Clinical Research Core Competencies Framework
Melanie Glaettli, Laura di Petto, Aurélie Fayet-Mello, Claudia Fila, Simone Kälin, Sandra Kohlmaier, Verena Küppers, Antoine Poncet
A reference for the education and career development of research professionals conducting clinical trials in Switzerland
Clinical research – and especially the development of medicinal products through clinical trials – is heavily regulated. This is primarily to ensure participants’ safety, protection and privacy as well as the quality of data and reliability of results. Despite strict regulations, national and international guidelines and laws set only vague requirements for the qualifications of sponsor-investigators, investigators and their research teams. For example, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guideline for good clinical practice only indicates that investigators need the appropriate “education, training, and experience to assume the responsibility for the proper conduct of the trial” (ICH GCP E6(R2) section 4.1.1). Likewise, Switzerland’s human research ordinances only refer generally to professional qualifications (Art. 4 of the Human Research Ordinance (HRO), Art. 6 of the Clinical Trials Ordinance (ClinO), and Art. 5 of the Ordinance on Clinical Trials with Medical Devices (ClinO-MD)). Consequently, despite innovative and successful research activities in Switzerland, ethics committees still encounter deficient clinical trials (e.g., incomplete study protocols), as pointed out by a 2014 report from the Federal Office of Public Health (FOPH) and the Swiss Academy of Medical Sciences (SAMS) (in French and in German).
The SAMS has also highlighted the importance of having well-trained, research-oriented physicians in its position papers “Medicine as a science” and “Culture of research and support for young scientists in medicine”. The FOPH addressed the most important recommendations from these position papers in the form of five work packages in its Roadmap 2016–2021 for Developing the Next Generation of Clinical Researchers (subsequently referred to as “Roadmap”). The Education Platform of the Swiss Clinical Trial Organisation (SCTO) was mandated to carry out the Roadmap’s second work package: to define the minimum skills and competencies required by physicians to undertake clinical research and therefore to provide training details not explicitly mentioned in national legislation and international guidelines. We hereby present the procedure followed by the Education Platform to fulfil this mandate, the results of this process, and the next steps and outlook.
Procedure
1. Review existing frameworks
A review of existing competencies frameworks showed that several of them are dedicated to a specific role (e.g., investigators, clinical research coordinators or study nurses). However, two of the frameworks reviewed are more general and applicable to entire research teams and the various roles involved in running a clinical trial: the Joint Task Force (JTF) Core Competency Framework for Clinical Research Professionals and the Training in Tropical Diseases and Researchers (TDR) Global Competency Framework for Clinical Research.
2. Adapt a framework to Swiss requirements
The Education Platform decided to create a framework based on the JTF Framework because it is updated regularly and because several institutions and countries use it to define role descriptions and comprehensive training programmes, to improve the quality of trials, and to facilitate regulatory compliance (see examples). Moreover, the JTF Framework includes three proficiency levels, thus making it possible to determine career perspectives. The Education Platform’s Clinical Research Core Competencies (CRCC) Framework is therefore an adaptation of the JTF Framework to the Swiss Human Research Act (HRA) and its ordinances on clinical trials. Like the JTF Framework (fig. 1), the CRCC Framework is comprised of eight main competency domains.
Figure 1. Competency domains for clinical research teams. Figure "JTF Core Competency Framework for Clinical Research Professionals" from https://mrctcenter.org/clinical-trial-competency, used with kind permission.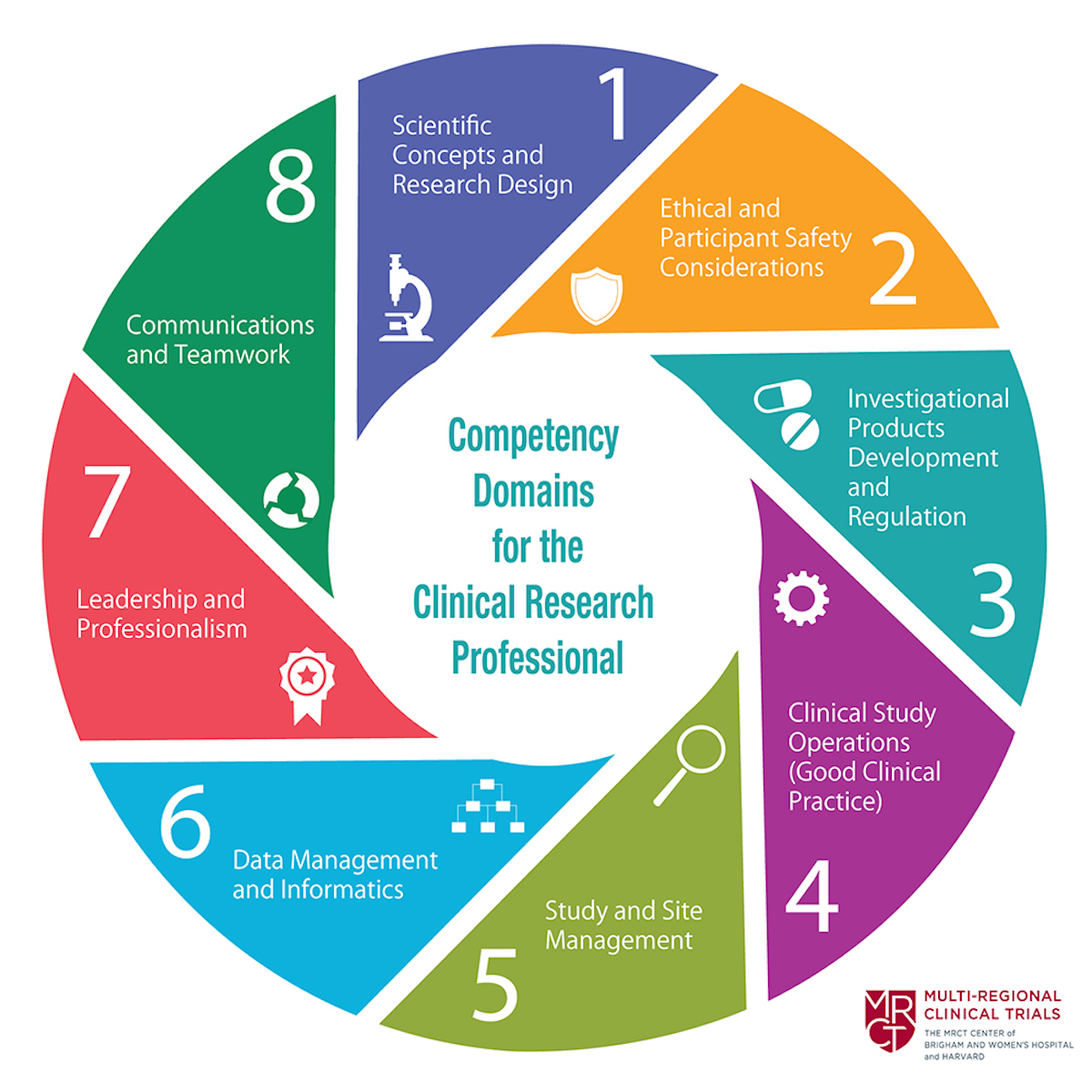
Each domain contains 5 to 10 competency statements, which are split into three proficiency levels and illustrated with examples. To take into account the FOPH’s mandate to focus on physician-scientists, the Education Platform used the three proficiency levels to define the competencies to be acquired by sub-investigators, investigators and sponsor-investigators (according to ICH GCP E6(R2)). However, this focus on investigator roles does not preclude applying the CRCC Framework to entire clinical research teams, since investigators are accountable for the conduct of research performed under their supervision.
3. Consult clinicians involved in clinical trials
In order to confirm that the CRCC Framework is comprehensive, a workshop was held with 11 junior and senior physician-scientists. In preparation for the workshop, participants were asked to assess the extent to which they delegate competencies to their research teams. Eight of them provided this information in written form. The domains dealing with clinical research operations and data management were delegated most often, and delegation increased with investigators’ experience (table 1) and most likely with the size of their research team.
| Table 1. Mean (standard deviation) percentage of competencies delegated by junior and senior physician-scientists. | ||
| Competency domain | Junior physician-scientists (n = 3) | Senior physician-scientists (n = 5) |
| Scientific concepts and research design | 5 (5) | 23 (27) |
| Ethics and participant rights | 2 (3) | 24 (17) |
| Healthcare intervention development and regulation | 30 (30) | 32 (18) |
| Clinical research operations (Good Clinical Practice) | 37 (21) | 64 (25) |
| Trial and site management | 20 (10) | 38 (26) |
| Data management | 52 (28) | 74 (18) |
| Leadership and professionalism | 10 (7) | 18 (22) |
| Communication and teamwork | 5 (5) | 46 (26) |
The workshop revealed how research competencies are acquired through both hands-on experience and theoretical training (table 2).
| Table 2. Mean (standard deviation) proportion of acquisition of research competencies through theoretical training and hands-on experience (n = 8). | ||
| Competency domain | Theoretical training | Hands-on experience |
| Scientific concepts and research design | 51 (16) | 49 (16) |
| Ethics and participant rights | 61 (24) | 39 (24) |
| Healthcare intervention development and regulation | 58 (22) | 42 (22) |
| Clinical research operations (Good Clinical Practice) | 60 (21) | 40 (21) |
| Trial and site management | 33 (18) | 67 (18) |
| Data management | 36 (18) | 64 (18) |
| Leadership and professionalism | 34 (21) | 66 (21) |
| Communication and teamwork | 25 (8) |
75 (8) |
Furthermore, participants recognised the importance of having a detailed competency framework since clinical research is a rigorous and highly regulated field of research.
4. Get stakeholder acknowledgment
The CRCC Framework was acknowledged by stakeholders responsible for setting up and writing the Roadmap. And a comparison of the CRCC Framework with all GCP courses taught by the Clinical Trials Unit (CTU) Network (covering learning objectives formulated by swissethics) showed that the CRCC Framework covers the content of the mandatory GCP courses for investigators and sponsor-investigators conducting clinical trials.
Results
The interactive CRCC Framework is available on the Clinical Research Careers (CR Careers) website and contains links to the SCTO’s database of academic clinical research postgraduate training options in Switzerland. The CR Careers website was developed by the SAMS, Médecine Universitaire Suisse (unimedsuisse), and the SCTO. It aims to support young and future physicians embarking on a clinical research career path. In addition to the CRCC Framework and the SCTO’s training database, the website contains a short description of career pathways, success stories of several physician-scientists, and searchable databases for career support and funding opportunities and for mentoring options.
Next steps and outlook
The CRCC Framework was initially developed for clinical trials since they are considered the most reliable form of scientific evidence and are the most regulated type of studies within clinical research. However, according to swissethics’ descriptive statistics on projects involving research on humans from 2018 to 2020, nearly 80% of studies submitted to Swiss ethics committees are observational prospective projects or studies that involve the further use of health-related data and/or biological material. Thus, most clinical research studies performed in Switzerland are governed by the HRO and are not subject to all the requirements in the ordinances governing clinical trials. Moreover, when young physician-scientists take their first steps in the complex and exciting field of research, they usually start with HRO-related projects. Therefore, the Education Platform is currently working on a simplified version of the CRCC Framework that would apply to human research projects only governed by the HRO. The CRCC Framework might be adapted in the future to better highlight the skills needed by other clinical research professionals (e.g., study nurses and/or coordinators).
Enhancing the quality and the outcomes of translational and clinical research is of high priority to allow Switzerland to remain internationally attractive and competitive as a research location. And an essential component of high-quality clinical research is having well-trained, multidisciplinary research teams. The CRCC Framework for clinical trials and its further development can allow educators to improve their training programmes, research professionals to better plan their career progression, and leadership at university and regional hospitals to better define research-specific job descriptions and clinical research positions. It therefore helps to develop clinical research in Switzerland, and it corresponds with future plans as described in the fifth goal of the SAMS’ White Paper Clinical Research: to strengthen multidisciplinary and integrated clinical research teams.
Acknowledgements
The authors thank collaborators from the JTF and TDR competencies frameworks for their availability and advice. We also appreciate the input provided by the following previous and current platform members: Andri Christen, François Curtin, Silke Ludwig, Barbara Peters, Cristiana Sessa, Fabian Tay, Sven Trelle, and Caecilia Schmid, who also critically reviewed the manuscript. We thank Meg Züblin for language editing and Annette Magnin for overall support. And we would like to thank the junior and senior physician-scientists who participated in the workshop (see procedure, step 3): Raphael Bernard-Valnet, Adalgisa Condoluci, Daniel Fuster, Philipp Kohler, Oriol Manuel, Marie-Anne Meier, Thomas Meinel, Victor Mergen, Dan Linh Nguyen-Kim, Jean-Luc Reny, and Chiara Zecca.
Potential competing interests
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
Melanie Glaettli, Swiss Clinical Trial Organisation, Bern, Switzerland, m.glaettli@scto.ch
Laura di Petto, Clinical Trial Unit, CTU-EOC Lugano, Lugano, Switzerland
Aurélie Fayet-Mello, Clinical Research Center (CRC), Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland
Claudia Fila, Clinical Trials Center, University Hospital Zurich, Zurich, Switzerland
Simone Kälin, Clinical Trials Unit, Kantonsspital St. Gallen, St. Gallen, Switzerland
Sandra Kohlmaier, Department of Clinical Research (DKF), University of Basel, Basel, Switzerland
Verena Küppers, Department of Clinical Research (DKF), University of Basel, Basel, Switzerland
Antoine Poncet, Centre de recherche clinique, Faculty of Medicine, University of Geneva, Geneva, Switzerland, Division of clinical epidemiology, Department of health and community medicine, University Hospitals of Geneva, Geneva, Switzerland
