, when MMP-9, which is typically released as an inactive preform, is activated and applied to the synapse, it chemically induces LTP [48, 50]. This supports the view that MMP-9 has an instructive role in a signalling pathway leading to LTP, although this role appears to vary depending on the pathways and receptor types involved [55]. Beyond LTP, synaptic structure changes induced by MMP-9 have been demonstrated [50]. As a possible pathway, it has been suggested that stimulation of the serotonin receptor 5-HT7R activates MMP-9, which cleaves the transmembrane protein CD44, a receptor for the ECM component hyaluron, and that this pathway, also involving the GTPase Cdc42, contributes to dendritic spine remodelling and LTP [56]. The extent to which other known proteolytic targets (e.g. growth factors and their precursors, cell surface receptors, several cell adhesion molecules) or non-proteolytic binding targets of MMP-9 contribute to this pathway remains unknown [57].

Figure 1 ECM and synaptic plasticity regulation. Different mechanisms underlying the impact of ECM components on synaptic plasticity are suggested.
While the aforementioned studies were conducted in slice preparations, there is also in vivo evidence for MMP-9’s involvement in learning and memory. In rodents, MMP inhibition reduces spatial and contextual learning [58, 59]. Regarding Pavlovian fear conditioning, one rodent study suggested that MMP-9 inhibition does not impair one-trial learning – a specific form of fear conditioning – but does inhibit the reconsolidation of fear memories acquired through a more standard protocol with four learning trials [60]. In humans, it has been shown that the broad spectrum MMP inhibitor doxycycline reduces Pavlovian fear acquisition/consolidation [61]. Since doxycycline is a clinically approved and relatively safe drug, it may provide a useful mechanism of action for the translational application of MMP-9 interference. Other forms of fear conditioning that require plasticity outside the amygdala are yet to be investigated. For example, hippocampal synaptic plasticity is required for trace conditioning (where the CS+ and US are separated by a time interval) [25] and context conditioning (where a spatial context predicts the US) [26].
PNNs are a particular ECM structure that is only found in the brain, and only around certain neurons and in certain brain regions. They were characterised by Golgi in 1898, but remained a specialist topic until the late 20th century [62]. PNN development is terminated during adolescence [63]. A wealth of research has demonstrated that PNN maturation ends critical periods in the visual system. For example, monocular deprivation in young animals impedes the development of vision in the deprived eye [64]. In adults, monocular deprivation has no such impact. The critical period for this impairment ends when PNNs become fully organised in the visual cortex, and chemical degradation of PNNs reinstates the critical period: monocular deprivation will then reduce vision for the deprived eye [65]. The deposition of PNNs appears to be related to neural activity [66]. Thus, the role of PNNs is often described as limiting neural plasticity after critical periods during adolescence [63]. This has driven exploration of the role of PNNs in other learning processes.
Indeed, a similar critical period is observed for Pavlovian fear conditioning. Adult rats (i.e. on postnatal day 23) pass tests for the stability of fear memory after extinction training. Younger rats, however, (i.e. postnatal day 16 days) appear to completely forget ('erase') fear memory during extinction, rather than establish competing memories [67–69]. This critical period is limited by the maturation of PNNs in basolateral amygdala, where synaptic plasticity is required for fear conditioning [5]. When adult PNNs in amygdala are chemically degraded before fear conditioning, the ensuing fear memories can be fully erased by extinction training [5]. Similarly, the auditory cortex is relevant for some forms of auditory learning, and degrading PNNs increases flexibility for re-learning [70]. On the other hand, PNNs may also be required for the initial learning process (which was unimpaired in the aforementioned studies). For example, fear memory with a simple auditory CS+ does not initially require the auditory cortex, but its consolidation takes place in the auditory cortex. Degrading auditory cortex PNNs appears to either block consolidation, or to make consolidated fear memory malleable for erasure [71]. All of these data suggest that PNNs play a profound role in memory formation and maintenance.
It is not yet known whether this role is permissive, i.e. enabling or protecting memory formation that takes place via other mechanisms, or instructive, i.e. providing the actual surrogate for memory [72]. PNN structures are predominantly observed around parvalbumin-expressing GABAergic interneurons [73], and GABAergic inhibitory transmission is fundamental in controlling neuronal excitability, dendritic integration and spike-timing, as well as neuronal synchronisation and the generation of oscillations. PNNs reduce GABAergic inhibition by acting as a physical barrier, limiting neurotransmitter diffusion. The identification of neuronal activity-dependent remodelling and/or degradation of PNNs and other ECM components has fostered several bold hypotheses regarding their function (figure 1) [74]. By compartmentalising the perisynaptic space, PNNs sequestrate neurotrophins and transcription factors and control ligand–cell surface receptor interactions [75]. As a diffusion barrier, the ECM/PNN affects the diffusion of receptors laterally within the plasma membrane and at the synaptic cleft, linking it to a particular form of plasticity known as synaptic scaling [76]. Synaptic scaling allows neurons to compensate for prolonged changes in firing rates by adjusting their synaptic strength. It is crucial for maintaining network stability. At glutamatergic synapses, the PNN protein NARP mediates scaling in an activity-dependent manner by clustering AMPA receptor subunits, while reelin signalling alters the lateral diffusion of NMDA receptors [77, 78]. Lateral diffusion of GABA-A receptors also contributes to synaptic scaling [79]. The mechanism underlying the ECM/PNN-dependent regulation of GABAergic synaptic strength is largely unknown. Interestingly, other ECM structures and enzymes have also been implicated in synaptic plasticity regulation [80, 81].
Synaptic plasticity regulation by both PNNs and MMPs occurs over various timescales. We discussed how MMPs are part of a signalling pathway that induces LTP in excitatory synapses on short timescales of 30-60 minutes. On the other hand, PNNs, which mainly surround inhibitory synapses (pyramidal neurons in hippocampal area CA2 appear to be an exception to this rule [82]), permanently inhibit synaptic plasticity. MMPs are involved in, and possibly required for, the degradation of the molecules that make up PNNs [83]. Many memories undergo systems-level consolidation on timescales of hours. This is accompanied by the electrophysiological phenomenon of sharp-wave ripple (SWR) events in the hippocampus [84]. Degradation of PNNs reduces the occurrence of this phenomenon [85], and thus PNNs also contribute to systems-level consolidation. Finally, on a timescale of a day, SWRs are intimately associated with sleep, which itself strongly facilitates memory consolidation. The processes involved here are likely twofold: the reinforcement of some synapses and the weakening of others [86]. It is possible that SWRs play a role in both processes [87, 88] through mechanisms that are not yet well understood. Roles for MMPs and PNNs here seem promising, but are unexplored. This long-term temporal regulation is preserved even on a cellular level: hippocampal LTP is regulated in circadian fashion both in vitro and in vivo, likely regulated by circadian MAPK signalling [89]. In turn, though the daily regulation of PNNs and neuronal MMPs is unknown, the regulation of MMPs by MAPK pathways during astrogliosis is well-documented [90].
Post-mortem studies have revealed that PNNs decrease in several brain areas in schizophrenia [91–93] and to a lesser extent in bipolar disorder [92]. PNN integrity may also be involved in the pathogenesis of depression [94, 95]. The expression of several ECM molecules, including chondroitin sulfate proteoglycans [92, 93] and reelin [96], is also decreased in schizophrenia. In a genome-wide association study, a genetic polymorphism in a region coding for the ECM enzyme MMP-16 was associated with schizophrenia [97]. Phenotype-based genetic association studies found a relationship between the early onset of schizophrenia and a polymorphism in the region coding for the cell adhesion molecule contactin-4, and also a relationship between the severity of chronic delusions and a polymorphism in a non-coding region related to the MMP-9 gene [98]. The latter polymorphism appears to relate to MMP-9 expression and dendritic spine morphology in vitro, and to phenotypic changes in mice [98].
Post-mortem studies have also suggested that reelin expression is reduced in autism [99]. Other disorders where MMP has been implicated in one way or another include addiction [100], as well as affective disorders and fragile X syndrome, for which MMP-inhibitors are being evaluated as a treatment (see [57, 94] for review). Since Fragile X syndrome is linked to sleep and circadian disturbances [101, 102], there is also the possibility that MMPs might play similar roles, though this has not been investigated.
ECM alterations could contribute to pathology in many different ways, ranging from developmental changes affecting cell migration and axonal guidance to the ECM's permissive role in providing a suitable environment for neuronal function, and to more specific influences on synaptic plasticity [63]. An influential theory has suggested that reduced PNN integrity in schizophrenia may increase the vulnerability of parvalbumin-positive interneurons to oxidative stress, which would reciprocally degrade PNNs further [103].
We have reviewed the literature on ECM contributions to learning and memory, and how this could be leveraged to understand the experience dependence of psychiatric conditions or contribute to learning-based treatments. A novel avenue for the treatment of pathological memories is reconsolidation blockade, which has – in different forms and with mixed success [39] – already been translated into clinical pilot trials. We suggest that ECM contributions to plasticity provide a mechanism by which to impact on memory consolidation and reconsolidation. Future work will focus on understanding the underlying pathways, their potential influence upon pathology, and their clinical translation to advance psychiatric treatment. Researchers have called for the increased integration of basic science findings in order to better understand treatment mechanisms and to increase the effectiveness of evidence-based treatments for psychiatric disorders [104]. Significant strides have been made in developing and refining psychotherapeutic treatments, which are recommended as first-line treatments for many psychiatric disorders, including anxiety and PTSD. However, there is significant room to improve these treatments, and they may benefit from innovations such as ECM modification.
This work was supported by the University of Zurich’s Clinical Research Priority Program for the CRPP "Synapse & Trauma".
No potential conflict of interest relevant to this article was reported.
1 Nicholson C , Syková E . Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21(5):207–15. doi:.https://doi.org/10.1016/S0166-2236(98)01261-2
2 Gundelfinger ED , Frischknecht R , Choquet D , Heine M . Converting juvenile into adult plasticity: a role for the brain’s extracellular matrix. Eur J Neurosci. 2010;31(12):2156–65. doi:.https://doi.org/10.1111/j.1460-9568.2010.07253.x
3 Ethell IM , Ethell DW . Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85(13):2813–23. doi:.https://doi.org/10.1002/jnr.21273
4 Huntley GW . Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13(11):743–57. doi:.https://doi.org/10.1038/nrn3320
5 Gogolla N , Caroni P , Lüthi A , Herry C . Perineuronal nets protect fear memories from erasure. Science. 2009;325(5945):1258–61. doi:.https://doi.org/10.1126/science.1174146
6 Whitlock JR , Heynen AJ , Shuler MG , Bear MF . Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–7. doi:.https://doi.org/10.1126/science.1128134
7 Stoker AK , Markou A . Neurobiological Bases of Cue- and Nicotine-induced Reinstatement of Nicotine Seeking: Implications for the Development of Smoking Cessation Medications. Curr Top Behav Neurosci. 2015;24:125–54. doi:.https://doi.org/10.1007/978-3-319-13482-6_5
8 Green JG , McLaughlin KA , Berglund PA , Gruber MJ , Sampson NA , Zaslavsky AM , et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67(2):113–23. doi:.https://doi.org/10.1001/archgenpsychiatry.2009.186
9 Li M , D’Arcy C , Meng X . Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: systematic review, meta-analysis, and proportional attributable fractions. Psychol Med. 2016;46(4):717–30. doi:.https://doi.org/10.1017/S0033291715002743
10 Agid O , Shapira B , Zislin J , Ritsner M , Hanin B , Murad H , et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4(2):163–72. doi:.https://doi.org/10.1038/sj.mp.4000473
11 Abel KM , Heuvelman HP , Jörgensen L , Magnusson C , Wicks S , Susser E , et al. Severe bereavement stress during the prenatal and childhood periods and risk of psychosis in later life: population based cohort study. BMJ. 2014;348(jan21 2):f7679. doi:.https://doi.org/10.1136/bmj.f7679
12 Mayford M , Siegelbaum SA , Kandel ER . Synapses and memory storage. Cold Spring Harb Perspect Biol. 2012;4(6):a005751. doi:.https://doi.org/10.1101/cshperspect.a005751
13 Hofmann SG , Otto MW , Pollack MH , Smits JA . D-cycloserine augmentation of cognitive behavioral therapy for anxiety disorders: an update. Curr Psychiatry Rep. 2015;17(1):532. doi:.https://doi.org/10.1007/s11920-014-0532-2
14Tulving E, Donaldson W. Episodic and semantic memory. In: Organization of Memory. Cambridge, MA: Academic Press Inc; 1972. pp 381–403.
15 Basu J , Siegelbaum SA . The Corticohippocampal Circuit, Synaptic Plasticity, and Memory. Cold Spring Harb Perspect Biol. 2015;7(11):a021733. doi:.https://doi.org/10.1101/cshperspect.a021733
16 LeDoux JE . Coming to terms with fear. Proc Natl Acad Sci USA. 2014;111(8):2871–8. doi:.https://doi.org/10.1073/pnas.1400335111
17 Calhoon GG , Tye KM . Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18(10):1394–404. doi:.https://doi.org/10.1038/nn.4101
18 Castegnetti G , Tzovara A , Staib M , Paulus PC , Hofer N , Bach DR . Modeling fear-conditioned bradycardia in humans. Psychophysiology. 2016;53(6):930–9. doi:.https://doi.org/10.1111/psyp.12637
19 Gale GD , Anagnostaras SG , Godsil BP , Mitchell S , Nozawa T , Sage JR , et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24(15):3810–5. doi:.https://doi.org/10.1523/JNEUROSCI.4100-03.2004
20 Herry C , Ferraguti F , Singewald N , Letzkus JJ , Ehrlich I , Lüthi A . Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31(4):599–612. doi:.https://doi.org/10.1111/j.1460-9568.2010.07101.x
21 Dunsmoor JE , Niv Y , Daw N , Phelps EA . Rethinking Extinction. Neuron. 2015;88(1):47–63. doi:.https://doi.org/10.1016/j.neuron.2015.09.028
22 Gershman SJ , Blei DM , Niv Y . Context, learning, and extinction. Psychol Rev. 2010;117(1):197–209. doi:.https://doi.org/10.1037/a0017808
23 Grewe BF , Gründemann J , Kitch LJ , Lecoq JA , Parker JG , Marshall JD , et al. Neural ensemble dynamics underlying a long-term associative memory. Nature. 2017;543(7647):670–5. doi:.https://doi.org/10.1038/nature21682
24 Gershman SJ , Jones CE , Norman KA , Monfils MH , Niv Y . Gradual extinction prevents the return of fear: implications for the discovery of state. Front Behav Neurosci. 2013;7:164. doi:.https://doi.org/10.3389/fnbeh.2013.00164
25 Connor DA , Gould TJ . The role of working memory and declarative memory in trace conditioning. Neurobiol Learn Mem. 2016;134(Pt B):193–209. doi:.https://doi.org/10.1016/j.nlm.2016.07.009
26 Chaaya N , Battle AR , Johnson LR . An update on contextual fear memory mechanisms: Transition between Amygdala and Hippocampus. Neurosci Biobehav Rev. 2018;92:43–54. doi:.https://doi.org/10.1016/j.neubiorev.2018.05.013
27 Nader K , Schafe GE , Le Doux JE . Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–6. doi:.https://doi.org/10.1038/35021052
28 Frankland PW , Ding HK , Takahashi E , Suzuki A , Kida S , Silva AJ . Stability of recent and remote contextual fear memory. Learn Mem. 2006;13(4):451–7. doi:.https://doi.org/10.1101/lm.183406
29 Kindt M . The surprising subtleties of changing fear memory: a challenge for translational science. Philos Trans R Soc Lond B Biol Sci. 2018;373(1742):20170033. doi:.https://doi.org/10.1098/rstb.2017.0033
30 Lin HC , Mao SC , Gean PW . Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Mem. 2006;13(3):316–21. doi:.https://doi.org/10.1101/lm.217006
31 Pedreira ME , Pérez-Cuesta LM , Maldonado H . Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn Mem. 2004;11(5):579–85. doi:.https://doi.org/10.1101/lm.76904
32 Misanin JR , Miller RR , Lewis DJ . Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160(3827):554–5. doi:.https://doi.org/10.1126/science.160.3827.554
33 Bustos SG , Maldonado H , Molina VA . Midazolam disrupts fear memory reconsolidation. Neuroscience. 2006;139(3):831–42. doi:.https://doi.org/10.1016/j.neuroscience.2005.12.064
34 Przybyslawski J , Roullet P , Sara SJ . Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19(15):6623–8. doi:.https://doi.org/10.1523/JNEUROSCI.19-15-06623.1999
35 Kroes MC , Tendolkar I , van Wingen GA , van Waarde JA , Strange BA , Fernández G . An electroconvulsive therapy procedure impairs reconsolidation of episodic memories in humans. Nat Neurosci. 2014;17(2):204–6. doi:.https://doi.org/10.1038/nn.3609
36 Kindt M , Soeter M , Vervliet B . Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12(3):256–8. doi:.https://doi.org/10.1038/nn.2271
37 Visser RM , Scholte HS , Kindt M . Associative learning increases trial-by-trial similarity of BOLD-MRI patterns. J Neurosci. 2011;31(33):12021–8. doi:.https://doi.org/10.1523/JNEUROSCI.2178-11.2011
38 Kindt M , Soeter M , Sevenster D . Disrupting reconsolidation of fear memory in humans by a noradrenergic β-blocker. J Vis Exp. 2014;(94). doi:.https://doi.org/10.3791/52151
39 Walsh KH , Das RK , Saladin ME , Kamboj SK . Modulation of naturalistic maladaptive memories using behavioural and pharmacological reconsolidation-interfering strategies: a systematic review and meta-analysis of clinical and ‘sub-clinical’ studies. Psychopharmacology (Berl). 2018;235(9):2507–27. doi:.https://doi.org/10.1007/s00213-018-4983-8
40 Kredlow MA , Unger LD , Otto MW . Harnessing reconsolidation to weaken fear and appetitive memories: A meta-analysis of post-retrieval extinction effects. Psychol Bull. 2016;142(3):314–36. doi:.https://doi.org/10.1037/bul0000034
41 Brunet A , Saumier D , Liu A , Streiner DL , Tremblay J , Pitman RK . Reduction of PTSD Symptoms With Pre-Reactivation Propranolol Therapy: A Randomized Controlled Trial. Am J Psychiatry. 2018;175(5):427–33. doi:.https://doi.org/10.1176/appi.ajp.2017.17050481
42 Jezierska A , Motyl T . Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit. 2009;15(2):RA32–40.
43 Watson SL , Lee MH , Barker NH . Interventions for recurrent corneal erosions. Cochrane Database Syst Rev. 2012;(9):CD001861.
44 Golub LM , Elburki MS , Walker C , Ryan M , Sorsa T , Tenenbaum H , et al. Non-antibacterial tetracycline formulations: host-modulators in the treatment of periodontitis and relevant systemic diseases. Int Dent J. 2016;66(3):127–35. doi:.https://doi.org/10.1111/idj.12221
45 Meighan SE , Meighan PC , Choudhury P , Davis CJ , Olson ML , Zornes PA , et al. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96(5):1227–41. doi:.https://doi.org/10.1111/j.1471-4159.2005.03565.x
46 Conant K , Wang Y , Szklarczyk A , Dudak A , Mattson MP , Lim ST . Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience. 2010;166(2):508–21. doi:.https://doi.org/10.1016/j.neuroscience.2009.12.061
47 Pastalkova E , Serrano P , Pinkhasova D , Wallace E , Fenton AA , Sacktor TC . Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313(5790):1141–4. doi:.https://doi.org/10.1126/science.1128657
48 Nagy V , Bozdagi O , Matynia A , Balcerzyk M , Okulski P , Dzwonek J , et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26(7):1923–34. doi:.https://doi.org/10.1523/JNEUROSCI.4359-05.2006
49 Meighan PC , Meighan SE , Davis CJ , Wright JW , Harding JW . Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J Neurochem. 2007;102(6):2085–96. doi:.https://doi.org/10.1111/j.1471-4159.2007.04682.x
50 Wang XB , Bozdagi O , Nikitczuk JS , Zhai ZW , Zhou Q , Huntley GW . Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci USA. 2008;105(49):19520–5. doi:.https://doi.org/10.1073/pnas.0807248105
51 Gorkiewicz T , Balcerzyk M , Kaczmarek L , Knapska E . Matrix metalloproteinase 9 (MMP-9) is indispensable for long term potentiation in the central and basal but not in the lateral nucleus of the amygdala. Front Cell Neurosci. 2015;9:73. doi:.https://doi.org/10.3389/fncel.2015.00073
52 Okulski P , Jay TM , Jaworski J , Duniec K , Dzwonek J , Konopacki FA , et al. TIMP-1 abolishes MMP-9-dependent long-lasting long-term potentiation in the prefrontal cortex. Biol Psychiatry. 2007;62(4):359–62. doi:.https://doi.org/10.1016/j.biopsych.2006.09.012
53 Dziembowska M , Milek J , Janusz A , Rejmak E , Romanowska E , Gorkiewicz T , et al. Activity-dependent local translation of matrix metalloproteinase-9. J Neurosci. 2012;32(42):14538–47. doi:.https://doi.org/10.1523/JNEUROSCI.6028-11.2012
54 Gawlak M , Górkiewicz T , Gorlewicz A , Konopacki FA , Kaczmarek L , Wilczynski GM . High resolution in situ zymography reveals matrix metalloproteinase activity at glutamatergic synapses. Neuroscience. 2009;158(1):167–76. doi:.https://doi.org/10.1016/j.neuroscience.2008.05.045
55 Wiera G , Nowak D , van Hove I , Dziegiel P , Moons L , Mozrzymas JW . Mechanisms of NMDA Receptor- and Voltage-Gated L-Type Calcium Channel-Dependent Hippocampal LTP Critically Rely on Proteolysis That Is Mediated by Distinct Metalloproteinases. J Neurosci. 2017;37(5):1240–56. doi:.https://doi.org/10.1523/JNEUROSCI.2170-16.2016
56 Bijata M , Labus J , Guseva D , Stawarski M , Butzlaff M , Dzwonek J , et al. Synaptic Remodeling Depends on Signaling between Serotonin Receptors and the Extracellular Matrix. Cell Rep. 2017;19(9):1767–82. doi:.https://doi.org/10.1016/j.celrep.2017.05.023
57 Vafadari B , Salamian A , Kaczmarek L . MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J Neurochem. 2016;139(Suppl 2):91–114. doi:.https://doi.org/10.1111/jnc.13415
58 Nagy V , Bozdagi O , Huntley GW . The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn Mem. 2007;14(10):655–64. doi:.https://doi.org/10.1101/lm.678307
59 Knapska E , Lioudyno V , Kiryk A , Mikosz M , Górkiewicz T , Michaluk P , et al. Reward learning requires activity of matrix metalloproteinase-9 in the central amygdala. J Neurosci. 2013;33(36):14591–600. doi:.https://doi.org/10.1523/JNEUROSCI.5239-12.2013
60 Brown TE , Wilson AR , Cocking DL , Sorg BA . Inhibition of matrix metalloproteinase activity disrupts reconsolidation but not consolidation of a fear memory. Neurobiol Learn Mem. 2009;91(1):66–72. doi:.https://doi.org/10.1016/j.nlm.2008.09.003
61 Bach DR , Tzovara A , Vunder J . Blocking human fear memory with the matrix metalloproteinase inhibitor doxycycline. Mol Psychiatry. 2018;23(7):1584–9.
62 Celio MR , Spreafico R , De Biasi S , Vitellaro-Zuccarello L . Perineuronal nets: past and present. Trends Neurosci. 1998;21(12):510–5. doi:.https://doi.org/10.1016/S0166-2236(98)01298-3
63 Sorg BA , Berretta S , Blacktop JM , Fawcett JW , Kitagawa H , Kwok JC , et al. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J Neurosci. 2016;36(45):11459–68. doi:.https://doi.org/10.1523/JNEUROSCI.2351-16.2016
64 Wiesel TN , Hubel DH . Effects of Visual Deprivation on Morphology and Physiology of Cells in the Cats Lateral Geniculate Body. J Neurophysiol. 1963;26(6):978–93. doi:.https://doi.org/10.1152/jn.1963.26.6.978
65 Pizzorusso T , Medini P , Berardi N , Chierzi S , Fawcett JW , Maffei L . Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298(5596):1248–51. doi:.https://doi.org/10.1126/science.1072699
66 Dityatev A , Brückner G , Dityateva G , Grosche J , Kleene R , Schachner M . Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol. 2007;67(5):570–88. doi:.https://doi.org/10.1002/dneu.20361
67 Kim JH , Richardson R . A developmental dissociation of context and GABA effects on extinguished fear in rats. Behav Neurosci. 2007;121(1):131–9. doi:.https://doi.org/10.1037/0735-7044.121.1.131
68 Kim JH , Richardson R . A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol Learn Mem. 2007;88(1):48–57. doi:.https://doi.org/10.1016/j.nlm.2007.03.004
69 Kim JH , Richardson R . The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. J Neurosci. 2008;28(6):1282–90. doi:.https://doi.org/10.1523/JNEUROSCI.4736-07.2008
70 Happel MF , Niekisch H , Castiblanco Rivera LL , Ohl FW , Deliano M , Frischknecht R . Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proc Natl Acad Sci USA. 2014;111(7):2800–5. doi:.https://doi.org/10.1073/pnas.1310272111
71 Banerjee SB , Gutzeit VA , Baman J , Aoued HS , Doshi NK , Liu RC , et al. Perineuronal Nets in the Adult Sensory Cortex Are Necessary for Fear Learning. Neuron. 2017;95(1):169–179.e3. doi:.https://doi.org/10.1016/j.neuron.2017.06.007
72 Tsien RY . Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc Natl Acad Sci USA. 2013;110(30):12456–61. doi:.https://doi.org/10.1073/pnas.1310158110
73 Härtig W , Derouiche A , Welt K , Brauer K , Grosche J , Mäder M , et al. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999;842(1):15–29. doi:.https://doi.org/10.1016/S0006-8993(99)01784-9
74 Frischknecht R , Chang KJ , Rasband MN , Seidenbecher CI . Neural ECM molecules in axonal and synaptic homeostatic plasticity. Prog Brain Res. 2014;214:81–100. doi:.https://doi.org/10.1016/B978-0-444-63486-3.00004-9
75 Berretta S , Pantazopoulos H , Markota M , Brown C , Batzianouli ET . Losing the sugar coating: potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res. 2015;167(1-3):18–27. doi:.https://doi.org/10.1016/j.schres.2014.12.040
76 Frischknecht R , Heine M , Perrais D , Seidenbecher CI , Choquet D , Gundelfinger ED . Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12(7):897–904. doi:.https://doi.org/10.1038/nn.2338
77 Chang MC , Park JM , Pelkey KA , Grabenstatter HL , Xu D , Linden DJ , et al. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci. 2010;13(9):1090–7. doi:.https://doi.org/10.1038/nn.2621
78 Groc L , Choquet D , Stephenson FA , Verrier D , Manzoni OJ , Chavis P . NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007;27(38):10165–75. doi:.https://doi.org/10.1523/JNEUROSCI.1772-07.2007
79 Bannai H , Lévi S , Schweizer C , Inoue T , Launey T , Racine V , et al. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62(5):670–82. doi:.https://doi.org/10.1016/j.neuron.2009.04.023
80 Conant K , Allen M , Lim ST . Activity dependent CAM cleavage and neurotransmission. Front Cell Neurosci. 2015;9:305. doi:.https://doi.org/10.3389/fncel.2015.00305
81 Sonderegger P , Matsumoto-Miyai K . Activity-controlled proteolytic cleavage at the synapse. Trends Neurosci. 2014;37(8):413–23. doi:.https://doi.org/10.1016/j.tins.2014.05.007
82 Carstens KE , Dudek SM . Regulation of synaptic plasticity in hippocampal area CA2. Curr Opin Neurobiol. 2019;54:194–9.
83 Pollock E , Everest M , Brown A , Poulter MO . Metalloproteinase inhibition prevents inhibitory synapse reorganization and seizure genesis. Neurobiol Dis. 2014;70:21–31. doi:.https://doi.org/10.1016/j.nbd.2014.06.003
84 Buzsáki G . Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25(10):1073–188. doi:.https://doi.org/10.1002/hipo.22488
85 Sun ZY , Bozzelli PL , Caccavano A , Allen M , Balmuth J , Vicini S , et al. Disruption of perineuronal nets increases the frequency of sharp wave ripple events. Hippocampus. 2018;28(1):42–52. doi:.https://doi.org/10.1002/hipo.22804
86 Niethard N , Burgalossi A , Born J . Plasticity during Sleep Is Linked to Specific Regulation of Cortical Circuit Activity. Front Neural Circuits. 2017;11:65. doi:.https://doi.org/10.3389/fncir.2017.00065
87 Khodagholy D , Gelinas JN , Buzsáki G . Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science. 2017;358(6361):369–72. doi:.https://doi.org/10.1126/science.aan6203
88 Norimoto H , Makino K , Gao M , Shikano Y , Okamoto K , Ishikawa T , et al. Hippocampal ripples down-regulate synapses. Science. 2018;359(6383):1524–7. doi:.https://doi.org/10.1126/science.aao0702
89 Xia Z , Storm D . Role of circadian rhythm and REM sleep for memory consolidation. Neurosci Res. 2017;118:13–20. doi:.https://doi.org/10.1016/j.neures.2017.04.011
90 Wang T , Liao Y , Sun Q , Tang H , Wang G , Zhao F , et al. Upregulation of Matrix Metalloproteinase-9 in Primary Cultured Rat Astrocytes Induced by 2-Chloroethanol Via MAPK Signal Pathways. Front Cell Neurosci. 2017;11:218. doi:.https://doi.org/10.3389/fncel.2017.00218
91 Mauney SA , Athanas KM , Pantazopoulos H , Shaskan N , Passeri E , Berretta S , et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74(6):427–35. doi:.https://doi.org/10.1016/j.biopsych.2013.05.007
92 Pantazopoulos H , Markota M , Jaquet F , Ghosh D , Wallin A , Santos A , et al. Aggrecan and chondroitin-6-sulfate abnormalities in schizophrenia and bipolar disorder: a postmortem study on the amygdala. Transl Psychiatry. 2015;5(1):e496. doi:.https://doi.org/10.1038/tp.2014.128
93 Pantazopoulos H , Woo TU , Lim MP , Lange N , Berretta S . Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67(2):155–66. doi:.https://doi.org/10.1001/archgenpsychiatry.2009.196
94 Pantazopoulos H , Berretta S . In Sickness and in Health: Perineuronal Nets and Synaptic Plasticity in Psychiatric Disorders. Neural Plast. 2016;2016:9847696. doi:.https://doi.org/10.1155/2016/9847696
95 Lorenzo Bozzelli P , Alaiyed S , Kim E , Villapol S , Conant K . Proteolytic Remodeling of Perineuronal Nets: Effects on Synaptic Plasticity and Neuronal Population Dynamics. Neural Plast. 2018;2018:5735789. doi:.https://doi.org/10.1155/2018/5735789
96 Guidotti A , Auta J , Davis JM , Di-Giorgi-Gerevini VJ , Dwivedi Y , Grayson DR , et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57(11):1061–9. doi:.https://doi.org/10.1001/archpsyc.57.11.1061
97 Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7. doi:.https://doi.org/10.1038/nature13595
98 Lepeta K , Purzycka KJ , Pachulska-Wieczorek K , Mitjans M , Begemann M , Vafadari B , et al. A normal genetic variation modulates synaptic MMP-9 protein levels and the severity of schizophrenia symptoms. EMBO Mol Med. 2017;9(8):1100–16. doi:.https://doi.org/10.15252/emmm.201707723
99 Fatemi SH , Snow AV , Stary JM , Araghi-Niknam M , Reutiman TJ , Lee S , et al. Reelin signaling is impaired in autism. Biol Psychiatry. 2005;57(7):777–87. doi:.https://doi.org/10.1016/j.biopsych.2004.12.018
100 Stefaniuk M , Beroun A , Lebitko T , Markina O , Leski S , Meyza K , et al. Matrix Metalloproteinase-9 and Synaptic Plasticity in the Central Amygdala in Control of Alcohol-Seeking Behavior. Biol Psychiatry. 2017;81(11):907–17. doi:.https://doi.org/10.1016/j.biopsych.2016.12.026
101 Bushey D , Tononi G , Cirelli C . The Drosophila fragile X mental retardation gene regulates sleep need. J Neurosci. 2009;29(7):1948–61. doi:.https://doi.org/10.1523/JNEUROSCI.4830-08.2009
102 Gatto CL , Broadie K . The fragile X mental retardation protein in circadian rhythmicity and memory consolidation. Mol Neurobiol. 2009;39(2):107–29. doi:.https://doi.org/10.1007/s12035-009-8057-0
103 Do KQ , Cuenod M , Hensch TK . Targeting Oxidative Stress and Aberrant Critical Period Plasticity in the Developmental Trajectory to Schizophrenia. Schizophr Bull. 2015;41(4):835–46. doi:.https://doi.org/10.1093/schbul/sbv065
104 Holmes EA , Craske MG , Graybiel AM . Psychological treatments: A call for mental-health science. Nature. 2014;511(7509):287–9. doi:.https://doi.org/10.1038/511287a
This work was supported by the University of Zurich’s Clinical Research Priority Program for the CRPP "Synapse & Trauma".
No potential conflict of interest relevant to this article was reported.